Abstract
Immunity and inflammation are well established factors in the pathogenesis of pulmonary arterial hypertension (PAH). We aimed to investigate whether dexamethasone (Dex), a potent immunosuppressant, could prevent the development of monocrotaline (MCT)-induced PAH in rats as compared with pyrrolidine dithiocarbamate (PDTC) and its effect on the immune mechanism. PAH in rats (n = 66) was induced by MCT (50 mg/kg) injected intraperitoneally. Two days after MCT treatment, Dex (1.0 mg/kg) and PDTC (100 mg/kg) were administered once daily for 21 days. Samples were collected at 7, 14, and 21 days. Dex effectively inhibited MCT-induced PAH and reduced the T-helper (Th) 1 dominant cytokine response (interferon-γ) but up-regulated the Th2 one (interleukin 4). It increased the number of CD4+ T cells and decreased the number of CD8+ T cells around pulmonary arteries, upregulated the mRNA expression of fractalkine and downregulated that of CX3CR1 in the lung. Serum levels of interferon γ and interleukin 4 did not significantly differ from that of controls. Dex attenuated the process of MCT-induced PAH through its immunomodulatory property. Dex could be an appropriate therapy for PAH, although more studies are needed to define the appropriate treatment regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monocrotaline (MCT) is a pyrrolizidine alkaloid extracted from seeds of Crotalaria spectabilis and has been shown to induce endothelial-cell membrane disruption in rat pulmonary arteries with progressive pulmonary arterial hypertension (PAH) [1, 2]. Inflammatory and immune mechanisms appear to play a significant role in the pathogenesis of PAH. For example, PAH has been increasingly recognized as a common and severe complication of connective tissue diseases (CTD) such as systemic scleorosis, systemic lupus erythematosus and mixed connective tissue diseases. As well, cases of HIV-related PAH have been recognized with increasing frequency [3–5]. Histopathological changes in the recognized forms of PAH are similar [6]. Many pathobiological mechanisms of PAH have been identified, but the exact initiation and perpetuation of the pathological process are not well understood. Endothelial dysfunction is firmly established as an initiation and propagation factor in PAH [7–9]. The chemokine fractalkine (FKN/CX3CL1) is largely synthesized by endothelial cells as a membrane protein [10]. CX3CR1, the specific receptor for FKN, is expressed by monocytes and T lymphocytes [11]. T cells may have an important role in PAH [12, 13].
Dexamethasone (Dex), a potent anti-inflammatory and immunosuppressive glucocorticoid, is widely used to treat inflammatory and autoimmune disorders [14]. It inhibits the secretion of T-helper 1 cells (Th1) and enhances the production of Th2 cytokines in vivo and in vitro [15–17]. Interferon γ (IFN-γ; Th1-type cytokine) and interleukin 4 (IL-4; Th2-type cytokine) have a role in the Th cytokine response [18]. Case reports or observations in small groups of patients of PAH associated with CTD reported an improvement of PAH after treatment with immunosuppressants combined with corticoids [19, 20]. An NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC) [21], was reported to ameliorate pulmonary arterial pressure [22]. NF-κB is well documented to be involved in expression of various genes, including that of inflammatory cytokines, chemokines, immunoreceptors, and cell adhesion molecules [23]. Likewise, inhibitors of NF-κB possess a great therapeutic potential in inflammatory diseases [24, 25]. Glucocorticoids could also suppress the activation of NF-κB coincident with their anti-inflammation and anti-immune property [26].
We hypothesized that Dex could prevent PAH induced by MCT through its anti-inflammation and immunosuppressant properties. To understand how T cells act in PAH, we sought to examine the distribution of CD4+ and CD8+ T cells around the pulmonary arteries in a rat PAH model. We tested whether Dex could improve the microenvironment of endothelia in a rat PAH model and examined the mechanisms involved in the infiltration of T cells and FKN in the pulmonary vasculature in the progression of this endothelial-related disorder.
Materials and methods
Animal protocols
We randomly divided 66 male Wistar rats (6–8 weeks, 150–180 g) into 5 groups for treatment: (1) normal control (NC) (n = 18), 0.9% saline intraperitoneally (i.p.) once on day 1; (2) PAH (n = 18), MCT (Sigma-Aldrich, St. Louis, USA), 50 mg/kg i.p. once on day 1; (3) PDTC (Sigma-Aldrich, St. Louis, USA) (n = 6), 100 mg/kg daily, 2 days after MCT exposure for 3 weeks; (4) Dex (Lukang Pharmaceutical co., Jinan, China) (n = 6), 1.0 mg/kg daily, 2 days after MCT exposure for 3 weeks; (5) vehicle (n = 18), 0.9% saline daily after the same MCT exposure as PAH group for 3 weeks (data not shown because their data did not significantly differ from that of the PAH group). The control and PAH groups were killed on days 7, 14 and 21 after treatment; the PDTC and Dex groups were killed on day 21 after treatment. All procedures were approved by the Institutional Animal Care Committee of Shandong University.
Measurement of pulmonary arterial pressure
On the last day of treatment, rats were anesthetized by pentobarbital injection (45 mg/kg). An incision was made in the neck, and the external jugular vein was exposed and cannulated with a 3F tubing. The tubing, containing heparinized saline (30 IU/ml), was inserted into the right ventricle (RV), and the pressure was recorded and analyzed with use of the PowerLab System (AD Instruments, Castle Hill, Australia). Right ventricular systolic pressure (RVSP) was used to indicate pulmonary arterial pressure (PAP). The right lung was removed, quickly frozen in liquid N2, and stored at −80°C for RNA extraction. The left lung was placed in 10% buffered formaldehyde for immunohistochemistry (IHC).
Assessment of right-ventricular hypertrophy
Each heart was trimmed of atrial appendages, and the free wall of the RV was separated from the left ventricle (LV) and the septum (S). The ratio of RV to LV + S was calculated to assess right-ventricular hypertrophy (RVH).
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted by use of Total RNA Isolation Reagent (Bipec Biopharma, Cambridge, USA), and 500 ng was reverse transcribed in a 10-μl system by use of a Transcriptor First Strand synthesis kit (TaKaRa Biotechnology (Dalian) Co., Dalian, China), according to the manufacturer’s instructions. The amplification mixture (20 μl) contained 4 μl cDNA, 5 μl primers and 11 μl Ex Taq™ SYBR Premix (Takara Bio Inc., Otsu, Japan). Amplification was performed at 95°C for 2 min, then 40 cycles of 95°C for 30 s, and 60°C for 1 min. The amplification was performed in triplicate. PCR involved use of an ABI 7500HT instrument (Applied Biosystems), and data analysis involved use of the cycle threshold (Ct) 2−∆∆Ct with normalization software (Applied Biosystems). The primers are in Table 1.
Serum levels of IL-4 and IFN-γ by enzyme linked immunosorbent assay (ELISA)
Total serum levels of IL-4 and IFN-γ were determined by an ELISA kit (Uscn Life Science & Technology, Wuhan, China). Each sample was measured in duplicate.
Immunohistochemistry (IHC)
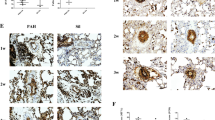
Formalin-fixed, paraffin-embedded lung tissue sections were analyzed by use of a streptavidine-biotin ABC kit (Zhongshan Co., Beijing). Briefly, sections (5 μm) were deparaffinized with turpentine and rehydrated through a graded ethanol series. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 15 min. Sections were heated at 98°C for 15 min in 10 mM sodium citrate buffer (pH 6.0) to retrieve antigen. Sections were incubated with primary antibodies against CD4 or CD8 (Biolegend, San Diego, CA, USA) overnight at 4°C, then incubated for 30 min with mouse-anti-rat secondary antibody (Zhongshan Co., Beijing) the next day. Immunoreactivity was visualized with use of diaminobenzidine, and sections were counterstained with hematoxylin. Brownish-yellow granular or linear deposits in the cells were interpreted as positive areas. The mean optical densities of CD4 or CD8 were measured at the original magnification (×400) in five randomly chosen fields.
Statistical analysis
Data are presented as mean ± SD. Statistical analysis involved use of SPSS v16.0 for Windows (SPSS Inc., Chicago, USA). The differences between groups were analyzed by ANOVA with post-hoc testing. Comparisons between two groups involved Student’s t test for parametric data and Mann–Whitney U test for nonparametric data. A P < 0.05 was considered statistically significant.
Results
Dex attenuated the establishment of PAH
As shown in Table 2, PAH induced in rats by MCT was evident at 2 weeks as measured by RVSP (37.34 ± 1.05 mmHg) and was further increased at 3 weeks (59.64 ± 2.64 mmHg). With PDTC treatment, RVSP was significantly attenuated (35.99 ± 1.26 mmHg) but was still higher than that in the control group (30.32 ± 0.62 mmHg) (P < 0.05). Dex restored PAP (31.99 ± 0.86 mmHg) (P > 0.05 vs. controls). Results for RVH were consistent with this trend.
mRNA expression of IFN-γ and IL-4 in the lung
With MCT treatment for 2 weeks to induce PAH, the mRNA expression of IFN-γ in lungs did not differ from that in controls but was increased at 3 weeks (P < 0.05). The mRNA expression of IFN-γ was lower with Dex treatment than 3-week PAH and PDTC treatment (Fig. 1a).
mRNA expression of IFN-γ, IL-4, FKN and CX3CR1 in rat lung. a IFN-γ. b IL-4. c FKN. d CX3CR1. * P < 0.05 vs. controls, # P < 0.05 vs. 3 week and PDTC treatment. NC controls (0.9% saline on day 1), 1, 2, 3 wk 1, 2, 3 weeks post-monocrotaline (MCT) treatment (50 mg/kg intraperitoneally on day 1), PDTC pyrrolidine dithiocarbamate treatment (100 mg/kg daily, 2 days after the MCT exposure for 3 weeks), Dex dexamethasone treatment (1.0 mg/kg daily 2 days after the MCT exposure for 3 weeks)
With MCT treatment for 2 weeks to induce PAH, the mRNA expression of IL-4 was downregulated in lungs but did not significantly differ from that in controls at 3 weeks. The IL-4 expression with Dex treatment was higher than that in 3-week PAH and with PDTC treatment (Fig. 1b).
Serum levels of IL-4 and IFN-γ
Because we found significant changes in mRNA levels of IFN-γ and IL-4 in the lung, we investigated whether the factors were also involved in systemic circulation in PAH. As shown in Table 3, serum levels of IFN-γ and IL-4 did not differ from controls at 1-, 2-, or 3-week PAH.
mRNA expression of FKN and CX3CR1 in lung
The mRNA expression of FKN was lower than that in controls with all treatments and was lowest at 3 weeks. With PDTC and Dex treatment, the expression of FKN was higher than that at 3 weeks (Fig. 1c).
The mRNA expression of CX3CR1 was high at 2 and 3 weeks as compared with controls. CX3CR1 level did not differ between PDTC and control treatment. Dex treatment produced minimal CX3CR1 expression (Fig. 1d).
CD4+ and CD8+ T cells around the pulmonary artery
As shown in Table 4, the number of CD8+ T cells increased significantly with MCT-induced PAH at 3 weeks, but treatment with PDTC and Dex prevented the increase in number of cells (Fig. 2). The number of CD4+ T cells gradually increased after MCT injection during the 3 weeks, with no significant difference in number of cells with PDTC and Dex treatment (Fig. 3).
Discussion
Hilliker et al. reported Dex reduced RVH in rats treated with MCT, but they were not sure whether this was related to Dex’s specific pharmacological actions [27]. Here, the study provide further evidence that Dex was efficient in preventing the development of MCT-induced PAH in rats. Dex treatment could significantly lower the PAP and suppress disease activities according to hemodynamic parameters and pathological changes of lung tissues. Dex was more potent than PDTC in preventing PAH in this model.
Dex treatment produced increased Th2-type cytokine expression (IL-4) accompanied by decreased Th1-type cytokine expression (IFN-γ) in the rat lung. However, our results were not consistent with those of Daley et al. [28], who reported lack of a polarized Th2 response in IL-4-deficient mice and significantly less severe pulmonary artery remodeling. In our study, the Th1 cytokine response was not activated during the first 2 weeks of PAH, and a Th2 cytokine response seemed to be suppressed from the beginning of treatment. Then the Th1 cytokine response was dominant at 3 weeks, which paralleled the peak of PAP (Fig. 1a, b). The groups did not differ in serum levels of IFN-γ and IL-4. Thus, Th-related cytokines were involved in part with the pathogenesis of PAH, and the Dex action was complicated in cellular and humoral immune responses [29].
The immune response in each tissue depends in part on lymphatic supply. Much research has demonstrated the potential pathologic role of T cells in PAH [13, 30]. Compelling evidence verified that CD4+ T cells specializing in the suppression of the immune response play a critical role in immune regulation [31]. CD4+ T cells can produce multiple cytokines. In our study, CD4+ T cells seemed to work by suppressing a Th1-type response, as was shown by reduced IFN-γ production with Dex treatment. Kumaraguru et al. [32] found that IFN-γ was critical in the response by CD4+ Th-dependent CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T cells can prevent CD8+ CTL activity in vivo through CD4+ regulatory T cells [33]. Our finding of increased number of perivascular CD8+ T cells with 3-week PAH reflected a particular phenomenon related to PAH evolution, and the infiltration into the pulmonary artery was reduced with Dex treatment (Fig. 2). In addition, we must point out that macrophages also played an important role in PAH [12] and we have found a time-dependent increase of them in the vicinity of remodeled pulmonary vessel walls in this model (Fig. S1 in Supplementary material).
We also aimed to explore the approaches for adhesion of T cells to endothelia of pulmonary arteries by examination of FKN and CX3CR1 levels. FKN can perform both adhesive and chemotactic functions [34]. Expression of chemokines are usually upregulated by inflammatory reaction, but interestingly, we found the highest level of FKN in normal rats (Fig. 1c). This finding contrasts with that of Perros et al. [35] whose research found FKN gene expression remained elevated during the process of PAH with the same animal models. One explanation for the difference is that the destruction of endothelial cells led to reduced expression of FKN. Although Dex did not completely rescue FKN expression, it diminished the level, which suggests its potential protection of endothelial cells. CX3CR1 is expressed by T lymphocytes and monocytes [36]. With disease progression, the expression of CX3CR1 is promoted. We found that the expression of CX3CR1 did not correspond to that of FKN with treatment. As compared with PDTC treatment, Dex downregulated CX3CR1 expression to much lower levels than that for FKN (Fig. 1d). This finding was probably a result of Dex inhibiting the migration of other inflammatory cells. Plasma-soluble FKN (sFKN) has potent chemoattractant activity for T cells and monocytes [11], but additional experiments are still needed to evaluate whether sFKN is expressed more and plays a more important role as compared with other kinds of chemokines in PAH. We speculated that FKN and CX3CR1, a new type of leukocyte trafficking regulators, have a complicated modulatory mechanism on T cells in rat lungs, and other chemokines and leukocyte adhesion molecules might be responsible for the invasion of T cells.
PDTC ameliorated PAH in rats by an NF-κB/vascular cell adhesion molecule 1 pathway [2]. However, Dex could also trigger other inflammatory or immunologic cascades through other pathways, which may be causally related to its inhibition of PAH [37, 38]. Further investigations are required to elucidate the mechanism by which Dex is more powerful than PDTC in the immune response.
Our study contains some limitations. First, although numerous studies of animal models with MCT-induced PAH and the effects of anti-inflammation treatment have stressed the importance of such therapeutic drugs [39, 40], no genetic evidence was demonstrated that inflammatory mechanism itself is necessary for the development of PAH in this trial. Second, a large, randomized, placebo-controlled clinical trial is essential to verify longterm efficacy and safety of Dex. Further studies are needed to address these questions. In addition, we didn’t evaluate the severity of pulmonary medial hypertrophy. As shown it was not difficult to find the difference among rats treated with MCT and Dex (Fig. 4). Despite these limitations, the significant improvements in pulmonary hemodynamics are strong evidence for a potent protective effect of Dex. Dex may have ameliorated the development PAH and pulmonary vascular disease in MCT-treated rats, at least in part by suppressing perivascular numbers of CD8+ T cells, decreasing the mRNA expression of IFN-γ and restoring the integrity of endothelial cells. Dex used as an immunosuppressive agent may contribute to the reduction of PAH.
References
Tanino Y (2001) Monocrotaline-induced pulmonary hypertension in animals. Nippon Rinsho 59:1076–1080
Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, Komada Y (2007) A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 132:1265–1274
Dorfmüller P, Perros F, Balabanian K, Humbert M (2003) Inflammation in pulmonary arterial hypertension. Eur Respir J 22:358–363
Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF (2005) Autoimmunity and pulmonary hypertension. Eur Respir J 26:1110–1118
Lederman MM, Sereni D, Simonneau G, Voelkel NF (2008) Pulmonary arterial hypertension and its association with HIV infection: an overview. AIDS 22:S1–S6
Chan SY, Loscalzo J (2008) Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44:14–30
Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF (2001) The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med 22:405–418
Budhiraja R, Tuder RM, Hassoun PM (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109:159–165
Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW (2009) Evidence for dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 180:780–787
Foussat A, Coulomb-L’Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D (2000) Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol 30:87–97
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ (1997) A new class of membrane bound chemokine with a CX3C motif. Nature 385:640–644
Tuder RM, Groves B, Badesch DB, Voelkel NF (1994) Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144:275–285
Taraseviciene-Stewart L, Scerbavicius DK, Burns N, Cool CD, Nicolls MR, Voelkel NF (2005) The protective role of T-lymphocytes in pulmonary vascular remodeling. Chest 128:571S–572S
Cupps TR, Fauci AS (1982) Corticosteroid-mediated immunoregulation in man. Immunol Rev 65:133–155
Ramírez F, Fowell DJ, Puklavec M, Simmonds S, Mason D (1996) Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol 156:2406–2412
DeKruyff RH, Fang Y, Umetsu DT (1998) Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 160:2231–2237
Guo C, Chu X, Shi Y, He W, Li L, Wang L, Wang Y, Peng J, Hou M (2007) Correction of Th1-dominant cytokine profiles by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. J Clin Immunol 27:557–562
Romagnani S (1997) The Thl/Th2 paradigm. Immunol Today 18:263–266
Sanchez O, Humbert M, Sitbon O, Simonneau G (1999) Treatment of pulmonary hypertension secondary to connective tissue diseases. Thorax 54:273–277
Bellotto F, Chiavacci P, Laveder F, Angelini A, Thiene G, Marcolongo R (1999) Effective immunosuppressive therapy in a patient with primary pulmonary hypertension. Thorax 54:372–374
Liu SF, Ye X, Malik AB (1999) Inhibition of NF-kB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 100:1330–1337
Huang J, Kaminski PM, Edwards JG, Yeh A, Wolin MS, Frishman WH, Gewitz MH, Mathew R (2008) Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol 294:L1250–L1259
Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866
Lee UJ, Choung SR, Prakash KV, Lee EJ, Lee MY, Kim YJ, Han CW, Choi YC (2008) Dual knockdown of p65 and p50 subunits of NF-kappaB by siRNA inhibits the induction of inflammatory cytokines and significantly enhance apoptosis in human primary synoviocytes treated with tumor necrosis factor-alpha. Mol Biol Rep 35:291–298
Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS Jr (1999) Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem 274:31868–31874
Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr (1995) Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270:283–286
Hilliker KS, Roth RA (1984) Alteration of monocrotaline pyrrole-induced cardiopulmonary effects in rats by hydrallazine, dexamethasone or sulphinpyrazone. Br J Pharmacol 82:375–380
Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G (2008) Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 205:361–372
Wilckens T, De Rijk R (1997) Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today 18:418–424
Pinto RF, Higuchi Mde L, Aiello VD (2004) Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol 13:268–275
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Kumaraguru U, Banerjee K, Rouse BT (2005) In vivo rescue of defective memory CD8+ T cells by cognate helper T cells. J Leukoc Biol 78:879–887
Trzonkowski P, Szmit E, Myśliwska J, Myśliwski A (2006) CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans impact of immunosenescence. Clin Immunol 119:307–316
Imaizumi T, Yoshida H, Satoh K (2004) Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb 11:15–21
Perros F, Dorfmüller P, Souza R, Durand-Gasselin I, Godot V, Capel F, Adnot S, Eddahibi S, Mazmanian M, Fadel E, Hervé P, Simonneau G, Emilie D, Humbert M (2007) Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J 29:937–943
Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O (1997) Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91:521–530
Kagoshima M, Ito K, Cosio B, Adcock IM (2003) Glucocorticoid suppression of nuclear factor-kappa B: a role for histone modifications. Biochem Soc Trans 31:60–65
Hayashi R, Wada H, Ito K, Adcock IM (2004) Effects of glucocorticoids on gene transcription. Eur J Pharmacol 500:51–62
Faul JL, Nishimura T, Berry GJ, Benson GV, Pearl RG, Kao PN (2000) Triptolide attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 162:2252–2258
Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN (1999) Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem 274:13443–13450
Acknowledgments
The authors are grateful to Fengqin Liu and Qingqing Wang for excellent secretarial work. We also greatly appreciate the staff of the Central Laboratory of our hospital.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Fig. S1
Immunohistochemical analysis of macrophages in rat lungs. Sections were from control treatment (a), 1 week post-MCT treatment (b), 2 weeks post-MCT treatment (c), 3 weeks post-MCT treatment (d), PDTC treatment (e), Dex treatment (f). Positive-stained cells are brown. Magnification ×400 (JPEG 1227 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Wang, Yl., Chen, Xy. et al. Dexamethasone attenuates development of monocrotaline-induced pulmonary arterial hypertension. Mol Biol Rep 38, 3277–3284 (2011). https://doi.org/10.1007/s11033-010-0390-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0390-x