Abstract
Combinations of effective leaf rust resistance genes that condition resistance to many leaf rust races are key to the development of wheat cultivars with long-lasting resistance to leaf rust caused by Puccinia triticina. A population of F6 recombinant inbred lines (RILs) derived from the cross ‘Thatcher*3/Americano 25e’ was evaluated for segregation of adult plant leaf rust resistance in three field plot tests and in a greenhouse test. A genetic map was constructed with 243 diversity array technology markers. Significant effects for reduction in leaf rust severity were found on chromosomes 1BL and 5BL. The sequence-tagged site marker csLV46, closely linked to Lr46, mapped to the logarithm of odds peak on chromosome 1BL, indicating that the RILs were likely segregating for this adult plant resistance gene. Lines with csLV46 had an average leaf rust severity of 35 % in the four tests. RILs with the resistance on chromosome 5BL designated as QLr.cdl-5BL had an average leaf rust severity of 47 %. RILs with both csLV46 and QLr.cdl-5BL had an average leaf rust severity of 22 %, close to the Thatcher*2/Americano 25e parental line. Lines that lacked both csLV46 and QLr-5BL had an average severity of 56 %. The QLr.cdl-5BL region enhanced the leaf resistance conditioned by Lr46 in an additive manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf rust of wheat (Triticum aestivum L.), caused by Puccinia triticina Erikss., is the most widely distributed rust on wheat (Roelfs et al. 1992). Long-lasting resistance to leaf rust in wheat has been difficult to achieve due to the high level of virulence diversity found worldwide (Germán et al. 2007; Kolmer et al. 2009). In the Great Plains of the USA, wheat cultivars with resistance genes Lr17, Lr39/41 and Lr21 have selected races of P. triticina (Kolmer and Hughes 2014) with virulence to these genes, eroding the resistance in many recent cultivars. Spring wheat cultivars with combinations of the seedling resistance genes Lr16, Lr23 and the adult plant gene Lr34 (Tsilo et al. 2014; Oelke and Kolmer 2005) have had effective leaf rust resistance for a number of years, even though these genes individually do not condition very high resistance. The identification and utilization of gene combinations that provide high levels of effective resistance that is long lasting is one strategy in the development of leaf rust resistant germplasm in wheat improvement programs.
The wheat cultivar ‘Americano 25e’ was derived from landraces in Uruguay in the early twentieth century (Kohli 1986) and was noted to have long-lasting leaf rust resistance (Roelfs et al. 1992). This cultivar was used as a parent in the wheat-breeding programs in Uruguay and Argentina in the 1920s. In a genetic study (Kolmer et al. 2007), Americano 25e was determined to have the seedling resistance genes Lr3a and Lr16, plus an unidentified seedling resistance gene that was not effective to many leaf rust races. In addition, Americano 25e was determined to have adult plant resistance that conditioned a partial level of resistance in field plot and greenhouse tests that did not phenotypically resemble the most effective and best-characterized adult plant resistance gene, Lr34 (Dyck 1987; Li et al. 2014). The objective of this research was to characterize and map the adult plant resistance derived from Americano 25e in a ‘Thatcher’ (Tc) spring wheat background for a direct comparison of this resistance with other Thatcher lines that are near-isogenic for adult plant and seedling leaf rust resistance genes (McIntosh et al. 1995).
Materials and methods
Population development and leaf rust assessment
Tc*2/Americano 25e F4 lines that had leaf rust severity of 30–40 % and response of moderately resistant to moderately susceptible in field plots (Kolmer et al. 2007) were advanced to F5 and F6 generations by single seed descent. These lines lacked any seedling resistance to P. triticina isolates with race designations BBBDB and SBDGG (Supplemental Table 1) (Long and Kolmer 1989). The F5 and F6 lines were also tested for resistance as adult plants in greenhouse tests with an isolate of P. triticina with race designation THBJG (Supplemental Table 1). Seed from a single F6 plant designated as Tc*2/Americano 25e 11-2A, that had clearly discernable adult plant resistance in a greenhouse test, was chosen for crossing with seed derived from a single plant selection of Tc. Tc*3/Americano 25e 11-2A recombinant inbred lines (RILs) were advanced to the F6 generation by single seed descent in a greenhouse. The greenhouse was set at 20 °C with 16 h daily of supplemental metal halide lighting. A single plant for each RIL/generation was grown in a 15-cm-dia pot filled with steamed topsoil and fertilized with Nutricote 13-13-13 NPK (Arysta Life Science America, New York) as needed. One hundred F6 lines and the two parental lines were evaluated for field resistance in St. Paul MN in 2009 and 2010, in Crookston MN in 2010 and in a greenhouse test in 2009. In the field plot tests, 50–60 seeds of the parents and each F6 RIL were planted in 2-m rows spaced 30 cm apart perpendicular to spreader rows of the wheat cultivars Tc, ‘Morocco,’ ‘Max,’ and ‘Little Club,’ that are all susceptible to leaf rust. A mixture of P. triticina isolates with race designations MLDSB, TDBGG, MFPSB, THBJG, TNRJJ, and MCRKG (Supplemental Table 1) from the USA in 2008 and 2009 (Kolmer et al. 2010, 2011) was inoculated to the spreader rows approximately 30 days after planting, when the plants were at jointing stage. The parents and F6 RILs were evaluated one time for leaf rust severity and response approximately 60 days after planting using the modified Cobb scale (Peterson et al. 1948). Leaf rust resistance response was rated R = necrosis surrounding small uredinia; MR = necrosis surrounding moderate-size uredinia; MS = distinct chlorosis surrounding moderate to large uredinia; and S = large uredinia lacking necrosis or chlorosis. The plots were rated when the susceptible parent Tc had leaf rust severity of 60–80 % with a susceptible response. Five flag leaves collected from different parts of the row were assessed for severity and response. In the greenhouse test, four seeds of the parents and each F6 RIL were planted in 15-cm pots filled with steamed topsoil. The plants were grown in a greenhouse set at 20 °C with a 16-h light period. The RIL population was inoculated at anthesis with the same mixture of P. triticina isolates used in the field plot tests. The flag leaves were inoculated individually with a mixture of Soltrol 170 oil and urediniospores. Eight to 10 flag leaves of each RIL and parent were inoculated with an atomized mixture of 5 mg of rust spores and 0.750 ml of oil. After inoculation, the plants were dried for at least 1 h and then placed in a closed chamber with a humidifier filled with distilled water running for 60 s every 5 min. The adult plants were removed the following morning and placed on a greenhouse bench. The RILs and parents were evaluated for leaf rust severity and infection type 14 days after inoculation. Severity was assessed using the primary flag leaf of each plant using the same scale as in the field plot tests. Infection types (IT) were assessed in the same manner as for seedling resistance with a 0–4 scale: IT 0 (no visible signs of infection); (hypersensitive flecks), 1 (small uredinia surrounded by necrosis), 2 (small- to moderate-size uredinia surrounded by chlorosis, 3 (moderate uredinia with no chlorosis or necrosis), and 4 (large uredinia with no necrosis or chlorosis). Larger and smaller uredinia were designated with + and −, respectively. IT of the F6 RILs were converted to numeric data for further quantitative analysis. Plants with easily discernable low IT of 12–23 were assigned a score of 0, plants with intermediate IT of 23 were scored as 1, and plants with no sign of resistance and IT 3+4 were scored as 2. The leaf rust severity (percentage of leaf area covered) of the RILs in the three field tests in St. Paul and Crookston in 2009 and 2010 and the greenhouse test were tested for correlation using Pearson’s coefficient with PROC CORR in Statistical Analysis Software (SAS, Cary NC).
Quantitative trait locus (QTL) analysis
Restriction quality DNA of the parents and 92 of the F6 RILs were isolated using the CTAB buffer method recommended by Diversity Arrays Technology (DArT) (Triticarte Pty Ltd, Canberra, Australia) (Akbari et al. 2006). DNA samples of the RILs and parents were genotyped with the DArT methodology. Polymorphic markers were indicated as present (1) or absent (0). Linkage groups of DArT markers were assembled with Mapmaker version 2.0 (Lincoln et al. 1992) for MacIntosh with the Kosambi mapping function with a logarithm of odds (LOD) of 3 and r = 0.3. Composite interval mapping (CIM) was done with QGENE (Nelson 1997) to calculate the coefficient of determination (R 2) and LOD scores for each marker interval at a significance level of α = 0.05 with 1,000 permutations of the dataset. The RILs and parents were also genotyped with the sequence-tagged site (STS) marker csLV46 (E. Lagudah, Commonwealth Scientific and Industrial Research Organization, Canberra, Australia, personal communication) that is linked to the adult plant leaf rust resistance gene Lr46 (Li et al. 2014; Singh et al. 1998). DNA of the parents and RILs were amplified with csLV46 using standard PCR techniques. Amplified products were separated in 1.0 % agarose gels and were visualized with ethidium bromide staining. DArT markers associated with leaf rust severity were identified by single factor regression with SAS. The datasets were also evaluated with PROC GLM in SAS to evaluate interactions between tests and marker regions associated with leaf rust resistance. Differences in leaf rust severity between RIL genotypes were assessed with Student’s t test in PROC GLM.
Results
The Tc*3/Americano 25e F6 lines had a wide segregation for leaf rust resistance in all four tests (Fig. 1). The leaf rust severities for the F6 lines ranged from 10 to 80 % in St. Paul 2009 and Crookston 2010 and from 10 to 70 % in St. Paul 2010 and the greenhouse test. The Tc*2/Americano 25e resistant parent had 10 % leaf rust severity in three of the tests and 20 % in St. Paul 2010. The susceptible parent Thatcher had 60–80 % leaf rust severity in all the tests. The leaf rust severity ratings of the Tc*3/Americano 25e F6 lines had a good general correlation between the four tests, ranging from 0.59 for the greenhouse—St. Paul 2010 tests, to 0.69 for the St. Paul 2009–Crookston 2010 tests (Table 1). The F6 RILs and the parents were also tested as seedlings with P. triticina isolates with race designations BBBDB and SBDGG that are highly avirulent to a number of Lr genes and isolates with race designations THBJG and MFPSB (Supplemental Table 1). The parents and RILs had high IT of 3 to 4 to all P. triticina isolates.
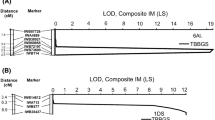
A total of 352 DArT markers segregated in the 92 F6 lines that were genotyped and in the parental lines. Markers that could not be linked to other markers were discarded which left 243 DArT markers to construct the map that spanned 1,196 cM over 21 linkage groups, with an average of 4.9 cM/marker. Composite interval mapping of the leaf rust severity of the F6 lines in the four tests identified two regions associated with leaf rust severity. DArT marker wPt-4651 on chromosome 1BL was highly associated with leaf rust resistance. The STS marker csLV46 that is linked to Lr46 on 1BL was then added to the linkage map. The marker for the Lr46 region on 1BL accounted for 25 to 42 % of the total variation in the four tests that measured leaf rust severity, with LOD scores of 5.7–10.5 (Table 2; Fig. 2a). The csLV46 marker had significant effects in the PROC GLM analysis (p < 0.001) in all four tests. The leaf rust infection types of the F6 lines were not significantly associated with the Lr46 region, with nonsignificant R 2 and LOD scores.
A second region on chromosome 5BL was also associated with adult plant leaf rust resistance. This quantitative trait locus designated as QLr.cdl-5BL spanned 4.4 cM between DArT markers wPt-4091 and wPt-2373 with the peak at 54 cM (Table 2; Fig. 2b). In St. Paul 2009 and Crookston 2010, this region accounted for 14 and 16 % of the total variation with LOD scores of 2.92 and 3.45, respectively. The QLr.cdl-5BL region had no effect in the St. Paul 2010 test. With the greenhouse severity ratings, this region was borderline significant with an R 2 of 12 % and LOD of 2.50. The QLr.cdl-5BL region was significantly associated with the greenhouse infection type ratings, with an R 2 of 0.20 and LOD of 4.3. In the PROC GLM analysis, the QLr.cdl-5BL region had significant effects (p < 0.01) in all tests, except for St. Paul 2010.
The 92 F6 lines that were genotyped were placed into four groups based on the presence of the csLV46 marker and QLr.cdl-5BL (Table 3). In the three field tests and the one greenhouse test, the lines that had both csLV46 and QLr.cdl-5BL significantly had the lowest leaf rust severity, ranging from 13.5 to 26.5 %. Lines with only csLV46 had the second lowest severity in all tests, ranging from 24.1 to 43.9 %. Lines with the QLr.cdl-5BL resistance singly had significantly lower leaf rust severity compared to the lines that lacked both loci in St. Paul 2009 and in the greenhouse test. In the greenhouse test, lines with the QLr.cdl-5BL resistance had significantly higher rust severity compared to lines with the csLV46 resistance. In St. Paul 2010 and Crookston 2010, there was no significant difference between lines with the QLr.cdl-5BL resistance only and lines that did not have any regions associated with resistance. In the PROC GLM analysis of the combined datasets, there was no significant interaction between the tests and either csLV46 (test × csLV46, p = 0.67) or QLr.cdl-5BL (test × QLr.cdl-5BL, p = 0.08) using Type III sum of squares. In the combined dataset, the lines with both csLV46 and QLr.cdl-5BL significantly had the lowest rust severity. Lines with csLV46 only had significantly lower rust severity compared to lines with the QLr.cdl-5BL resistance only. Lines with the QLr.cdl-5BL resistance only had significantly lower rust severity compared to lines that lacked any regions associated with resistance.
Twenty of the 24 F6 lines with both csLV46 and QLr.cdl-5BL had low IT that ranged from 12 to 23 in the greenhouse test (Supplemental Table 2). Similarly, 14 of the 25 lines that lacked the two regions associated with leaf rust resistance had high IT of 3–4. Twelve of the 24 lines that only had csLV46 had IT of 12–23, and twelve of the 19 lines that only had QLr.cdl-5BL for resistance had the same range of low IT. The distribution of F6 genotypes for IT was highly significant with χ 2 = 32.5 and p < 0.001.
Discussion
In this study, the leaf rust resistance on the 1BL region of Lr46 was enhanced in an additive manner by the presence of the QLr.cdl-5BL resistance. The RILs with combinations of Lr46 and QLr.cdl-5BL always had significantly lower leaf rust severity compared to lines with only Lr46, although the QLr.cdl-5BL resistance was not always effective when present singly as in Crookston 2010 and St. Paul 2010. It is not clear why the QLr.cdl-5BL resistance was less effective in these two tests. In assessing RILs that are segregating for partial adult plant resistance, the timing of the readings is critical. If the plots are assessed too early or too late, it can be difficult to consistently distinguish different levels of resistance in the segregating lines. It may be that the plots in St. Paul 2010 and Crookston 2010 were assessed at times that were not optimal to distinguish the resistance in lines that only had the QLr.cdl-5BL resistance.
In the previous genetic study of leaf rust resistance in Americano 25e (Kolmer et al. 2007), the Tc*2/Americano 25e F2 families segregated in a three gene ratio for leaf rust resistance in a field plot rust nursery test. These genes were likely Lr16, Lr46, and the QLr.cdl-5BL resistance. For many years, Americano 25e has had good leaf rust resistance in rust nursery plots. Other cultivars in South America developed from germplasm with Americano 25e in the pedigree (Kohli 1986) may also have Lr46. The Uruguayan cultivars Estanzuela Calandria and Estanzuela Federal had unidentified adult plant resistance (Germán and Kolmer 2013) that could be Lr46. CIMMYT cultivars Pavon 76, Saar, Pastor, and Oligo were also determined to have Lr46 (Li et al. 2014). In these studies, the R 2 values for effects due to Lr46 ranged from 0.13 to 0.53. The R 2 values for Lr46 derived from Americano 25e in this study fell within this range. This gene may also be present in winter wheat germplasm, as the 1BL region of Lr46 was highly associated with adult plant leaf rust resistance in Thatcher lines derived from the US winter wheat line CI13227 (Kolmer et al. 2012). In field plots in Minnesota, Thatcher lines with Lr46 can vary between 20 and 40 MRMS when Thatcher is at 60–70S. This level of resistance would not be sufficient by itself, but may be useful in combination with other genes such Lr16, Lr23, or Lr34 that have conditioned good levels of resistance in Minnesota spring wheat cultivars (Oelke and Kolmer 2005) and breeding lines (Tsilo et al. 2014).
QLr.cdl-5BL is located in the distal region of chromosome 5BL. DArT marker wPt-2373 mapped to the distal region of 5BL in a consensus map that incorporated DArT markers, single nucleotide polymorphic markers, genotype by sequence markers and simple sequence repeat markers (Yu et al. 2014). Other leaf rust resistance QTL have been found on chromosome 5BL. Chu et al. (2009) mapped a QTL for adult plant leaf rust resistance in the distal region of 5BL that was derived from a synthetic hexaploid wheat. Other QTL on 5BL were identified in the winter wheat cultivars Forno (Messmer et al. 2000) and Capo (Buerstmayr et al. 2014). The relationship between these different QTL on 5BL can only be resolved by development and characterization of RIL populations that are segregating for a single QTL. Tc*3/Americano 25e lines that have only QLr.cdl-5BL will be crossed with Thatcher to develop a RIL population segregating for this single leaf rust resistance QTL.
In this study, it was possible to distinguish the adult plant resistance in a greenhouse test. The csLV46 region affected rust severity in the greenhouse test as did QLr.cdl-5BL. However, the csLV46 region had no significant effect on rust IT, while the 5BL QTL did. Lines with Lr46 have been noted to have a high infection type in field plots tests, although with lower rust severity (Singh et al. 1998). In the absence of other major genes for leaf rust resistance, it may be possible to select germplasm lines for adult plant resistance in a greenhouse test in addition to field plots by evaluating rust severity if susceptible controls are also included.
Adult plant leaf rust resistance genes often condition only a partial, incomplete level of resistance by themselves. In this test, lines with Lr46 and the QLr.cdl-5BL resistance singly had intermediate to high levels of leaf rust severity.
However, lines with the combination of Lr46 plus the QLr.cdl-5BL resistance had resistance equivalent or better than the Thatcher lines with Lr34 (Dyck 1987), the most effective adult plant resistance gene yet characterized. In another study, Lan et al. (2014) determined that RILs with combinations of Lr46 plus other regions with QTL for leaf rust resistance did not have lower leaf rust severity when compared to lines with Lr46 only. They attributed the lack of additive enhancement to the low severity level of lines that had Lr46 singly. Genes Lr34 and Lr46 and other adult plant resistance genes in wheat also condition resistance to stripe rust and stem rust (Li et al. 2014). Testing of the Tc*3/Americano 25e RILs in plots with stripe and stem rust will allow QLr.cdl-5BL to be evaluated for effectiveness to these two rusts.
References
Akbari M, Wenzel P, Craig V, Carling J, Yang S, Usynski G, Mohler V, Lehemensiek A, Huchel H, Hayden MJ, Howes N, Sharp P, Vaughn P, Rathnell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Buerstmayr M, Matiasch L, Masher F, Vida G, Ittu M, Robert O, Holdgate S, Flath K, Neumayer A, Buerstmayr H (2014) Mapping of quantitative adult plant field resistance to leaf and stripe rust in two European winter wheat populations reveals co-location of three QTL conferring resistance to both pathogens. Theor Appl Genet 127:2011–2028
Chu CG, Friesen TL, Xu SS, Faris JD, Kolmer JA (2009) Identification of novel QTLs for seedling and adult plant leaf rust resistance in a wheat double haploid population. Theor Appl Genet 119:263–269
Dyck PL (1987) The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome 29:467–469
Germán SE, Kolmer JA (2013) Leaf rust resistance in selected late maturity, common wheat cultivars from Uruguay. Euphytica 195:57–67
Germán SE, Barcellos A, Chaves M, Kohli M, Campos P, de Viedma L (2007) The situation of common wheat rusts in the Southern Cone of America and perspectives for contol. Aust J Agric Res 58:620–630
Kohli MM (1986) Wheat varieties of the Southern Cone Region of South America. Names: pedigrees and origins. CIMMYT, Mexico
Kolmer JA, Hughes ME (2014) Physiologic specialization of Puccinia triticina on wheat in the United States in 2012. Plant Dis 98:1145–1150
Kolmer JA, Oelke LM, Liu JQ (2007) Genetics of leaf rust resistance in three Americano landrace-derived wheat cultivars from Uruguay. Plant Breeding 126:152–157
Kolmer J, Chen X, Jin Y (2009) Diseases which challange global wheat production—the wheat rusts. In: Carver BF (ed) Wheat: science and trade. Wiley, Ames, pp 89–124
Kolmer J, Long DL, Hughes ME (2010) Physiologic specialization of Puccinia triticina in the United States in 2008. Plant Dis 94:775–780
Kolmer J, Long DL, Hughes ME (2011) Physiologic specialization of Puccinia triticina in the United States in 2009. Plant Dis 95:935–940
Kolmer JA, Lin M, Bai G (2012) Genetics of leaf rust resistance in the winter wheat line CI13227. Crop Sci 52:2166–2172
Lan C, Rosewarne GM, Singh RP, Herrera-Foessel SA, Huerta-Espino J, Basnet BJ, Zhang Y, Yang E (2014) QTL characterization of resistance to leaf rust and stipe rust in the spring wheat line Francolin#1. Mol Breed 34:789–803
Li Z, Lan C, He Z, Singh RP, Rosewarne GM, Chen X, Xia X (2014) Overview and application of QTL for adult plant resistance to leaf rust and powdery mildew in wheat. Crop Sci 54:1907–1925
Lincoln SE, Daly MJ, Lander E (1992) Constructing genetic maps with Mapmaker version 2.0. Whitehead Inst, Cambridge
Long DL, Kolmer JA (1989) A North American system of nomenclature for Puccinia recondita f.sp. tritici. Phytopathology 79:525–529
McIntosh RA, Wellings CR, Park RF (1995) Wheat Rusts: an altas of resistance genes. CSIRO Australia, Kluwer Academic Publishers, Dordrecht
Messmer MM, Seyfarth R, Keller M, Schachermayr G, Winzeler M, Zanetti S, Feuillet C, Keller B (2000) Genetic analysis of durable leaf rust resistance in winter wheat. Theor Appl Genet 100:419–431
Nelson JC (1997) QGENE: software for marker-based genomic analysis and breeding. Mol Breed 3:239–245
Oelke LM, Kolmer JA (2005) Genetics of leaf rust resistance in spring wheat cultivars Norm and Alsen. Phytopathology 95:773–778
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res 26:496–500
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico
Singh RP, Mujeebkazi A, Huerta-Espino J (1998) Lr46—a gene conferring slow rusting resistance to leaf rust in wheat. Phytopathology 88:890–894
Tsilo TJ, Kolmer JA, Anderson JA (2014) Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 104:865–870
Yu LX, Barbier H, Rouse MN, Singh S, Singh RP, Bhavani S, Huerta-Espino J, Sorrels ME (2014) A consensus map for Ug99 stem rust resistance loci in wheat. Theor Appl Genet 127:1561–1581
Acknowledgments
I thank K. Xiao and M. Hughes for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolmer, J.A. A QTL on chromosome 5BL in wheat enhances leaf rust resistance of Lr46 . Mol Breeding 35, 74 (2015). https://doi.org/10.1007/s11032-015-0274-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0274-9