Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most devastating diseases worldwide and is also an important disease in China. The wheat translocation line H9014-121-5-5-9 was originally developed from interspecific hybridization between wheat (Triticum aestivum L.) line 7182 and Psathyrostachys huashanica Keng. This translocation line showed resistance to predominant stripe rust races in China when it was tested with nine races of Pst. To determine the inheritance and map the resistance gene, segregating populations were developed from the cross between H9014-121-5-5-9 and the susceptible cultivar Mingxian 169. The seedlings of the F1, F2, and F2:3 generations were tested with race CYR31. The results showed that the resistance in H9014-121-5-5-9 was conferred by a single dominant gene. Bulked segregant analysis and simple sequence repeat (SSR) markers were used to identify polymorphic markers associated with the resistance gene locus. Seven polymorphic SSR markers were linked to the resistance gene. A linkage map was constructed according to the genotypes of the seven SSR markers and the resistance gene. Based on the SSR marker positions on the wheat chromosome, the resistance gene was assigned on chromosome 1AL, temporarily designated YrHA. Based on chromosomal location, reaction patterns and pedigree analysis, YrHA should be a novel resistance gene to stripe rust. The molecular markers of the new resistance gene in H9014-121-5-5-9 could be useful for marker-assisted selection in breeding programs against stripe rust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stripe rust (yellow rust), caused by Puccinia striiformis f. sp. tritici (Pst), is one of the most devastating diseases of wheat (Triticum aestivum L.) worldwide. China is the largest region of epidemic wheat stripe rust in the world, especially the northwest, southwest and Yellow-Huai wheat regions of China. Stripe rust has become a major threat to wheat production due to the appearance of new virulent races (Wan et al. 2004; Wellings 2011). Growing resistant cultivars is the most effective, economic and environment-friendly strategy for controlling the disease. However, the race-specific resistance gene(s) are usually overcome by new virulent Pst races shortly after the genes are widely deployed in commercial cultivars (Li and Zeng 2002; Lin and Chen 2008). It is therefore an important task to identify new stripe rust resistance genes and develop molecular markers for efficient incorporation and pyramiding of the genes into new wheat varieties.
To date, 53 stripe rust resistance genes have been officially named, of which 14 genes were introgressed into wheat from alien species, viz. Yr5, Yr7, Yr8, Yr9, Yr15, Yr17, Yr24, Yr26, Yr28, Yr35, Yr36, Yr37, Yr38 and Yr40. These genes enrich the stripe rust resistance gene pool, and some genes (e.g. Yr9, Yr15) have made great contributions to wheat improvement (Li and Zeng 2002). Chinese geneticists and breeders have successfully introgressed the chromosomes or chromosomal fragments from alien species into wheat and developed several valuable cultivars. For example, Xiaoyan 6 and Guinong 22, which carry stripe rust resistance genes from Elytrigia elongate and Haynaldia illosa, respectively, remained resistant for many years (Shang 1998; Jing et al. 2007; Zhang et al. 1998). Our group has been working on genetics of alien species resistance to wheat stripe rust since the 1990s. In genetic stock resistance screening, over 40 resistant lines from progenies of wheat hybridizations with Psaythyrostachys huashanica, Haynaldia villosa, Leymus mollis (Trin.) Hara, Elytrigia elongata and Thinopyrum intermedium were identified (Jing et al. 1999). Sixteen genes from these genetic stock lines have been mapped, viz. YrZL93444 (Wang et al. 2011a), Yr93447 (Yang et al. 2010b), Yr88375 (Yang et al. 2008c) and YrZhong22 (Yang et al. 2008b) derived from Thinopyrum intermedium; YrElm1-4 (Yang et al. 2010a), YrEml4 (Yang et al. 2009a), YrElm2 (Yang et al. 2008a), YrLm2 (He et al. 2010) and YrLm1 (Song et al. 2009) derived from L. mollis (Trin.) Hara; YrWV (Wang et al. 2011b) and YrV1 (Zhou et al. 2008) derived from H. villosa; Yrcy54-1 (Hou et al. 2010) and Yrxyh (Yang et al. 2009b) derived from E. elongata; and YrHy (Yao et al. 2010), Yrhua (Cao et al. 2008) and YrH9020 (Li et al. 2012) derived from P. huashanica, and these genes are effective against the current Pst population in China.
Psathynrostachys huashanica Keng (2n = 2x = 14, NN) is a perennial wild grass native to Huashan Mountain in Shaanxi Province, China. P. huashanica is a useful breeding material for wheat improvement due to its valuable characters such as early maturity, tolerance to drought stress and disease resistances. Various types of wheat—P. huashanica hybrid genetic stocks have been successfully created through interspecific hybridization and chromosomal engineering, including nullisomic heptaploid lines, alien chromosomal substitution lines, addition lines and translocation lines (Chen et al. 1991, 1996; Hou et al. 1997). In our disease resistance assessments, we found most of the translocation lines had a high level of resistance to Chinese Pst races (Jing et al. 1999). To make the resistance sources more useful for breeding programs, our group studied the inheritance of these resistance genes, and identified and molecular-mapped four stripe rust resistance genes from P. huashanica (Tian et al. 2011; Yao et al. 2010; Cao et al. 2008; Liu et al. 2008).
Wheat line H9014-121-5-5-9 was developed from interspecific hybridization between common wheat line 7182 and P. huashanica accession 0503383 and subsequent backcrossing with 7182 (Cao et al. 2008). The objective of this study was to identify the stripe rust resistance gene(s) in H9014-121-5-5-9, map the resistance gene using simple sequence repeat (SSR) markers and develop molecular markers in wheat breeding.
Materials and methods
Plant and pathogen materials
The translocation line H9014-121-5-5-9, which originated from interspecific hybridization between Triticum aestivum line 7182 and P. huashanica accession 0503383, was created and kindly provided by Professor Jie Fu (Northwest A & F University, Yangling, China). The segregating populations were made from the cross between H9014-121-5-5-9 and Mingxian 169. H9014-121-5-5-9 is a resistant donor. Mingxian 169 is a Chinese winter wheat cultivar and susceptible to all Chinese Pst races identified so far. The F1, F2 and F2:3 progenies were developed in a greenhouse. Chinese Spring (CS) and its complete set of 21 nulli-tetrasomic lines were used to determine the chromosomal position of the molecular markers related to stripe rust resistance gene in H9014-121-5-5-9. Four wheat—P. huashanica translocation lines, H9020-17-15, H9020-1-6-8-3, H9020-20-12-1-8 and H122, were used to determine the gene’s origin and relationships.
Nine predominant Chinese Pst races (CYR25, CYR27, CYR29, CYR30, CYR31, CYR32, CYR33, Su11-4 and Su11-11) representing various virulence combination patterns reported in China in recent years were used to test H9014-121-5-5-9 and Mingxian 169 at the seedling stage in a greenhouse.
Seedling tests
The seedling tests were conducted under a controlled greenhouse condition. About 10 seeds for each parent and the F1, 228 seeds for the F2 and 20 seeds for each of the F3 lines were used for tests. Seeds were planted in several 10-cm-diameter pots. About 10 days after planting, seedlings were inoculated by dusting with a mixture of urediniospore and talc at a 1:50 ratio and kept in a dark dew chamber at 10 °C for 24 h. The inoculated plants were transferred to an environmentally controlled greenhouse with 16 h light/8 h dark photoperiod at 12–17 °C. Infection types (IT) were recorded 17–18 days after inoculation when rust had fully developed on the susceptible check Mingxian 169. Infection type was based on a 0–4 scale where the plants with IT 0–2+ were considered to be resistant and those with IT 3−–4 susceptible (Li et al. 2006).
SSR analysis
Genomic DNA was extracted from green leaves using the CTAB method as modified by Yan et al. (2003). Two hundred and twenty-eight F2 plants and the corresponding F3 lines from the cross Mingxian 169/H9014-121-5-5-9 were used as a mapping population. Based on the F3 disease phenotypic data, DNA from 10 resistant lines and 10 susceptible lines was used for constructing resistant and susceptible bulks, respectively. Each bulk was composed of equal amounts of F2 plant DNA (Michelmore et al. 1991).
The SSR procedure was used to identify markers linked to the resistance loci. SSR primer sequences were obtained from the GrainGenes website (http://wheat.pw.usda.gov). A total of 660 wheat SSR markers were screened on the two parents and the resistant and susceptible bulks for detecting polymorphisms. A 15 μL reaction mixture consisted of 1.5 μL PCR 10× buffer (containing 5 mmol Mg2+), 2.0 μL DNA template (25 ng/μL), 1.2 μL dNTPs (2.5 mM each), 1.5 μL each of forward and reverse primers (5 μM), 0.3 μL Taq DNA polymerase (5 U/μL) and 7.0 μL ddH2O. PCR amplification of SSRs was performed in a MJ Research PTC-200 thermal cycler. The PCR conditions were the following: initial denaturation at 94 °C for 5 min, followed by 35 cycles of 30 s denaturation at 94 °C, 1 min annealing at 50–68 °C depending on primers and 45 s extension at 72 °C, and a final extension at 72 °C for 10 min. After amplification, 6 μL of formamide loading buffer [98 % formamide, 10 mM EDTA (pH 8.0), 0.5 % (w/v) xylene cyanol and 0.5 % (w/v) bromophenol blue] was added to the PCR product. About 5–7 μL mixture of the PCR product and loading buffer for each sample was loaded for electrophoresis on 8 % polyacrylamide gels. The gel was stained and visualized as previously described (Bassam et al. 1991).
Data analysis and linkage map construction
Segregation of markers and resistance gene locus was tested for goodness-of-fit to the expected ratio using the Chi squared test with the Excel data analysis software of Microsoft Office 2007. Linkage analysis was performed with MAPMAKER 3.0b (Lincoln et al. 1992). The recombination frequency was converted to map distance (in centiMorgans) according to the Kosambi mapping function (Kosambi 1943). The linkage map was drawn using the software Mapdraw v2.1 (Liu and Meng 2003).
Validation of the close marker in wheat—P. huashanica translocation lines
The closest SSR markers to the stripe rust resistance gene in H9014-121-5-5-9 were used to test four wheat translocation lines (H9020-17-5, H9020-1-6-8-3, H9020-20-12-1-8 and H122), which also contained the stripe rust resistance genes from P. huashanica (Tian et al. 2011; Yao et al. 2010; Cao et al. 2008; Liu et al. 2008). The DNA isolation of the four translocation lines and P. huashanica accession 0503383 were as previously described.
Results
Genetic character of stripe rust resistance in line H9014-121-5-5-9 in the seedling tests
H9014-121-5-5-9 was resistant (IT 0–1) to nine Pst races. The reaction to seven races was light necrosis with IT 0. The reaction to race Su11-4 was immune. Only a few tiny unopened stripe rust pustules of race Su11-11 were formed on H9014-121-5-5-9. The resistant donor P. huashanica accession 0503383 had a similar reaction to stripe rust as H9014-121-5-5-9. Wheat line 7182, as recurrent parent for developing H9014-121-5-5-9, was highly susceptible, with IT 4. Mingxian 169, as the susceptible control, was also highly susceptible (IT 4). When inoculated with CYR31, F1 plants had IT 0, and in the 228 F2 plants 173 plants were resistant and 55 susceptible, fitting a resistant:susceptible ratio of 3:1 (χ2 = 0.09, P = 0.76). Of 192 F3 families, 50 were homozygous resistant, 96 segregating and 46 homozygous susceptible lines, showing a 1:2:1 ratio (χ2 = 0.17, P = 0.92). Both F2 and F3 segregation data indicated that a single dominant gene conferred the stripe rust resistance to CYR31 in H9014-121-5-5-9 (Table 1). We tentatively designated this gene as YrHA.
Molecular linkage map for YrHA
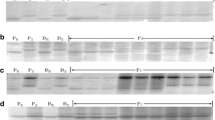
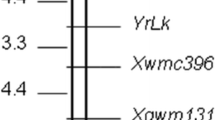
A total of 660 SSR markers covering all 21 wheat chromosomes were screened for polymorphisms between H9014-121-5-5-9 and Mingxian 169. Seven markers, Xbarc240, Xwmc312, Xwmc93, Xwmc9, Xwmc469, Xgwm497 and Xwmc59, showed clear polymorphisms between the resistant and susceptible DNA bulks as well as the parents. The entire F2 population was then genotyped with the seven polymorphic markers. All of them are co-dominant markers except Xwmc469 and Xwmc312 (Table 2). The genotypes of the seven SSR markers fitted 3:1 (dominant) or 1:2:1 (co-dominant) ratio, indicating that each of the seven SSR markers inherited as the single locus. A linkage group was constructed using these markers and the resistance gene YrHA. The linkage map spanned 25.1 cM, and the resistance gene YrHA was closely linked to Xwmc469 and Xgwm497 with map distances of 3.4 and 4.6 cM, respectively (Table 2, Fig. 2). The markers Xwmc469 and Xgwm497 are shown in Fig. 1 as an example. Based on the wheat SSR consensus map (http://wheat.pw.usda.gov), the seven linked markers were located on the long arm of chromosome 1A. The marker Xwmc312 was used to test 21 CS nulli-tetrasomic lines. The target fragment was present in all lines except N1AT1B, confirming that the resistance gene in H9014-121-5-5-9 was located on chromosome 1A (fig. S1).
Origin of the stripe rust resistance gene YrHA
The wheat line H9014-121-5-5-9 was derived from consecutive backcross between P. huashanica accession 0503383 and common wheat line 7182. Seedling tests showed that the recurrent parent 7182 was susceptible to nine stripe rust races and pathotypes, whereas H9014-121-5-5-9 and P. huashanica accession 0503383 were resistant to all the stripe rust races and pathotypes. Therefore, from the pedigree and the resistance to stripe rust, we conclude that the resistance gene in H9014-121-5-5-9 was derived from P. huashanica accession 0503383. To determine the origin of the resistance gene in H9014-121-5-5-9, the two closest flanking SSR markers (Xwmc469, Xgwm497) were tested with the four translocation lines, 7182 and P. huashanica accession 0503383. SSR analyses showed that P. huashanica accession 0503383 possessed the same bands as that of H9014-121-5-5-9, whereas the target bands were absent in 7182 and the four translocation lines H9020-17-5, H9020-1-6-8-3, H9020-20-12-1-8 and H122 (Fig. 3). These results indicated that the resistance gene in H9014-121-5-5-9 was originated from P. huashanica accession 0503383 and different from the other translocation lines that also derived from P. huashanica. The resistance gene in H9014-121-5-5-9 was a novel effective gene to current stripe rust pathogen population, temporarily designated YrHA.
Discussion
To date, none of the officially named Yr genes is located on chromosome 1A. Only one temporarily named gene, YrDa1 from cultivar Daws, has been located on chromosome 1A by monosomic analysis (Chen et al. 1995). The wheat cultiver Daws is developed from a three-way cross, CI14484/CI13645/PI178383 (Zeven and Zeven-Hissink 1976). The gene in Daws could have been from CI14484, CI13645, and/or Washington Selection 101, which was used to develop CI14484. All of the wheat genotypes in the Daws pedigree are common wheat. YrHA is derived from P. huashanica and should be different from YrDa1.
Our group has identified four stripe rust resistance genes from wheat—P. huashanica translocation lines (Tian et al. 2011; Yao et al. 2010; Cao et al. 2008; Liu et al. 2008). All of these four genes have a high level of resistance to the tested races. Yrhua is identified in the translocation line H9020-17-5 against CRY30, and mapped on chromosome 6AL. YrH9020, identified from translocation line H9020-1-6-8-3, is resistant to CYR32 and located on chromosome 2D. YrHy is identified in translocation line H9020-20-12-1-8 and confers resistance to CYR32, which is located on chromosome 2BS. YrH122 in translocation line H122 is resistant to Pst pathotype Sun11-4 and mapped on chromosome 1DL. In this study, we identified a new resistance gene YrHA in translocation line H9014-121-5-5-9. Comparing the SSR genotypes with the other identified Yr genes from P. huashanica, YrHA is different from other Yr genes and is deduced to be a novel resistance gene to stripe rust.
Zhao infers that chromosomes of P. huashanica can substitute for the wheat chromosomes 5A, 3B or 3D to generate a common wheat genotype (Zhao et al. 2003), which means that it is easy to create a relatively stable substitution lines between P. huashanica and common wheat. In other words, the chromosomes of P. huashanica can be transferred to common wheat and could be recognized as relatively fixed common wheat chromosome groups. Resistance assessments over several years also confirm that resistance of H9014-121-5-5-9 to stripe rust is stable, and YrHA is stably inherited.
Wheat wild relatives have various disease resistances and play important roles in wheat improvement. The wheat cultivars derived from wheat-rye (Secale cereal L.) 1BL/1RS translocation lines carry several disease resistance genes such as Lr26, Sr31, Yr9 and Pm8 which provide many benefits to modern wheat breeding (Singh et al. 1990; Villareal et al. 1991). Almost 90 % of predominant Chinese wheat cultivars in 1990s are developed from this translocation line (Li and Zeng 2002). In China, another wild wheat relative, Elytrigia elongata, is used in wheat improvement and batches of cultivars benefit from the wheat—E. elongate translocation. The wheat—E. elongate translocation lines carry both race-specific and non-race-specific resistance. Wheat cv. Xiaoyan 6 derived from this translocation has high-temperature durable rust resistance and has remained resistant over 20 years since its release in the 1980s (Ma and Shang 2000).
Psathynrostachys huashanica has similar resistance characteristics to E. elongate against multiple races at seedling stage and also remains resistant in multiple-year rust evaluation in the field. However, it is not known whether P. huashanica has high-temperature resistance. P. huashanica possesses several stripe-rust resistance genes, but they have not been deployed in current wheat cultivars. In our studies we found that several wheat—P. huashanica translocation lines not only have disease resistance but also have other favorable agronomic traits such as drought tolerance and good seed quality. They are a valuable genetic source for wheat improvement. With the SSR markers in this study, the resistance gene YrHA will be easily introgressed into elite wheat lines in breeding programs.
References
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196(1):80–83
Cao Z, Deng Z, Wang M, Wang X, Jing J, Zhang X, Shang H, Li Z (2008) Inheritance and molecular mapping of an alien stripe-rust resistance gene from a wheat-Psathyrostachys huashanica translocation line. Plant Sci 174(5):544–549
Chen S, Zhang A, Fu J (1991) The hybridization between Triticum aestivum and Psathyrotachys huashanica. Acta Genetica Sinica 18(6):508–512
Chen X, Line RF, Jones SS (1995) Chromosomal location of genes for resistance to Puccinia striiformis in winter wheat cultivars Heines VII, Clement, Moro, Tyee, Tres, and Daws. Phytopathology 85(11):1362–1367
Chen S, Hou W, Zhang A, Fu J, Yang Q (1996) Breeding and cytogenetic study of Triticum aestivum-Psathyrostachys huashanica alien addition lines. Acta Genetica Sinica 23(6):447–452
He M, Song X, Wang Y, Yao Q, Li Y, Jing J (2010) Molecular mapping of stripe rust resistance gene in wheat translocation line M853–4 derived from Leymus mollis (Trin.) Hara. Acta Phytophylacica Sinica 37:119–122
Hou W, Zhang A, Yang Q, Fu J, Chen S (1997) Breeding and cytogenetic study of Triticum aestivum-Psathyrostachys huashanica alien substitution lines. Acta Botanica Boreali-Occidentalia Sinica 17(3):368–373
Hou D, Zhou X, Wang L, Wang W, Ma D, Jing J (2010) Molecular mapping of high temperature adult plant stripe rust resistance gene Yrxy54-1 with RGAP markers. Acta Phytophylacica Sinica 006:487–492
Jing J, Fu J, Yuan H, Wang M, Shang H, Li Z (1999) A preliminary study on heredity of the resistance to stripe rust in three wild relatives of wheat. Acta Phytopathologica Sinica 29:147–150
Jing J, Xu Z, Wang D, Wang M, Yao Q, Shang H, Li Z (2007) Genetic analysis of gene conferring resistance to stripe rust in Xiaoyan6. Scientia Agricultura Sinica 40(3):499–504
Kosambi D (1943) The estimation of map distances from recombination values. Ann Hum Genet 12(1):172–175
Li Z, Zeng S (2002) Wheat rust in China. China Agriculture Press, Beijing
Li G, Li Z, Yang W, Zhang Y, He Z, Xu S, Singh R, Qu Y, Xia X (2006) Molecular mapping of stripe rust resistance gene YrCH42 in Chinese wheat cultivar Chuanmai 42 and its allelism with Yr24 and Yr26. Theor Appl Genet 112(8):1434–1440
Li Q, Huang J, Hou L, Liu P, Jing J, Wang B, Kang Z (2012) Genetic and molecular mapping of stripe rust resistance gene in wheat-Psathyrostachys huashanica translocation line H9020–1-6-8-3. Plant Dis 96(10):1482–1487
Lin F, Chen X (2008) Molecular mapping of genes for race-specific overall resistance to stripe rust in wheat cultivar Express. Theor Appl Genet 116(6):797–806
Lincoln S, Daly M, Lander E (1992) Constructing genetic maps with Mapmarker/EXP3.0. In: Whitehead Institute Techn Rep, 3rd edn. Whitehead Institute, Cambridge
Liu R, Meng J (2003) MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas 25(3):317
Liu P, Yang M, Zhou X, Wu H, Jing J (2008) Genetic analysis and molecular mapping of stripe rust resistance of wheat translocation line H9020–1-6-8-3 derived from Psathyrostachys huashanica Keng. Acta Phytopathologica Sinica 38(1):104–107
Ma Q, Shang H (2000) High-temperature resistance of wheat cultivar Xiaoyan series to wheat stripe rust. Acta Agriculturae Boreali-Occidentalis Sinica 9:39–42
Michelmore RW, Paran I, Kesseli R (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88(21):9828–9832
Shang H (1998) High temperature resistance of wheat to stripe rust. Scientia Agricultura Sinica 31(4):46–50
Singh NK, Shepherd KW, MeIntosh RA (1990) Linkage mapping of genes for resistance to leaf, stem and stripe rusts and ω-secalins on the short arm of rye chromosome 1R. Theor Appl Genet 80:609–616
Song X, Hou L, Yang M, He M, Yan Y, Jing J (2009) Genetic analysis and molecular mapping of stripe rust resistance gene in wheat translocation line M8657–1 derived from Leymus mollis Trin. Hara. Acta Phytopathologica Sinica 38(6):652–655
Tian Y, Huang J, Li Q, Hou L, Li G, Wang B (2011) Inheritance and SSR mapping of a stripe-rust resistance gene YrH122 derived from Psathyrostachys huashanica Keng. Acta Phytopathologica Sinica 41:64–71
Villareal R, Rajaram S, Mujeeb‐Kazi A, Toro E (1991) The effect of chromosome 1B/1R translocation on the yield potential of certain spring wheats (Triticum aestivum L.). Plant Breed 106(1):77–81
Wan A, Zhao Z, Chen X, He Z, Jin S, Jia Q, Yao G, Yang J, Wang B, Li G (2004) Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis 88(8):896–904
Wang L, Hou D, Wang W, Zhou X, Song J, Jing J (2011a) Genetic analysis and molecular mapping of stripe rust resistance gene in wheat line Zhongliang93444. Acta Phytophylacica Sinica 38(2):109–115
Wang R, Zhang S, Xu Z, Chen J, Li Q, Hou L, Jing J (2011b) Genetic analysis and SSR molecular mapping of new stripe-rust resistance gene YrWV derived from Triticum aestivum-Haynaldia villosa translocation line V9125–2. Scientia Agricultura Sinica 44:9–19
Wellings CR (2011) Global status of stripe rust: a review of historical and current threats. Euphytica 179(1):129–141
Yan GP, Chen XM, Line RF, Wellings CR (2003) Resistance gene analog polymorphism markers co-segregating with the Yr5 gene for resistance to wheat stripe rust. Theor Appl Genet 106:636–643
Yang M, Jing J, Liu P, Hou L, Hu M, Song X, Wang M, Li Z (2008a) Genetic analysis and microsatellite makers of a resistance gene YrElm2 in wheat from Elymus mollis (Trin.) Hara. J Northwest A & F University (Natural Science Edition) 36:187–192
Yang M, Xu Z, Wang M, Song J, Jing J, Li Z (2008b) Genetic analysis and molecular mapping of stripe rust resistance gene in wheat cultivar Zhongliang 22. Acta Agronomica Sinica 34:1280–1284
Yang M, Xu Z, Wang M, Song J, Jing J, Li Z (2008c) Molecular mapping of stripe rust resistance gene in wheat line Zhongliang88375. Scientia Agricultura Sinica 41:2931–2936
Yang M, Yao Q, He M, Hou L, Jing J (2009a) Genetic analysis and SSR location of stripe rust resistance of wheat translocation line M853–4 derived from Elymus mollis (Trin.) Hara. J Agric Biotechnol 17(4):695–700
Yang W, Chen H, Jing J (2009b) Genetic analysis of Xiaoyan6 resistance to stripe rust under high-temperature. Acta Phytophylacica Sinica 36(1):95–96
Yang M, Peng Y, Yao Q, He M, Jing J (2010a) Genetic analysis and SSR location of stripe rust resistance of wheat translocation line M8657–1 derived from Triticum aestivum-Leymus mollis (Trin.) Hara. J Agric Biotechnol 18(5):861–866
Yang Y, Ma D, Wang W, Song J (2010b) Genetic analysis and molecular mapping of stripe rust resistance gene in Zhong-liang 93447. Acta Phytopathologica Sinica 005:482–488
Yao Q, Wang Y, He M, Li Y, Zhou X, Wang B (2010) SSR molecular mapping of stripe rust resistance gene of wheat translocation line H9020–20-12-1-8 derived from Psathyrostachys huashanica Keng. J Agric Biotechnol 18(4):676–681
Zeven AC, Zeven-Hissink NC (1976) Genealogies of 14,000 wheat varieties. Netherlands Cereal Center and International Maize and Wheat Improvement Center
Zhang Q, Zhang L, Zhu W (1998) Utilization of Haynaldia Villosa (L.) Shur. in resistance breeding in wheat. Acta Phytophylacica Sinica 25(1):41–45
Zhao J, Chen X, Wang X, Wu J, Fu J, He B, Song Y, Sun Z (2003) C-Banding identification of alien substitution lines and alien additional lines in Triticum-Psathyrrostachys. J Northwest Sci-Tech Univ Agric For (Nat Sci Ed) 31:1–4
Zhou X, Wu H, Zhang R, Liu P, Jing J (2008) Microsatellite tagging of stripe-rust resistance gene YrV1 derived from Haynaldia-villosa. Acta Phytopathologica Sinica 38(1):69–74
Acknowledgments
The authors are grateful to Dr. Meinan Wang for editorial review of manuscript and suggestions for its improvement. The financial support of projects “the 111 Project from the Education Ministry of China” (No. B07049), “National 11th Five-Year Plan Key Project” (2006BAD08A05) and “Toxicity Variation of Wheat Stripe Rust Pathogen and Demonstration of Integrated Management of Stripe Rust, China” (No. 200903035-02) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dongfang Ma and Xinli Zhou made equal contributions to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, D., Zhou, X., Hou, L. et al. Genetic analysis and molecular mapping of a stripe rust resistance gene derived from Psathynrostachys huashanica Keng in wheat line H9014-121-5-5-9. Mol Breeding 32, 365–372 (2013). https://doi.org/10.1007/s11032-013-9876-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-9876-2