Abstract
Breast carcinoma (BC) is a prevalent malignant tumour occurring in women. Many studies have indicated the role of human papilloma virus type 16 (HPV16) in the pathogenesis of BC; however, the correlations of HPV16 infection with the clinicopathologic features of BC and the expressions of c-erbB-2 and bcl-2 have not yet been elucidated. In this study, HPV16 was detected by amplifying the HPV16 E6 gene by the polymerase chain reaction method, and the expressions of c-erbB-2 and bcl-2 in 40 BCs and 20 normal breast tissue samples, obtained from Shaanxi Province, were examined using the streptavidin-peroxidase method with monoclonal antibodies specific to c-erbB-2 and bcl-2. The infection rate of HPV16 E6 and the positive expression rate of c-erbB-2 were significantly higher in the BCs than in the normal tissues (HPV16 E6: 60% vs. 5%; c-erbB-2: 42.5% vs. 5%, P < 0.05). However, the positive expression rate of bcl-2 was significantly lower in the BCs than in the normal tissues (67.5% vs. 95%, P < 0.05). The infection rate of HPV16 did not correlate with any of the pathological features observed (P > 0.05). HPV16 infection correlated with bcl-2 expression (P = 0.015) but not with c-erbB-2 expression (P = 0.747) in the BCs. Interestingly, HPV16 infection correlated with bcl-2 expression in grade I BCs (P = 0.018) but not in grade II–III BCs (P = 0.633). Our data suggest that HPV16 infection is correlated with bcl-2 expression in BCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human papilloma virus (HPV) is an important tumour-related virus, usually detected in cervical cancer. Recent studies have demonstrated HPV18 and, in particular, HPV16 in breast cancers (BCs) [1–3]. HPV16 E6 is an oncoprotein synthesized by HPV16. Substantial studies have shown the potential of HPV16 E6 to induce cell proliferation and apoptosis [4–6]. It can bind to the tumour suppressor p53 and promote its degradation, consequently stimulating cell proliferation [7–9]. A recent study also suggested that the E6 activity downregulates P21, which is important for the induction of apoptosis [10]. It is unclear whether the oncoprotein HPV16 E6 modulates other P53-independent pathways besides P21 and thus induces carcinogenesis. c-erbB-2 and bcl-2 are involved in cell proliferation and apoptosis. Therefore, in this study, we investigated the correlations of HPV16 infection with the clinicopathologic features of BCs and the expressions of c-erbB-2 and bcl-2; the results obtained may help us further understand the function of HPV16 in BCs.
Materials and methods
Tissue samples
During 2006–2007, 40 primary BC samples and 20 normal breast tissue (NBT) samples were obtained postoperatively from the Second Affiliated Hospital of Xi’an Jiaotong University. The median age of the BC patients was 51 years (range, 41–65 years). The histopathological types of the primary tumours that were re-evaluated according to the World Health Organization (WHO) criteria comprised invasive ductal carcinoma (n = 31, 77.5%), invasive lobular carcinoma (n = 1, 2.5%), invasive ductal carcinoma with predominantly intraductal components (n = 4, 10.0%), and medullary carcinoma (n = 4, 10.0%). All the carcinomas were classified into grade I, II, or III according to a histological grading system [11] in which codifications were given to the WHO classification. Pathological examination of the BC and NBT samples was strictly performed at the department of pathology of the respective hospital. None of the BC patients received any adjuvant chemotherapy or radiotherapy prior to the operation. The acquisition of patient tissue samples was approved by The Human and Ethical Committee for Medical Research at Xi’an Jiaotong University School of Medicine. All the samples were snap-frozen in liquid nitrogen and stored at −70°C until use.
DNA extraction and polymerase chain reaction
The BC and NBT samples were minced and homogenized in lysis buffer (300 mmol/l NaCl, 50 mmol/l Tris–HCl [pH 8.0], 0.2% sodium dodecyl sulphate [SDS], and 200 mg/l proteinase K). The homogenate was incubated at 55°C overnight. DNA was extracted using phenol/chloroform, precipitated with cold alcohol, and dissolved in ion-free water. DNA concentration was determined from its optical density (OD) at 260 nm. A set of specific primers (P1: 5′CGG AAT TCA TGC ACC AAA AGA GAA CTG CA3′, P2: 5′CCC AAG CTT ACA GCT GGG TTT CTC TAC G3′) was designed to amplify the HPV16 E6 gene [12]. PCR was performed as follows: initial denaturation at 94°C for 5 min followed by denaturation at 94°C for 50 s, annealing at 55°C for 50 s, extension at 72°C for 1 min for 30 cycles, and final amplification at 72°C for 10 min. The product size was approximately 494 bp. The pBR322/hpv16 plasmid containing the full-length HPV16 genome was used as the positive control, and the pUC19 plasmid was used as the negative control. The success of PCR was assessed by electrophoresis of the PCR products in 1.5% agarose and compared with SD002 (Dingguo Biological, Beijing, China).
Immunohistochemistry
Immunoperoxidase staining of formalin-fixed, paraffin-embedded tissue sections was performed using the streptavidin-peroxidase (SP) method. After deparaffinization and rehydration in descending alcohol dilution, the sections were heated in an 800-W microwave oven at maximal power for 5 min in 0.01 M citrate buffer (pH 6.0) for antigen retrieval. After cooling to room temperature, the activity of endogenous peroxidase in the treated sections was blocked by 0.3% hydrogen peroxide in methanol for 10 min at room temperature. The sections were then blocked with 5% normal mouse serum in phosphate-buffered saline (PBS) for 10 min followed by incubation with mouse anti-c-erbB-2 or anti-bcl-2 monoclonal antibody (Xiuwei Biotechnology Co Ltd, Guangdong, China) overnight at 4°C. The subsequent steps were performed using an ultrasensitive SP kit (Maixin Biological, Fuzhou, China) according to the manufacturer’s protocols. At the completion of the procedure, the sections were counterstained with haematoxylin. Negative controls were obtained by omitting the use of the primary antibodies. Normal ductal epithelial cells or lymphocytes were used as internal positive controls for bcl-2 expression. BC samples showing c-erbB-2 amplification were also used as positive controls [13].
Evaluation of immunostaining
Positive staining for c-erbB-2 and bal-2 was detected in the cell membrane and membrane/cytoplasm, respectively. The intensity of bcl-2 protein expression was compared with the immunostaining findings in the normal ductal epithelial cells and lymphocytes, which were used as internal controls. The expression was classified into the following three categories by using the results of the comparison: strong, when the intensity of the bcl-2 expression was similar to or greater than that of the internal controls; weak, when the intensity was inferior to that of the internal controls; and negative, when there was no staining. According to the staining intensity and the proportion of cancer cells showing positive immunoreactivity, bcl-2 expression could be classified into 3 grades: (−) = no staining or staining in less than 10% cancer cells; (+) = strong staining in more than 10% but less than 50% cancer cells, or weak staining in more than 10% cancer cells; and (++) = strong staining in more than 50% cancer cells.
The c-erbB-2 expression were scored as follows: 0 = no staining or weak and incomplete membrane staining in less than 10% cancer cells; (+) = incomplete and faint membrane staining in more than 10% cancer cells; (++) = moderate and complete membrane staining in more than 10% cancer cells; and (+++) = strong and complete membrane staining in more than 10% cancer cells. According to the criteria described above, cases with scores (++) and (+++) were expected to show c-erbB-2 protein overexpression. Further, the c-erbB-2 protein was considered positively expressed (+) or overexpressed when more than 10% cancer cells revealed distinct nuclear and membrane staining, respectively; otherwise, they were regarded as negative (−).
Statistical analysis
Statistical significance was determined by chi-squared and Fisher’s exact tests. Analysis was performed using the SPSS 11.5 software for Windows. A value of P < 0.05 was considered statistically significant.
Results
HPV16 E6 gene in BCs
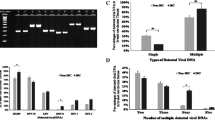
The HPV16 E6 gene was detected in 24 of the 40 BC samples (60.0%) but in only 1 of the 20 (5.0%) NBT samples by the PCR method. The infection rate of HPV16 was higher in the BCs than in the NBTs (P = 0.001; Fig. 1, Table 1).
Part of results of detecting HPV16 E6gene by PCR method. A: DNA molecular weight standard SD002 marker. B: PCR of pUC19 plasmid as negative control. C: PCR of pBR322/HPV16 plasmid as positive control. D: PCR of CaSKi DNA as positive control. E: negative PCR in BCs. F: No template as negative control. G–I: positive PCR in BCs
Correlations between HPV16 infection in BCs and histological parameters
HPV16 infection did not exhibit statistical correlations with age, lymph node metastasis, histological grade, ER and PR (P > 0.05; Table 2).
The expressions of c-erbB-2 and bcl-2 in BCs
The positive expression rates of c-erbB-2 and bcl-2 were 42.5%(17/40) and 67.5%(27/40) in the 40 BC samples, respectively, and 5% (1/20) and 95% (19/20) in the 20 NBT samples, respectively (Fig. 2). The positive expression rate of c-erbB-2 was significantly higher in the BC samples than in the NBT samples (P = 0.003), whereas the positive expression rate of bcl-2 was significantly lower in the BC samples than in the NBT samples (P = 0.023; Table 1).
The relationship between HPV16 infection and the expressions of c-erbB-2 and bcl-2 in BCs
Immunoreactivity of the bcl-2 protein was significantly higher in the HPV16-infected BCs than in the HPV16-noninfected BCs (P = 0.015). However, there was no difference in the c-erbB-2 immunoreactivity between these two groups (P = 0.747; Table 3).
The relationship between HPV16 infection and the expressions of c-erbB-2 and bcl-2 in different grades of BCs
There was no difference in the c-erbB-2 expression level between the HPV16-positive and HPC16-negative BC samples, irrespective of their pathological grade (P = 1.000 and P = 0.607, respectively). In contrast, the bcl-2 expression in grade I BC samples significantly differed between the HPV16-positive and HPV16-negative BC samples (P = 0.018). However, the expression of bcl-2 in grade II–III BC samples did not show a significant difference (P = 0.633; Table 3).
Discussion
In 1990, Band et al. [14] proposed that products of the HPV genome could induce immortalization in human breast epithelial cells. Since then, researchers have focussed on the relationship between HPV and BCs. In our study, HPV16 infection was found to be more frequent in BCs than in NBTs. This result indicates that HPV16 might correlate with the occurrence of BC. Previous studies have shown that the HPV16 infection rate varied from 0 to 46% [1−3, 15−20]; these rates are lower than that observed in our study. After reviewing the relevant literature, we attribute this discrepancy in the infection rate to two factors. Firstly, some of these studies used paraffin-embedded specimens to investigate the HPV levels; this could have decreased the sensitivity of the detection. However, in the present study, we used frozen tissue specimens. Secondly, since the HPV16 E6 gene exists stably in BC cells, we used this gene as the marker gene and not the L1 gene which was used in previous studies. During HPV integration into the host genome, the L1 gene is frequently lost [21]. Therefore, in studies using the L1 gene as the marker gene, the infection rate of HPV in BCs might have been underestimated. In addition, our results indicated that HPV16 was detectable in the BC samples irrespective of the patients’ age, lymph node metastasis, and histological grade; this suggests that HPV16 infection may function as a biological promoter in the pathogenesis of BC.
The bcl-2 protein, which inhibits apoptosis, is normally expressed in some adult progenitor cells from tissues such as blood and the intestine. This protein is also expressed in hormonally regulated epithelium of tissues such as the breast and prostate, although its functional role in these tissues is not distinct. The in vitro expression of bcl-2 is associated with resistance to chemotherapeutic agents in haematogenous malignancies, where its expression probably indicates poor prognosis. In solid tumours, however, its role is more complex. While bcl-2 expression might predict a poor prognosis in haematogenous malignancies, the reverse appears to be true in the case of BCs. A number of retrospective analyses have shown that bcl-2 is associated with favourable prognostic factors and increased survival in BCs [22, 23]. In the present study, we observed a lower bcl-2 expression in the BCs than in the NBTs. One explanation for this paradox is that the bcl-2 protein is involved in the regulation of tumour growth by prolonging the cell cycle, and this effect differs from its antiapoptotic function [24].
In the present study, we found a significant positive correlation between HPV infection and bcl-2 expression in the BCs. Our results also suggest that HPV16 infection might induce bcl-2 expression in the early stage of BC. Grace VM and Sidransky [25, 26] proposed that p53 inactivation by the oncoprotein E6 of the high-risk HPV subtypes releases the repressed bcl-2 gene, leading to the overexpression of both the bcl-2 protein and the nonfunctional p53 protein in cervical cancer. It is not clear whether the upregulation of the bcl-2 protein is a direct effect of HPV infection or an indirect effect through p53 protein inactivation in BCs. Further studies detailing the interaction between E6 and bcl-2 are therefore necessary.
The c-erbB-2 gene, also named HER-2, is a proto-oncogene that encodes a transmembrane glycoprotein similar to the human epidermal growth factor receptor known as the HER-2 protein. This gene is well known for its cell proliferation capacity. A literature review shows a controversy regarding the relationship between HPV and c-erbB-2. Hennig [27] reported no significant correlation was found between HPV16 and c-erbB-2 expression in BCs. Similar results were reported in cervical carcinomas [28, 29]. However, Lam [30] has reported a correlation between c-erbB2 overexpression and HPV in oesophageal carcinoma in Egyptian patients. In the present study, c-erbB2 overexpression was detected in a large proportion of BCs (42.5%), with no association between c-erb2 overexpression and the presence of HPV16 in the BCs. A further study is required to investigate whether or not c-erbB2 is another target of HPV.
In general, our findings provide some information on the relationship between HPV16 E6 and bcl-2 expression; these findings may be beneficial in enhancing our understanding of the pathogenesis of BC.
Abbreviations
- BC:
-
Breast carcinoma
- HPV 16:
-
Human papillomavirus type 16
- PCR:
-
Polymerase chain reaction
- SP:
-
Streptavidin-Peroxidase
References
Liu Y, Klimberg VS, Andrews NR et al (2001) Human papillomavirus DNA is present in a subset of unselected breast cancers. J Hum Viral 4:329–334
Damin AP, Karam R, Zettler CG et al (2004) Evidence for an association of human papillomavirus and breast carcinomas. Breast Cancer Res Treat 84:131–137
Kan CY, Iacopetta BJ, Lawson JS et al (2005) Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer 93:946–948
Sima N, Wang S, Wang W (2007) Antisense targeting human papillomavirus type 16 E6 and E7 genes contributes to apoptosis and senescence in SiHa cervical carcinoma cells. Gynecol Oncol 106:299–304
Hamada K, Shirakawa T, Gotoh A et al (2006) Adenovirusmediated transfer of human papillomavirus 16 E6/E7 antisense RNA and induction of apoptosis in cervical cancer. Gynecol Oncol 103:820–830
DeFilippis RA, Goodwin EC, Wu L et al (2003) Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol 77:1551–1563
Mantovani F, Banks L (2001) The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874–7887
Scheffner M, Werness BA, Huibregtse JM et al (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129–1136
Werness BA, Levine AJ, Howley PM (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76–79
Fan X, Liu Y, Chen JJ (2005) Down-regulation of p21 contributes to apoptosis induced by HPV E6 in human mammary epithelial cells. Apoptosis 10:63–73
Tsuda H, Hirohashi S, Shimosato Y et al (1990) Correlation between histologic grade of malignancy and copy number of c-erbB-2 gene in breast carcinoma: a retrospective analysis of 176 cases. Cancer 65:1794–1800
Liu WK, Chu YL, Zhang F et al (2005) The relationship between HPV16 and expression of CD44v6, nm23H1 in esophageal squamous cell carcinoma. Arch Virol 150:991–1001
Tsuda H, Hirohashi S, Shimasato Y et al (1990) Immunohistochemical study on overexpression of c-erbB-2 proteinin human breast cancer: its correlation with gene amplification and long-term survival of patients. Jpn J Cancer Res 81:327–332
Band V, Zajchowski D, Kulesa V (1990) Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA 87:463–467
de Villiers EM, Sandstrom RE, zur Hausen H et al (2005) Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res 7:R1–R11
Kroupis C, Markou A, Vourlidis N et al (2006) Presence of high-risk human papillomavirus sequences in breast cancer tissues and association with histopathological characteristics. Clin Biochem 39:727–731
Gumus M, Yumuk PF, Salepci T et al (2006) HPV DNA frequency and subset analysis in human breast cancer patients’ normal and tumoral tissue samples. Exp Clin Cancer Res 25:515–521
Bratthauer GL, Tavassoli FA, O’Leary TJ (1992) Etiology of breast carcinoma: no apparent role for papillomavirus types 6/11/16/18. Pathol Res Pract 188:384–386
Lindel K, Forster A, Altermatt HJ et al (2007) Breast cancer and human papillomavirus (HPV) infection: no evidence of a viral etiology in a group of Swiss women. Breast 16:172–177
Gopalkrishna V, Singh UR, Sodhani P et al (1996) Absence of human papillomavirus DNA in breast cancer as revealed by polymerase chain reaction. Breast Cancer Res Treat 39:197–202
Lavergne D, de Villiers EM (1999) Papillomavirus in esophageal papillomas and carcinomas. Int J Cancer 80:681–684
Lipponen P (1995) Apoptosis suppressing protein bcl-2 is expressed in well-differentiated breast carcinomas with favourable prognosis. J Pathol 1:177–180
Gee JM, Robertson JF, Ellis IO (1994) Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer 59:619–628
Knowlton K, Mancini M, Creason S (1998) Bcl-2 slows in vitro breast cancer growth despite its antiapoptotic effect. J Surg Res 76:22–26
Grace VM, Shalini JV, Lekha TT et al (2003) Co-overexpression of p53 and bcl-2 proteins in HPV-induced squamous cell carcinoma of the uterine cervix. Gynecol Oncol 91:51–58
Sidransky D, Hollstein M (1996) Clinical implications of the p53 gene. Annu Rev Med 47:285–301
Hennig EM, Kvinnsland S, Holm R (1999) Significant difference in p53 and p21 protein immunoreactivity in HPV 16 positive and HPV negative breast carcinomas. Acta Oncol 38:931–938
Bar JK, Harłozińska A, Sedlaczek P (2001) Relations between the expression of p53, c-erbB-2, Ki-67 and HPV infection in cervical carcinomas and cervical dysplasias. Anticancer Res 21:1001–1006
Ngan HY, Cheung AN, Liu SS (2001) Abnormal expression of epidermal growth factor receptor and c-erbB2 in squamous cell carcinoma of the cervix: correlation with human papillomavirus and prognosis. Tumour Biol 22:176–183
Bahnassy AA, Zekri AR, Abdallah S (2005) Human papillomavirus infection in Egyptian esophageal carcinoma: correlation with p53, p21, mdm2, C-erbB2 and impact on survival. Pathol Int 55:53–62
Acknowledgements
We appreciate Dr. Wen-kang Liu for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Q., Zhang, SQ., Chu, YL. et al. The correlations between HPV16 infection and expressions of c-erbB-2 and bcl-2 in breast carcinoma. Mol Biol Rep 36, 807–812 (2009). https://doi.org/10.1007/s11033-008-9249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9249-9