Abstract
Submergence is a widespread problem of rice production, especially in low-lying areas in South and Southeast Asia. Despite the success of Sub1 mega varieties, repeated instances of prolonged and severe flooding in stress-prone areas suggests that the SUB1 gene is no longer sufficient in those regions and requires improved varieties with increased tolerance. A study was conducted to identify quantitative trait loci (QTLs) associated with submergence tolerance using 115 F7 recombinant inbred lines (RILs) derived from the cross of Ciherang-Sub1, a popular Indonesian cultivar carrying the SUB1 gene that has relatively higher tolerance to submergence compared to the performance of most other Sub1 lines and the submergence and stagnant flooding tolerant IR10F365. As the tolerant allele at SUB1A on chromosome 9 was fixed in this mapping population, additional QTLs responsible for submergence tolerance were expected to be revealed. Genotyping with an Infinium 6K SNP chip resulted in 469 polymorphic markers that were then used for QTL mapping. Phenotyping was performed under complete submergence with two replicates. A major QTL for submergence derived from Ciherang-Sub1, named qSUB8.1, was detected on chromosome 8 with a LOD score of 10.3 and phenotypic variance of 27.5%. Additionally, a smaller QTL, also derived from Ciherang-Sub1, was detected on chromosome 2 with a LOD score of 3.5 and phenotypic variance of 12.7%. There was no digenic interaction detected between these QTLs suggesting their independent action. The QTLs detected in this study can be used in marker-assisted selection to further improve the tolerance of other Sub1 varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is life for most people living in Asia, and nearly half of the world’s population depends on rice as a staple food. However, there are many constraints in rice production. One of the most serious is flash flooding, where complete submergence during rice growth can cause widespread losses to farmers’ crops. Submergence is an unavoidable abiotic stress that affects 22 million hectares of the lowland rainfed rice farms worldwide, accounting for 18% of the global supply of rice (Khush 1984). In addition, an estimation of up to one billion US dollars economic loss has been reported (Herdt 1991; Dey and Upadhyaya 1996; Mackill et al. 1996). It is a re-occurring scenario that has escalated recently due to the influence of extreme and erratic weather conditions. The development of varieties tolerant to submergence is essential to provide stable yields in flood-prone rice production areas.
Several independent studies have identified quantitative trait loci (QTLs) for submergence tolerance obtained from different populations or new potential submergence donors (Nandi et al. 1997; Siangliw et al. 2003; Toojinda et al. 2003; Xu and Mackill 1996; Septiningsih et al. 2012; Gonzaga et al. 2016; Iftekharuddaula et al. 2016a). Among the discovered QTLs, the SUB1 QTL from chromosome 9 derived from FR13A is known to be the most significant, contributing up to 2–3 weeks of submergence tolerance depending on the genetic background and the environmental conditions at the time of flooding. SUB1 was previously fine mapped, the FR13A-derived line was sequenced for the SUB1 region, and a cluster of three ethylene response factor (ERF) genes, namely SUB1A, SUB1B, and SUB1C, were unveiled (Xu et al. 2000; Xu et al. 2006). SUB1A was found in a subset of indica and aus accessions, while SUB1B and SUB1C were detected in all indica and japonica accessions screened for these genes (Xu et al. 2006; Li et al. 2010). Moreover, SUB1A was confirmed as the causal gene providing submergence tolerance at the Sub1 QTL (Septiningsih et al. 2009; Singh et al. 2010; Xu et al. 2006). The activation of SUB1A under complete submergence stimulates the expression of the slender rice-1 (SLR1) and SLR like-1 (SLRL1) genes through the suppression of ethylene consequently inhibiting gibberellic acid (GA)-mediated shoot elongation (Fukao and Bailey-Serres 2008). Thereby, leaf starch and sugar consumption are deliberately slower compared to the intolerant lines lacking SUB1A that rapidly consume carbohydrate energy reserves for shoot elongation due to the activation of ethylene that signals GA accumulation. Hence, intolerant lines die after the water subsides because all the carbohydrate energy reserves were spent during submergence, while lines containing the tolerant SUB1A allele retain enough carbohydrate reserves to recover after the water subsides (Fukao and Bailey-Serres 2008).
The discovery of the SUB1 gene gave rise to the eight upgraded mega varieties (Swarna-Sub1, Samba Mahsuri-Sub1, IR64-Sub1, BR11-Sub1, Thadokkam 1-Sub1, CR1009-Sub1, PSBRc18-Sub1, and Ciherang-Sub1) that were developed at the International Rice Research Institute (IRRI) through a precise marker-assisted backcrossing (MABC) strategy (Iftekharuddaula et al. 2011; Neeraja et al. 2007; Septiningsih et al. 2009, 2013, 2015). Upgraded varieties largely retained the recurrent parent background, so they are nearly identical to the original parent with the advantage of submergence tolerance. Desirable agronomic characteristics of the mega varieties such as high yield, optimal plant height, and grain quality were unaffected and were made possible through the precise MABC strategy. Many of these varieties have been released in Asian countries, providing farmers with stable yields even after experiencing flash flood events.
The development of Sub1 varieties has been a key breakthrough in marker-assisted breeding and has provided rice-producing countries with an exceptional tool against submergence stress (Iftekharuddaula et al. 2016b; Singh et al. 2016). In most cases, the upgraded varieties have a reasonably high tolerance to submergence; however, the level of tolerance varies among different genetic backgrounds (Septiningsih et al. 2009, 2013). Moreover, the effectiveness of the SUB1 gene depends on environmental conditions, such as water turbidity, temperature, and extent of light penetration, that cause different levels of plant injury and survival rates under complete submergence. In severe conditions, water turbidity and high temperatures can cause Sub1 lines to succumb after just 1–2 weeks of submergence, while even under the best conditions, Sub1 lines cannot survive floods longer than 3 weeks. Therefore, greater submergence tolerance than Sub1 alone is needed for severe flood conditions, especially under the threat of future intensification of weather events leading to more common and more severe flooding across rainfed rice production areas. Hence, the SUB1 gene is not by itself sufficient to fight against extreme and recurring submergence problems.
Research efforts have recently begun to search for novel QTLs that might enhance the effect of SUB1 to contribute greater levels of submergence tolerance. One constraint when trying to identify additional QTLs in populations where Sub1 is segregating is that the large Sub1 effect will mask smaller QTLs, disrupting the identification of non-Sub1 QTLs and preventing an accurate estimate of the effects of non-Sub1 QTLs. The current study, therefore, employs a fixed Sub1 population, derived from a cross of Ciherang-Sub1 with the submergence and stagnant flooding tolerant line IR10F365, both of which contain the tolerant allele at the SUB1A gene. Ciherang-Sub1 previously had demonstrated higher levels of submergence tolerance than other Sub1 lines, suggesting the presence of additional QTLs that might be complementary to the SUB1 gene, which in turn may be used to develop improved rice varieties with even greater levels of tolerance to submergence stress. Moreover, the high levels of tolerance in IR10F365 to stagnant flooding, where water levels remain above 50–60 cm even up to harvesting time, provides an opportunity to combine tolerance to both submergence and stagnant flooding in a high-yielding genetic background (Collard et al. 2013a).
Materials and methods
Development of the mapping population
Ciherang-Sub1, an upgraded variety of Ciherang, a mega variety from Indonesia, through a marker-assisted backcrossing (MABC) strategy (Septiningsih et al. 2015), carries the SUB1 QTL from chromosome 9, originally derived from FR13A. It was crossed with IR10F365 (IRGC 87439; parentage: IR 70215-4-CPA 3-1-3-1/NSIC RC 158) which also contains SUB1 QTL and is a donor for stagnant flooding tolerance (Collard et al. 2013a). The F1 plants were confirmed to be a true hybrid using SSR markers, and the mapping population was developed using rapid generation advance (RGA) for fast and efficient progress. A total of 156 recombinant inbred lines (RILs) were generated.
Field trials and testing for stagnant flooding tolerance
Field trials were conducted under normal (i.e., optimal) field conditions and stagnant flooding conditions in 2012 during dry and wet seasons following standard operating protocols used at IRRI. Further details of the entire submergence breeding scheme and process are described in Collard et al. (2013b). A total of 80 entries were tested including elite breeding lines from the submergence breeding program, Sub1 varieties, and recipient non-Sub1 variety parents. Ciherang-Sub1 and IR10F365 were included along with Swarna-Sub1 and IRRI 154 as susceptible and tolerant check varieties, respectively. The stagnant flooding trial management was followed as described in Kato et al. (2014). Yield and agronomic data were analyzed using mixed model analysis using Genstat (16th edition, VSN International). Yield performance was compared by calculating each entry compared to the site mean yield of the trial (SMY) expressed as a percentage. Least significant difference (α = 0.05) was calculated for comparing entries.
Submergence screening of the mapping population
Submergence screening of the Ciherang-Sub1/IR10F365 mapping population was performed in the BG-02 outdoor tank at the International Rice Research Institute (IRRI) at Los Baños, Philippines. Only 115 RILs were subjected to phenotyping due to the space limitation of the tank. Twenty pre-germinated seeds of each lines along with the parents, positive (IR64-Sub1 and FR13A) and negative (IR42) checks, were sown in black trays (53.3 × 38.1 × 10.2 cm) in an alpha lattice design using two replicates. Each tray had 20 entries with positive and negative controls. Upon 14 days from seeding, the plants were completely submerged with a water depth of approximately 1.5 m. The IR42 susceptible check was assessed during the tenth day of submergence and continued on a daily basis and trays were de-submerged upon 90–100% damage of the IR42 plants were observed. Plant survival was scored at 21 days after de-submergence, and percent survival was computed for QTL analysis.
6K SNP genotyping
Total genomic DNA of Ciherang-Sub1, IR10F365, and the F7 progenies were extracted using mini-prep CTAB method according to Zheng et al. (1995) with slight modifications. The isolated DNA were treated with RNase and quantified using nano-drop spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and the quality check was completed through agarose gel electrophoresis (1.2% agarose gel). The final concentration of the DNA samples was normalized to 50 ng/uL. SNP genotyping was accomplished with an Illumina Infinium 6K SNP chip developed by S. McCouch at Cornell University (named “Cornell_6k_Array_Infinium_Rice”), providing a total of 5274 SNP markers, and run at the Genotyping Services Lab, IRRI (Thomson, 2014). Illumina Bead chip kits were used for the Infinium II whole-genome genotyping assay and amplification and hybridization steps as per the standard protocol. Subsequently, enzymatic base extension conferred allelic specificity and the products are consequently stained fluorescently. Illumina Bead Array Reader detected the intensities of the beads’ fluorescence and analyzed by Illumina’s software for automated genotype calling. Raw intensity values were analyzed using the GenomeStudio software V1.1.0 (Illumina), and nucleotide calls were accomplished using a custom cluster file developed at the Genotyping Services Lab, IRRI (Thomson 2014). The data was transformed manually into ABH calls based on the two parental alleles at each locus.
Linkage map construction and QTL analysis
The linkage map was constructed using Map Manager QTX, vQTXb20 (Manly et al. 2001) with Kosambi map function to calculate genetic distances. The QTL analysis was performed using QGene v4.3.10 (Joehanes and Nelson 2008; http://www.qgene.org/qgene/), Windows QTLCartographer (WinQTLCart) v2.5 (Wang et al. 2010), IciMapping (Meng et al. 2015), and QTLNetwork (Yang and Zhu 2005; Yang et al. 2007, 2008) while digenic interactions were analyzed with QTLNetwork and IciMapping software. Permutation of 1000 iterations was used to determine the threshold at p ≤ 0.05 and p ≤ 0.01 to declare significance level of the detected QTLs in both interval mapping (IM) and composite interval mapping (CIM) methods of QTL Cartographer v2.5 software. The parameter for CIM in forward-backward stepwise regression was set to F-in = 0.01 and F-out = 0.01. For QTL comparison, data were analyzed in QGene and IciMapping software in IM and CIM methods with 10,000 and 1000 permutations, respectively. The stepwise cofactor selection for QGene in CIM was set to F to add = 0.01 and F to drop = 0.01. QTLNetwork with 1000 permutations in 0.05 level of significance was used for F value and heritability measurements. Standard rice QTL nomenclature was followed in QTL designation (McCouch 2008).
Results
Submergence screening and stagnant flooding tolerance of IR10F365
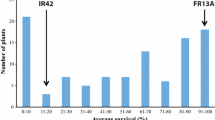
The survival rates of 115 RILs screened under complete submergence in an outdoor tank at IRRI ranged from 32.3 to 100%, with a mean of 77.6% (Fig. 1). Ciherang-Sub1 and IR10F365 both possess the SUB1 QTL from chromosome 9, resulting in a skewed distribution of the population under complete submergence stress. The survival rates of the susceptible check IR42 and tolerant check FR13A were 0 and 87.4%, respectively. For the parents of the mapping population, survival rates were 74.1% for Ciherang-Sub1 and 49.1% for IR10F365. The data showed that the survival rates of the population were all above the IR42 susceptible check, 6 out of the 115 families (5.2%) had a lower survival rate than IR10F365, while 75 (65.2%) had a higher survival rate than Ciherang-Sub1. Twenty-two lines (19.1%) even had a higher percentage of survival than the tolerant check FR13A (Fig. 1).
Phenotypic evaluation of F7 Ciherang-Sub1/IR10F365 RILs under submergence stress. Evaluation of the survival rate of the mapping population was conducted under complete submergence in an outdoor concrete tank. The phenotypic distribution of the 115 RILs is shown with the average survival of the parents (Ciherang-Sub1 and IR10F365), susceptible (IR42), and tolerant (FR13A) checks
Aside from submergence tolerance, the progenies might also possess stagnant flooding tolerance since the donor parent IR10F365 has high levels of tolerance to stagnant flooding conditions (Table 1). The trial results indicated that IR10F365 has higher yield potential and stagnant flooding tolerance compared to Ciherang-Sub1 and also comparable performance to the check variety (IRRI 154). The trial under stagnant flooding tolerance conditions in the 2012 wet season was quite severe as indicated by the complete death of the sensitive check, Swarna-Sub1. During the wet season, trials show wide variation in performance, especially in stagnant flooding conditions (Table 1).
Linkage map construction and QTL analysis
Genotyping was performed with an Illumina Infinium 6K SNP chip designed at Cornell University (Cornell_6k_Array_Infinium_Rice), providing a total of 5274 SNP markers, of which 469 (8.9%) high-quality SNP markers were identified as polymorphic between Ciherang-Sub1 and IR10F365 and used for the analysis. The linkage map had a total length of 1147.8 cM with an average distance of 3.2 cM between markers which provides a well-covered map for the QTL mapping analysis (Fig. 2).
Mapping of submergence-tolerant QTLs derived from the Ciherang-Sub1/IR10F365 population. The molecular linkage map was constructed based on 6K Infinium SNP chip platform with 469 polymorphic SNP markers. The position of the two significant submergence-tolerance QTLs on chromosomes 2 and 8 are shown in black bars next to the chromosomes. Centromeres are shown as black boxes in each chromosome
Results were compared across four QTL mapping software packages using permutation analysis to declare empirical significance thresholds. QTLs were detected for submergence tolerance based on 10,000 permutations in QGene (Joehanes and Nelson 2008) with LOD threshold scores of 2.87 (p ≤ 0.05) and 3.61 (p ≤ 0.01) for interval mapping (IM) and 3.83 (p ≤ 0.05) and 5.12 (p ≤ 0.01) for composite interval mapping (CIM). One QTL was detected above the empirical significance threshold at p ≤ 0.01, located on chromosome 8 (qSUB8.1) with a maximum LOD score of 8.1 and phenotypic variance (R 2) of 27.5%. Additional QTLs identified but below the threshold from chromosome 2 (qSUB2.1) with a maximum LOD score of 2.7 and R 2 of 10.2% detected in both IM and CIM, and chromosome 3 QTL (qSUB3.1) with a maximum LOD score of 3.1 and R 2 of 11.7% identified in CIM method only. All the QTLs came from the female parent Ciherang-Sub1. Using QTL Cartographer v2.5 (Wang et al. 2010) with 1000 permutations, LOD scores of 2.94 and 3.87 for IM, and 3.10 and 4.02 for CIM were defined at the significance levels p ≤ 0.05 and p ≤ 0.01, respectively. Likewise, based on 1000 iterations using QTL IciMapping (Meng et al. 2015), the declared threshold levels at p ≤ 0.05 were 3.41 and 3.39, for IM and CIM, respectively, and at p ≤ 0.01 were 4.55 for IM and 3.52 for CIM. QTL analysis result of QTL Cartographer and IciMapping software were similar with Qgene that detected the major QTL on chromosome 8 but QTL Cartographer had identified chromosome 2 QTL above the threshold at p ≤ 0.05 with LOD score of 3.5 and R 2 of 8.19%. Also, QTL qSUB3.1 was identified only in QGene although it was below the threshold (Fig. 2; Table 2). QTLs slightly below the threshold are reported for the purpose of comparison with other QTLs identified in previous studies. In QTL Network (Yang and Zhu 2005; Yang et al. 2007, 2008), the declared threshold for QTL identification based on 1000 permutations was at p ≤ 0.05 and F value at 13.1. Using this software, qSUB2.1 and qSUB8.1 were both detected above the threshold with F = 13.50 and 8 F = 33.80, and heritability of 10.10 and 23.26%, respectively which is similar with QTL Cartographer result. There were no significant digenic interactions detected in this population using QTLNetwork and IciMapping software (Table 3; Suppl. Fig. 1).
Tolerant lines with survival rates above the Ciherang-Sub1 and tolerant check FR13A
Seventy-five tolerant lines (65.2%) with survival rates higher than Ciherang-Sub1 parent were identified. Survival rates ranged from 75 to 100%. Interestingly, 22 (19.1%) of those lines have even higher survival rates than the tolerant check FR13A (Suppl. Table 1). In addition to SUB1, 54 (72%) of the lines had the chromosome 8 QTL, 42 (56%) lines had chromosomes 2 QTL, and 46 (61.33%) lines had chromosome 3 QTL all derived from the Ciherang-Sub1 parent. Thirty lines had chromosome 2 and 8 QTL combinations and 32 lines had chromosome 3 and 8 QTL combination.
Discussion
Submergence tolerance expression is classified as genetically complex and identified to be reliant on environmental conditions (Suprihatno and Coffman 1981; Mohanty and Khush 1985; Sinha and Saran 1988; Haque et al. 1989; Adkin et al. 1990; Setter et al. 1997). Earlier findings using different populations mapped QTLs linked to submergence tolerance on genomic regions and revealed the recurring detection of a major QTL on chromosome 9, the SUB1 QTL (Nandi et al. 1997; Siangliw et al. 2003; Toojinda et al. 2003; Xu and Mackill 1996; Septiningsih et al. 2012; Gonzaga et al. 2016). SUB1 has been the largest and most important submergence tolerant QTL identified to date. Hence, the QTL had been sequenced and associated diagnostic DNA markers had been developed which facilitates the development of mega varieties tolerant to submergence through the use of marker-assisted technology (Iftekharuddaula et al. 2011, 2012, 2016b; Neeraja et al. 2007; Septiningsih et al. 2009, 2013, 2015). Most of the upgraded mega varieties have been released in some countries in Asia and provide outstanding solutions against flash flooding problems worldwide. However, recent erratic weather conditions have caused persistent and severe flooding that heavily affects rice farmers, especially in Asia. Hence, this study aims to identify QTLs which are complementary to the SUB1 gene and has a significant contribution to submergence tolerance. Ciherang-Sub1 and IR10F365 were used as parents to fix the SUB1 gene in order to identify smaller QTLs that are otherwise masked by the effect of SUB1. The use of IR10F365 is an advantage as it is also a donor for stagnant flooding resulting with progenies containing both SUB1 gene and stagnant flooding tolerance (Table 1).
QGene, QTL Cartographer, IciMapping, and QTLNetwork software with different algorithms were used to ensure a comprehensive and precise QTL mapping analysis. All software had identified a highly significant QTL on chromosome 8 (qSUB8.1), while the chromosome 2 QTL (qSUB2.1) was detected significantly in QTLCartographer and QTLNetwork while below the threshold in other software (Table 1; Fig. 1). In addition to interval mapping, QGene and QTL Cartographer also employ composite interval mapping with stepwise cofactor selection across individual markers; IciMapping uses modified algorithm of CIM called inclusive composite interval mapping (ICIM) (Meng et al. 2015); and QTLNetwork utilizes marker-pair selection of cofactors that is advantageous on the overfitting problem and results to the identification of the best-fitting models (Yang et al. 2007). Moreover, QTLNetwork provides a more stringent measure for declaring a significant QTL due to a more conservative cofactor selection with a genome-wise false positive rate control. One distinct advantage of QTLNetwork is the ability for epistasis detection, although there were no digenic interactions detected in this mapping population. The heritability of qSUB8.1 was relatively high (0.233) (Table 3; Suppl. Fig. 1).
A comparison with previous independent studies for mapping QTLs for submergence tolerance (Nandi et al. 1997; Siangliw et al. 2003; Toojinda et al. 2003; Xu and Mackill 1996; Septiningsih et al. 2012; Gonzaga et al. 2016) revealed that none of the QTLs detected in this study are directly co-located with the earlier QTLs reported, suggesting that Ciherang-Sub1contains novel QTLs. The highly significant QTL on chromosome 8 (qSUB8.1) in this study is located in a different region from the QTL identified by Gonzaga et al. (2016) derived from FR13A with a distance between id8005815 and id8007472 which is positioned lower than qSUB8.1.
There were 75 RILs that exceeded the survival rate of Ciherang-Sub1 with a range of 75–100%. Fifty-four lines (72%) contain chromosome 8 QTL (qSUB8.1) and the majority are combinations of 2–3 QTLs, in addition to the fixed Sub1 QTL on chromosome 9. Twenty-two (19.1%) RILs had even outperformed the tolerant FR13A check (above 87.4%); 18 of which (81.8%) had qSUB8.1 and majority with 2–4 QTL combinations (Suppl. Table 1). This suggests the substantial impact of the identified QTLs, especially qSUB8.1 with the highest LOD and explained phenotypic variation. A large number of the tolerant RILs contained combinations of SUB1, qSUB8.1, and qSUB2.1 or SUB1, qSUB8.1, and qSUB3.1. In this study, where the lines were subjected to severe stress, the best available submergence tolerant check FR13A (Vergara and Mazaredo 1975) had only 87.4% survival while some of the progenies exceeded this rate up to 100%, indicating that the identified QTLs have the ability to enhance the effect of the Sub1 QTL in those particular RIL genetic backgrounds. It is worth mentioning as well that FR13A has a high rate of survival since this variety also carries other smaller QTLs in addition to SUB1 (Nandi et al. 1997; Toojinda et al. 2003; Gonzaga et al. 2016).
In conclusion, the QTL qSUB8.1 on chromosome 8 is a novel QTL for submergence tolerance during vegetative stage derived from Ciherang-Sub1. This QTL provides a valuable target for marker-assisted breeding and gene discovery efforts to identify alternative molecular pathways for tolerance to submergence in addition to SUB1. QTL validation through the development of near-isogenic lines will be initiated and pursued for future breeding programs. The complementary action of qSUB8.1 with the SUB1 gene is therefore a promising target for QTL pyramiding for improved tolerance of the current Sub1 varieties to withstand prolonged and severe flooding. In addition, this trait can also be combined with other abiotic stress tolerance QTLs, such as anaerobic germination, stagnant flooding, and salinity to provide more resilient rice varieties for areas that experience different stresses. Since one of the parents of the current population, IR10F365, also has tolerance to stagnant flooding, this RIL population will be useful for future studies to dissect the molecular control of stagnant flooding as well.
References
Adkin SW, Shiraishi T, McComb JA (1990) Submergence tolerance of rice-a new glasshouse method for the experimental submergence of plants. Physiol Plant 80:642–646
Collard BCY, Septiningsih EM, Das SR, Carandang JJ, Pamplona AM, Sanchez DL, Kato Y, Ye G, Reddy JN, Singh US, Iftekharuddaula KM, Venuprasad R, Vera-Cruz CN, Mackill DJ, Ismail AM (2013a) Developing new flood-tolerant varieties at the International Rice Research Institute (IRRI). SABRAO J Breed Genet 45:42–56
Collard BCY, Kato Y, Septiningsih IAM, Mackill DJ (2013b) Defining IRRI’s role in the EIRLSBN: current status and future directions. In: Collard BCY, Ismail AM, Hardy B (eds) EIRLSBN: twenty years of achievements in rice breeding. International Rice Research Institute, Los Baños, pp 135–144
Dey MM, Upadhyaya HK (1996) Yield loss due to drought, cold and submeMappingrgence in Asia. In: Everson RE (ed) Rice research in Asia: progress and priorities. International Rice Resreach Institute, Manila, pp 291–303
Fukao T, Bailey-Serres J (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellins responses in rice. Proc Natl Acad Sci 105:16814–16819
Gonzaga ZJ, Carandang J, Sanchez DL, Mackill DJ, Septiningsih EM (2016) Mapping additional QTLs from FR13A to increase submergence tolerance beyond SUB1. Euphytica 209:627–636
Haque QA, Hille Ris Lambers D, Tepora NM, Dela Cruz QD (1989) Inheritance of submergence tolerance in rice. Euphytica 41:247–251
Herdt RW (1991) Research priorities for rice biotechnology. In: Khush GS, Toenniessen GH (eds) Rice biotechnology. CAB International, Oxon, pp 19–54
Iftekharuddaula KM, Newaz MA, Salam MA, Ahmed HU, Mahbub MAA, Septiningsih EM, Collard BCY, Sanchez DL, Pamplona AM, Mackill DJ (2011) Rapid and high-precision marker assisted backcrossing to introgress the SUB1 QTL into BR11, the rainfed lowland rice mega variety of Bangladesh. Euphytica 178:83–97
Iftekharuddaula KM, Salam MA, Newaz MA, Ahmed HU, Collard BCY, Septiningsih EM, Sanchez DL, Pamplona AM, Mackill DJ (2012) Comparison of phenotypic versus marker-assisted background selection for the SUB1 QTL during backcrossing in rice. Breed Sci 62:216–222
Iftekharuddaula KM, Ghosal S, Gonzaga ZJ, Amin A, Barman HN, Yameen R, Carandang J, Collard BYC, Septiningsih EM (2016a) Allelic diversity of newly characterized submergence-tolerant rice (Oryza sativa L.) germplasm from Bangladesh. Genet Resour Crop Evol 63:859–867
Iftekharuddaula KM, Ahmed HU, Ghosal S, Amin A, Moni ZR, Ray BP, Barman HN, Siddique MA, Collard BCY, Septiningsih EM (2016b) Development of early maturing submergence-tolerant rice varieties for Bangladesh. Field Crops Res 190:44–53
Joehanes R, Nelson JC (2008) QGene 4.0, an extensible java QTL-analysis platform. Bioinformatics 24:2788–2789
Kato Y, Collard BCY, Septiningsih EM, Ismail AM (2014) Physiological analyses of traits associated with tolerance of long-term partial submergence in rice. AoB PLANTS 6:plus058.
Khush GS (1984) Terminology of rice growing environments. International Rice Resreach Institute, Manila, pp 5–10
Li Z-X, Septiningsih EM, Quiloy-Mercado SM, McNally KL, Mackill DJ (2010) Identification of Sub1A alleles from wild rice Oryza rufipogon Griff. Genet Resour Crop Evol 58:1237–1242
Mackill DJ, Coffman WR, Garrity DP (1996) Rainfed lowland rice improvement. International Rice Research Institute, Los Baños
Manly KF, Cudmore RH Jr, Meer JM (2001) Map manager QTX, cross-platform software for genetic mapping. Mamm Genome 12:930–932
McCouch SR (2008) Gene nomenclature system for rice. Rice 1:72–84
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:368–283
Mohanty HK, Khush GS (1985) Diallel analysis of submergence tolerance in rice, Oryza sativa L. Theor Appl Genet 70:467–473
Nandi S, Subudhi PK, Senadhira D, Manigbas NL, Sen-Mandi S, Huang N (1997) Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Mol Gen Genet 255:1–8
Neeraja CN, Maghirang-Rodriquez R, Pamplona A, Heuer S, Collard BCY, Septiningsih EM, Vergara G, Sanchez D, Xu K, Ismail AM, Mackill DJ (2007) A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor Appl Genet 115:767–776
Septiningsih EM, Pamplona AM, Sanchez DL, Neeraja CN, Vergara GV, Heuer S, Ismail AM, Mackill DJ (2009) Development of submergence tolerant rice cultivars: the Sub1 locus and beyond. Ann Bot 103:151–160
Septiningsih EM, Sanchez DL, Singh N, Sendon PMD, Pamplona AM, Heuer S, Mackill DJ (2012) Identifying novel QTLs for submergence tolerance in rice cultivars IR72 and Madabaru. Theor Appl Genet 124:867–874
Septiningsih EM, Collard BCY, Heuer S, Bailey-Serres J, Ismail AM, Mackill DJ (2013) Applying genomics tools for breeding submergence tolerance in rice. In: Varshney RK, Tuberosa R (eds) Translational genomics for crop breeding: abiotic stress, yield and quality, 1st edn. John Wiley and Sons, Chichester, pp 9–30
Septiningsih EM, Hidayatun N, Sanchez DL, Nugraha Y, Carandang J, Pamplona AM, Collard BCY, Ismail AM, Mackill DJ (2015) Accelerating the development of new submergence tolerant rice varieties: the case of Ciherang-Sub1 and PSBRc18-Sub1. Euphytica 202:259–268
Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S (1997) Physiology and genetics of submergence tolerance in rice. Ann Bot 79:67–77
Siangliw M, Toojinda T, Tragoonrung S, Vanavichit A (2003) Thai jasmine rice carrying QTLchr9 (SubQTL) is submergence tolerant. Ann Bot 91:255–261
Singh N, Dang T, Vergara G, Pandey D, Sanchez D, Neeraja C, Septiningsih E, Mendioro M, Teeson-Mendoza R, Ismail A, Mackill D, Heuer S (2010) Molecular marker survey and expression analyses of the rice submergence-tolerance genes SUB1A and SUB1C. Theor Appl Genet 121:1441–1453
Singh R, Singh Y, Xalaxo S, Verulkar S, Yadav N, Singh S, Singh N, Prasad KSN, Kondayya K, Rao PVR, Rani MG, Anuradha T, Suraynarayana Y, Sharma PC, Krishnamurthy SL, Sharma SK, Dwivedi JL, Singh AK, Singh PK, Nilanjay SNK, Kumar R, Chetia SK, Ahmad T, Rai M, Perraju P, Pande A, Singh DN, Mandal NP, Reddy JN, Singh ON, Katara JL, Marandi B, Swain P, Sarkara RK, Singh DP, Mohapatra T, Padmawathi G, Ram T, Kathiresan RM, Paramsivam K, Nadarajan S, Thirumeni S, Nagarajan M, Singh AK, Vikram P, Kumar A, Septiningsih E, Singh US, Ismail AM, Mackill D, Singh NK (2016) From QTL to variety-harnessing the benefits of QTLs for drought, flood, and salt tolerance in mega varieties of India through a multi-institutional network. Plant Sci 242:278–287
Sinha MM, Saran S (1988) Inheritance of submergence tolerance in lowland rice. Oryza 25:351–354
Suprihatno B, Coffman WR (1981) Inheritance of submergence tolerance in rice (Oryza sativa L.). SABRAO J 13:98–108
Thomson MJ (2014) High-throughput SNP genotyping to accelerate crop improvement. Plant Breed Biotech 2:195–212
Toojinda T, Siangliw M, Tragroonrung S, Vanavichit A (2003) Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Ann Bot 91:243–253
Vergara BS, Mazaredo A (1975) Screening for resistance to submergence under greenhouse conditions. In: Proceedings International Seminar on Deepwater Rice. Bangladesh Rice Research Institute, Dhaka, Bangladesh pp 67–70
Wang S, Basten CJ, Zeng Z-B (2010) Windows QTL Cartographer 2.5. Department of Statistics. North Carolina State University, Raleigh
Xu K, Mackill DJ (1996) A major locus for submergence tolerance mapped on rice chromosome 9. Mol Breed 2:219–224
Xu K, Xu X, Ronald PC, Mackill DJ (2000) A high-resolution linkage map of the vicinity of the rice submergence tolerance locus Sub1. Mol Gen Genet 263:681–689
Xu K, Xia X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene response factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Yang J, Zhu J (2005) Methods for predicting superior genotypes under multiple environments based on QTL effects. Theor Appl Genet 110:1268–1274
Yang J, Zhu J, Williams RW (2007) Mapping the genetic architecture of complex traits in experimental populations. Bioinformatics 23:1527–1536
Yang J, Hu CC, Hu H, Yu RD, Xia Z, Ye XZ, Zhu J (2008) QTL network: mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 24:721–723
Zheng K, Subudhi PK, Domingo J, Magpantay G, Huang N (1995) Rapid DNA isolation for marker assisted selection in rice breeding. Rice Genet Newslett 12:255–258
Acknowledgements
We thank R. Garcia, E. Suiton, J. Mendoza, G. Perez, J. Borgonia, R. Formaran, V. Bartolome and the IRRI GSL team for technical assistance. The work reported here was supported in part by a grant from the Bill and Melinda Gates Foundation (BMGF) through the project on “Stress-tolerant rice for Africa and South Asia (STRASA)”, Global Rice Science Partnership (GRiSP), and the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project 1009300.
Authors’ contributions
E.M.S. designed the experiments. B.C.Y.C developed the RIL population using rapid generation advance (RGA). J.C. performed the seed multiplication of the mapping population. Z.J.C.G and A.S. performed the DNA extraction, and J.C. and Z.J.C.G performed the phenotyping under the supervision of E.M.S. M.J.T. supervised the SNP genotyping in the Genotyping Service Lab (GSL). Z.J.C.G., A.S., and E.M.S. analyzed the data. Z.J.C.G. and E.M.S. wrote the manuscript. B.C.Y.C. and M.J.T. edited the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
Supplementary Table 1
Recombinant Inbred Lines (RILs) with average survival rates above the tolerant check FR13A containing combinations of the identified QTLs (90–100% survival rates). (DOCX 13 kb)
Supplementary Figure 1
A representative figure showing the QTLs for submergence tolerance from Ciherang-sub1/IR10F365 population identified by QTLNetwork. The red balls indicate the QTLs with additive effects. (DOCX 49 kb)
Rights and permissions
About this article
Cite this article
Gonzaga, Z.J.C., Carandang, J., Singh, A. et al. Mapping QTLs for submergence tolerance in rice using a population fixed for SUB1A tolerant allele. Mol Breeding 37, 47 (2017). https://doi.org/10.1007/s11032-017-0637-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0637-5