Abstract
Interleukin-22 (IL-22) is a member of the IL-10 family. Its potential in clinical use has been highlighted for its important roles in promoting antimicrobial defense and preventing epithelial damages. Previous studies have reported that IL-22 can be expressed using prokaryotic systems and purified from inclusion bodies, however the recovery rate was poor. To produce functional IL-22 with a high yield, human IL-22 was inserted into the eukaryotic expression vector pPICZαA and transformed into Pichia pastoris. The expression of recombinant human IL-22 (rhIL-22) was induced by methanol and accounted for about 85% of the total secreted proteins. A simple purification strategy was established to purify the rhIL-22 from the culture supernatant, yielding 100 mg/l at 90% purity by chromatography with a SP Sepharose FF column. Bioactivity analysis showed the purified rhIL-22 demonstrated a specific activity that was comparable with the commercial one. This study provides a new strategy for large-scale production of bioactive IL-22 for use in basic studies and therapeutic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interleukin-22 (IL-22) was originally identified in interleukin-9 stimulated T cells and in mast cells from mice [1]. Recently, IL-22 has been found to be preferentially produced by T helper 17 cells [2–4]. The expression of its receptor has been found to be restricted to skin, respiratory and digestive tissues, but not immune tissues. Therefore, it is proposed that IL-22 should not act directly on immune cells, but act as a multifunctional effector to promote the innate non-specific immunity of tissues [5]. IL-22 plays an important role in host defense against extracellular pathogens and preventing damages of the mucosal epithelial tissue in the respiratory and digestive systems [6, 7]. Moreover, IL-22 is also involved in the pathogenesis of some autoimmune diseases in the skin [4]. These findings indicate that IL-22 might be a novel type of immune mediator and a potential therapeutic target for autoimmune diseases. In fact, IL-22 and its antagonist have been proved to be effective in the treatment of ulcerative colitis and psoriasis, respectively [8, 9]. With the increasing potential of IL-22 in clinical applications, more interests are focused on the high-level expression and preparation of IL-22.
Although IL-22 can be produced in prokaryotic expression systems [10], the target protein forms inclusion bodies and makes the following purification process involving a complicated refolding step with a low recovery rate. In addition, there is report of recombinant IL-22 expression in insect cells line, which is not only costly but also unsuitable for commercial large-scale production of therapeutic proteins [11].
The Pichia pastoris expression system is being used successfully for the production of various recombinant heterologous proteins. Most important, P. pastoris is a eukaryote, and thereby provides the potential for producing soluble, correctly folded recombinant proteins that have undergone all the post-translational modifications required for functionality. In this study, we established a more efficient method to produce soluble and bioactive recombinant human IL-22 (rhIL-22) using a eukaryotic expression system of Pichia pastoris, which lays the basis for further studying its role in the pathogenesis of immune diseases as well as in the clinical applications.
Materials and methods
Construction of the expression vector
The DNA (460 bp) fragment coding for human IL-22 was amplified from the recombinant expression plasmid pBV-IL22 which was previously constructed for Escherichia coli expression in our laboratory. The forward and reverse primers were 5′-ATCTCGAGAAAAGAGCGCCCATCAGCTCC-3′ containing an XhoI site (underlined) and 5′-GTTCTAGATCAAATGCAGGCATTTCT-3′ containing an XbaI site (underlined), respectively. The PCR product was digested with XhoI and XbaI, and then inserted into the expression vector pPICZαA (Invitrogen, USA). The recombinant plasmid pPICZαA-IL22 was confirmed by restriction analysis and sequencing.
Transformation and selection of rhIL-22 expression strain
Pichia pastoris KM71H were transformed with a linearized expression vector digested with PmeI. Transformation was performed using the electroporation method as described in the P. pastoris expression manual (Invitrogen, USA). The transformants were analyzed by colony PCR to verify the integration of the recombinant gene [12]. Positive transformants were tested for their ability to secrete rhIL-22 into the cell culture supernatants as follows. Cells were inoculated into 5 ml of buffered minimal glycerol (BMGY) medium and allowed to grow for 24 h, then 1% (v/v) methanol was added to the culture at intervals of 12 h during an incubation period of 120 h. The supernatants were collected and analyzed by 15% SDS–PAGE.
Expression and purification of rhIL-22
Positive transformants were picked up from the plates and inoculated into a flask containing 10 ml BMGY medium. After shaking at 30°C, 300 rpm for 24 h, the culture was centrifuged at 4°C, 3,000 rpm for 5 min. The collected cells were resuspended in 2 ml BMMY medium and allowed to grow at 30°C for 6 days, and then the methanol was added every 24 h. To obtain the highest yield of protein, different culture parameters including pH and concentration of methanol added daily were evaluated. In addition, 2% (w/v) Casamino acids was added to the induction medium to enhance protein accumulation [13]. Scale-up expression was performed under the optimized cultivation conditions. The culture was centrifuged at 5,000 rpm for 15 min and the supernatant was concentrated by ultrafiltration. The concentrated sample was loaded onto SP Sepharose FF column (Pharmacia Biotech Inc., Sweden) and eluted with linear gradient of 0–2.0 M NaCl in PBS. The eluted fractions were analyzed by 15% SDS–PAGE.
Western blot analysis
Protein samples were separated by 15% SDS–PAGE and electroblotted onto a nitrocellulose membrane (Amersham Biosciences, USA). The membrane was blocked with 5% (w/v) milk in 10 mM Tris–HCl with 150 mM NaCl (pH 8.0) and 0.1% (v/v) Tween-20 (TBST) for 2 h at room temperature (RT). The membrane was then incubated for 2 h at RT with anti-human IL-22 monoclonal antibody (R&D, USA). After washing three times, the membrane was incubated for 1 h with peroxidase-conjugated goat anti-mouse IgG (ZhongShan Ltd. China). The membrane was washed with TBST and the specific protein bands were visualized by ECL detection kit (Amersham Pharmacia Biotech, Canada).
MTT proliferation assay
The growth-promoting effects of rhIL-22 and its commercial product (R&D, USA) on HepG2 cells were determined using MTT assay. HepG2 cells were cultured in RPMI1640 medium (Gibco, USA) with 10% (v/v) fetal bovine serum (FBS) and kept in an incubator with 5% CO2 at 37°C. Cells were adjusted to a concentration of 1 × 105 cells/ml and seeded in 96-well flat-bottom plates. Then, different concentrations (5–4,000 ng/ml) of rhIL-22 and commercial IL-22 were added to each well and cultured for 72 h. Cell proliferation was analyzed by the MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide) proliferation assay as described [14]. Absorbance at 570 nm was measured using a Bio-Rad model 550 microplate reader (BioRad Molecular Bioscience Group, USA).
Results
Construction of the recombinant expression plasmid
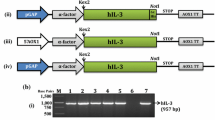
The DNA fragment encoding IL-22 was inserted between the XhoI and XbaI sites of P. pastoris expression vector pPICZαA which contains a Saccharomyces cerevisiae alpha factor leader and an alcohol oxidase1 (AOX1) promoter (Fig. 1). Sequencing of the recombinant plasmid pPICZαA-IL22 confirmed that the reading frame of IL-22 was correct.
Transformation and expression of rhIL-22
The recombinant vector pPICZαA-IL22 was transformed into P. pastoris KM71H cells by electroporation. The transformed strains were analyzed by colony PCR to evaluate the integration of IL-22 in P. pastoris transformants. A 460 bp insert was detected in positive transformed KM71H cells.
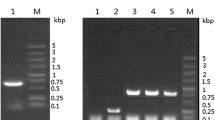
The positive colonies were cultured and induced to express the recombinant proteins. SDS–PAGE analysis showed that rhIL-22 was successfully expressed in the P. pastoris KM71H transformant. The culture supernatant was harvested from the culture every 24 h after methanol induction. As shown in Fig. 2a, a band corresponding to rhIL-22 with an expected molecular weight about 16 kDa was observed at 24 h after induction and the maximal expression level was reached at 96 h (Fig. 2a). This result was confirmed by Western blot analysis (Fig. 2b). Optimum conditions leading to maximum IL-22 yield were: pH 5.0, methanol daily addition of 0.5% (v/v) and induction time of 96 h. The addition of 2% (w/v) Casamino acids had no effect on IL-22 production.
Expression of rhIL-22 in P. pastoris KM71H at different time points. a 15% SDS–PAGE analysis of rhIL-22 expressed in P. pastoris after induction by methanol. Lane M low molecular weight protein marker; lane 1 culture supernatants from P. pastoris transformed with pPICZαA; lanes 2–6 culture supernatants from P. pastoris transformed with pPICZαA-IL22 at 24, 48, 72, 96 and 120 h after methanol induction, respectively. The arrow indicates the location of rhIL-22. b Western blot analysis of rhIL-22 expression. Lanes 1–5 culture supernatants collected from P. pastoris transformed with pPICZαA-IL22 at 24, 48, 72, 96 and 120 h after methanol induction, respectively
Purification of rhIL-22
The culture supernatant was collected and the concentration of rhIL-22 was estimated to be 150 mg/l according to the analysis using 15% SDS–PAGE. rhIL-22 was subsequently purified by a SP Sepharose FF column, and identified by Western blot using anti-hIL22 monoclonal antibody (Fig. 3, lane 3). The purified rhIL-22 appeared as a single band on 15% SDS–PAGE with a purity of 90% (Fig. 3, lane 2). In total, 100 mg of rhIL-22 were obtained from 1 l of culture supernatant, representing a recovery rate of 66% of the total rhIL-22 from the culture supernatant.
Activity assay of rhIL-22
The biological activity of rhIL-22 was detected by the MTT method and was compared with that of commercially available IL-22. The result showed that rhIL-22 could stimulate the growth of HepG2 cells over a range from 5 to 4,000 ng/ml, and the cell proliferation of HepG2 was significantly stimulated with a peak effect at the concentration of 1,000 ng/ml (Fig. 4). The activity of the purified rhIL-22 was comparable with the commercially available one derived from E. coli.
The effects of rhIL-22 on HepG2 cells. Cells (1 × 105 cells/ml) were seeded in 96-well plates and incubated with the indicated amount of recombinant proteins at 37°C for 72 h. Cell growth was evaluated by the MTT assay, and the absorbance of 570 nm was measured. The data represent the means of three independent experiments
Discussion
IL-22 is a cytokine secreted by activated T cells, which plays an important role in regulating the immune response. Some studies have suggested that IL-22 is involved in the pathogenesis of various immune diseases [15–17]. The promising value of IL-22 in clinical applications has been highlighted, particularly for the treatment of diseases such as ulcerative colitis and mucosal infections [6, 18].
IL-22 is mainly produced in prokaryotic expression systems and is purified from the inclusion bodies [10]. However, this process involves a complicated refolding step of the inclusion bodies. Moreover, the contamination of pyrogenic components derived from E. coli is a significant concern for clinical applications. The eukaryotic expression system of P. pastoris has become increasingly popular for its ease of genetic manipulation and ability to grow to a high cell density, which allows high yield. In addition, pyrogenic contamination is not a concern. Many pharmaceutical proteins have been successfully produced using this system [19].
To produce large amounts of bioactive human IL-22 for both basic and clinical studies, we testsed P. pastoris as an expression system. After optimization of the expression conditions, rhIL-22 accounted for about 85% of the total secretory proteins. After purification by SP Sepharose FF column, 100 mg of rhIL-22 was obtained from 1 l of culture supernatant, which is higher than the yield of refolded rhIL-22 (33 mg from 1 l culture) previously produced by E. coli in our laboratory (unpublished data). The in vitro activity assay confirmed that biological activity of the purified rhIL-22 was comparable with the commercial available one.
In conclusion, we have developed a novel and highly efficient way of producing biologically active rhIL-22, which could be adopted for large-scale production of functional rhIL-22. Also this work will help to promote studies on the clinical application of IL-22 as well as clarification of its role in immune system.
References
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 1:488–494
Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C (2006) Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16:902–907
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coex-pressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648–651
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21:241–254
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K (2008) IL-22 mediates mucosal host defense against gram-negative bacterial pneumonia. Nat Med 14:275–281
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289
Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118:534–544
Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA (2008) IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 118:597–607
Dumoutier L, Lejeune D, Colau D, Renauld JC (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 166:7090–7095
Xu T, Logsdon NJ, Walter MR (2004) Crystallization and X-ray diffraction analysis of insect cell derived IL-22. Acta Crystallog sect D Biol Crystallog sect D 60:1295–1298
Braud S, Bon C, Wisner A (2000) Snake venom proteins acting on hemostasis. Biochimie 82:851–859
Shi X, Karkut T, Chamankhah M, Alting-Mees M, Hemmingsen SM, Hegedus D (2003) Optimal conditions for the expression of a single-chain antibody (scFv) gene in Pichia pastoris. Protein Expr Purif 28:321–330
vandeLoosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM (1994) A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174:311–320
Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36:1309–1323
Wolk K, Witte E, Wallace E, Döcke WD, Endesfelder S, Asadullah K, Volk HD, Sterry W, Sabat R (2007) IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J.Immunol 178:5973–5981
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y (2005) Expression of interleukin-22 in rheumatoid arthritis: potential role a proinflammatory cytokine. Arthritis Rheum 52:1037–1046
Radaeva S, Sun R, Pan HN, Hong F, Gao B (2004) Interleukin22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39:1332–1342
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Author information
Authors and Affiliations
Corresponding author
Additional information
Xin Cai and Jinfeng Wang contributed equally to this work as co-first authors.
Rights and permissions
About this article
Cite this article
Cai, X., Wang, J., Wang, Y. et al. Expression, purification and characterization of recombinant human interleukin-22 in Pichia pastoris . Mol Biol Rep 37, 2609–2613 (2010). https://doi.org/10.1007/s11033-009-9785-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9785-y