Abstract
Glutathione (GSH), a low-molecular-weight tripeptide molecule that plays an important role in cell function and metabolism as an antioxidant, is synthesized by γ-glutamylcysteine synthetase and glutathione synthetase. To investigate the functional role of GSH in the adaptation of plants to abiotic stresses, we developed Brassica juncea L. ECS (BrECS)-expressing transgenic rice plants (BrECS1 and BrECS2) under the regulation of a stress-inducible Rab21 promoter. BrECS1 and BrECS2 transgenic rice plants with BrECS overexpression tolerated high salinity by maintaining a cellular glutathione (GSH)/glutathione disulfide redox buffer, which prevented unnecessary membrane oxidation. BrECS1 and BrECS2 rice plants also showed lower ion leakage and higher chlorophyll-fluorescence than wild-type (WT) rice plants in the presence of methyl viologen (MV) and salt, resulting in enhanced tolerance to abiotic stresses. During germination, BrECS overexpression increased growth and development, resulting in an increased germination rate in the presence of salt conditions, but not under salt-free normal conditions. Furthermore, BrECS1 and BrECS2 rice plants displayed a moderate increase in biomass and rice grain yield under general paddy field conditions when compared to WT rice plants under general paddy field conditions. Therefore, our results suggest that BrECS-overexpression was critical for cellular defense from reactive oxygen species attacks produced by salt and MV, promotion of germination, and metabolic processes involved in natural environmental stress tolerance, thereby enhancing growth development and rice grain yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are constantly subjected to abiotic and biotic stresses, including high salinity, extreme temperatures, heavy metals, flooding, drought, nutrient depletion, and pathogen infection, which affect germination, growth development, and grain production (Foyer et al. 1994; Asada 1999). Rice (Oryza sativa L.) is a major crop that is grown worldwide in both tropical and temperate climate zones. These environmental changes cause reactive oxygen species (ROS)-induced oxidative stress directly and indirectly following disruption of redox homeostasis. Oxidative stress leads to cellular damage such as membrane instability by lipid oxidation, enzyme inactivation by protein carbonylation, and DNA oxidation, which decreases photosynthetic ability and metabolic capacity, resulting in cell death (Mittler 2002; Apel and Hirt 2004). To overcome and withstand these oxidative conditions, plants have developed a wide range of cell rescue systems, including antioxidant molecules and enzymes, molecular chaperones, and metabolic solutes such as proline and trehalose (Singh et al. 2011). Specifically, plants detoxify ROS by the AsA–GSH cycle via a combination of antioxidants such as ascorbate (AsA) and glutathione (GSH), as well as antioxidative enzymes such as ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR). Components of the AsA–GSH pathway are localized in different organelles, including chloroplasts, cytosol, and mitochondria (Potters et al. 2002).
Among these antioxidant systems, tripeptide glutathione (Glu–Cys–Gly; GSH) is one of the most crucial metabolites in plants and is considered the most important intracellular defense against ROS-induced oxidative damage. This tripeptide occurs abundantly in reduced form (GSH) in its plant tissues and is localized in all cellular compartments such as the cytosol, chloroplasts, mitochondria, peroxisomes, vacuoles, endoplasmic reticulum (ER), and apoplast, and plays a central role in several physiological processes, including regulation of sulfur transport, signal transduction, conjugation of metabolites, neutralization of xenobiotics (Gill and Tuteja 2010), and the expression of stress-responsive genes (i.e., PR1, GST, GPX, CHS, PAL, SOD, etc.) in GSH-fed suspension cells or leaves of a range of plant species (Mullineaux and Rausch 2005). It is well established that GSH also plays an important role in several growth and development-related events in plants, including cell death and senescence, cell differentiation, pathogen defense, and enzyme regulation (Gill and Tuteja 2010). In addition, GSH accumulates in response to environmental stresses such as low temperature, heavy metals, and oxidants in levels that vary depending on the stress types and periods of stress exposure (Noctor and Foyer 1998; Aravind and Prasad 2005). In plants, GSH synthesis is mainly mediated by two enzymes, γ-glutamylcysteine synthetase (γ-ECS) and GSH synthetase (GS) in the presence of amino acids and ATP. First, γ-ECS catalyzes formation of γ-glutamylcysteine from Cys and Glu; this is thought to be the rate-limiting step of the pathway. Second, GS adds Gly to γ-glutamylcysteine to give GSH (Gill and Tuteja 2010). As synthesized, GSH provides a substrate for multiple cellular reactions that yield the oxidized form (GSSG). The balance between GSH and GSSG is a central component in maintaining the cellular redox state. GSH is necessary to maintain the reduced redox state of cells so as to counteract the inhibitory effects of ROS-induced oxidative conditions (Gill and Tuteja 2010; Szarka et al. 2012). γ-ECS and GS are upregulated in peas, tobacco, maize, and tomatoes exposed to abiotic stresses (Rennenberg et al. 1981; Chen and Goldsbrough 1994; Kocsy et al. 2001). In this regard, the levels of GSH determine the capacity of a plant to respond to stress.
Brassica species comprise the second most important crop after rice. Brassicas are primarily used as food and feed, but have recently gained interest as a source of by-products such as biodiesel (Nesi et al. 2008). Brassicas are abundant in minerals (calcium, phosphorus, and iron), fiber, phytochemicals and antioxidant molecules such as AsA, GSH, and tocopherols (Goraj et al. 2012). The concentration of antioxidative compounds such as phenolics in Brassica spp. is at least 30 times higher than that in soybeans and rice (Nesi et al. 2008), which may result from environmental conditions and their genetic background. Currently, these compounds are of special interest due to their antioxidant activity and beneficial effects on human health (Francisco et al. 2012). Although numerous studies of dietary effects have been reported, there is little information regarding high solute accumulation and crop quality through overexpression of genes involved in antioxidant synthesis in transgenic plants.
Currently, a great deal of effort has been put into the development of stress-resistant crop plants; however, excessive overexpression of genes of interest can result in metabolic abnormalities following feedback inhibition. This problem can be resolved by manipulating the expression of regulatory genes under stress conditions. On the other hand, although much research into the responses of GSH to environmental stresses has been conducted, adaptation in paddy field conditions has not yet been investigated. Integration of physiological, biochemical, and molecular approaches would allow us to gain deeper insights into the role and mechanism of GSH in adaptation or resistance to environmental conditions. Based on this information, we demonstrated that overexpression of the γ-glutamylcysteine synthetase gene from B. juncea L. (BrECS) under the control of a stress-inducible responsive ABA protein 21 (Rab21) promoter enhanced tolerance to salt and methyl viologen (MV) and improved germination in the presence of these compounds by minimizing cellular damage via creation of an enhanced GSH redox state and increased biomass and rice grain yield under paddy field conditions. The results presented herein provide valuable information for improving rice grain yield under abiotic stress conditions that limit agricultural productivity.
Materials and methods
Plasmid construction and transformation into rice plants
Full-length cDNA (accession no. AJ563921.1) encoding the ECS gene in B. juncea L. was cloned by reverse-transcription PCR (RT-PCR) using the following primer set: sense, 5′-GGGATCCATGGCGTTATTGTCTCAGGC-3′; antisense, 5′-GCTCGAGTCATCCTCAGTAAAGCAGTTC-3′. The generated cDNA was designated BrECS. The p600-Rab21 vector was then made using the pSB11 vector (accession No. AB027256) as a backbone, after which the cDNA was cloned between the stress-inducible Rab21 promoter and nos terminator using BamHI and XhoI restriction endonucleases; this was denoted pBrECS. The nucleotide sequences of the stress-inducible Rab21 promoter and BrECS were then determined to ensure that the open reading frame was combined without any frame shifts or nucleotide conversions. The complete BrECS coding sequence contained 1,545 base pairs. The p600-Rab21 binary vector was then introduced into Agrobacterium strain LBA 4404, which was used to transform rice calli produced from the scutella of mature seeds of Oryza sativa cv. Ilmi. Agrobacterium-mediated rice transformation was conducted as previously described (Hiei et al. 1994; Kang et al. 1998).
Plant materials and growth conditions
Seeds were surface-sterilized with 0.05 % pesticide (Bayer CropScience, South Korea) for 24 h, rinsed completely, and then soaked in distilled water for 4 days. Following seed germination, seedlings were transplanted into soil and then grown in a glasshouse (16-h light/8-h dark cycles) at 28–32 °C. Transgenic and control wild-type (WT) rice plants were grown in pots (two plants/pot). Four-week-old transgenic and WT rice seedlings were subjected to 100 mM NaCl solution for 30 days in the glasshouse and then recovered for another 14 days by irrigation in salt-free water. Leaves of the rice plants were then collected at 0, 6, and 12 days after salt treatment, frozen quickly in liquid nitrogen, and stored at –70 °C for subsequent experiments.
DNA extraction and genotyping
About 0.5 g of leaf samples was placed into a microfuge tube and finely ground, after which 500 μL of DNA extraction buffer containing 100 mM Tris–HCl (pH 9.0–9.5), 10 mM EDTA, and 1 M KCl was added, and the samples were vortexed for 5 min. Sample solutions were then incubated at 70 °C for 1 h with vortexing every 10 min, after which 500 μL of distilled water was added. The solutions were mixed with shaking for 10 min and then centrifuged at 12,000 rpm for 10 min at 4 °C. The cleared supernatants were then transferred to a fresh microfuge tube and used as a template for genotype PCR. PCR was conducted using the extracted genomic DNA in a PCR PreMix (Bioneer, South Korea). Genomic DNA integration of the BrECS transgene with the stress-induced promoter in transgenic rice plants was verified using the Rab21-F (5′-CCACCTTCACCTGCTTGCACACCA-3′) and BrECS-R (5′-CCCTTAGGCATGGTGGGAATGTCC-3′) primer set. The genotype PCR conditions were as follows: initial denaturation at 94 °C for 3 min followed by 34 cycles of 94 °C for 40 s, 54 °C for 40 s, and 72 °C for 50 s and then a final extension for 5 min at 72 °C.
RNA extraction and semi-quantitative RT-PCR
Total RNA was isolated from the leaf tissue of rice plants. Four-week-old transgenic and WT rice seedlings grown in a glasshouse (16-h light/8-h dark cycles) at 28–30 °C were subjected to 100 mM NaCl solution for 12 days under the same conditions. Total RNA was then extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. For semi-quantitative RT-PCR, first-strand cDNA was synthesized from DNase I-treated total RNA using SuperScript II reverse transcriptase (Invitrogen, USA) according to the manufacturer’s instructions. To monitor BrECS expression, the BrECS-F1 (5′-ATGGCGTTATTGTCTCAGGCAGG-3′) and BrECS-R (5′-CCCTTAGGCATGGTGGGAATGTCC-3′) primer set was used. The rice tubulin gene with the Tub-F (5′-TACCGTGCCCTTACTGTTCC-3′) and Tub-R (5′-CGGTGGAATGTCACAGACAC-3′) primer set was used as a positive housekeeping gene under the same PCR conditions. The semi-quantitative RT-PCR conditions were as follows: initial denaturation at 93 °C for 5 min followed by 22–25 cycles of 93 °C for 30 s, 54 °C for 30 s, and 72 °C for 50 s, and then a final extension for 5 min at 72 °C. Semi-quantitative PCR products were resolved on a 1.5 % agarose gel, stained with ethidium bromide, and then photographed with a Gel Image Analysis System (Core Bio system, South Korea).
Measurement of GSH contents
To determine the total GSH contents of rice plant leaves (0.5 g), the leaves were frozen in liquid nitrogen, homogenized in 5 % trichloroacetic acid, and then centrifuged at 13,000 rpm for 20 min at 4 °C. Total glutathione (GSH + 2GSSG) was determined by adding either GSH as a standard or properly diluted samples to a reaction mixture containing 100 mM phosphate buffer (pH 7.4), 6.3 mM EDTA, 0.6 mM 5,5′-dithiol-bis-nitrobenzoic acid (DTNB), 0.3 mM NADPH, 0.09 % 5-sulfosalicylic acid (SSA), and 1.2 units of glutathione reductase (GR) in a final volume of 1 mL. After incubation for 20 min at room temperature in the dark, the concentrations were measured at 412 nm. An additional reaction was carried out to determine the oxidized form (GSSG) of GSH. In this reaction, any GSH present in the sample was first removed by vortexing for 1 h at room temperature with 0.05 % 2-vinylpyridine. Following centrifugation, the GSSG levels were measured using the same methods used to determine the total GSH (Rahman et al. 2006).
MV-mediated hydroperoxide detection with DAB staining and stress responses to H2O2
Solutions of 50 μM MV containing 0.1 % Tween-20 were sprayed onto the leaves of whole rice plants and incubated for 12 h, after which the leaves were cut, placed on 3,3′-diaminobenzidine (DAB; 1 mg/mL)—HCl solution (pH 3.8), incubated in a growth chamber for 8 h at 25–28 °C, and cleaned in boiling ethanol (96 %) for 10 min before photographing. H2O2 produced by MV is visualized as a reddish-brown coloration (Sung and Hong 2010). Four-week-old rice seedlings were exposed to 50 mM H2O2 for 30 days at 25–28 °C in a growth chamber (Kwon et al. 2003; Eltayeb et al. 2007) and then photographed.
Ion leakage analysis under MV treatment
Leaves were collected from 8-week-old rice seedlings grown in soil, after which ten leaf discs (about 1 cm) were cut from five leaves. The leaf discs were then immediately placed in a 10-μM MV solution in a 9.0-cm diameter Petri dish as described by Kwon et al. (2002) and incubated in darkness for 12 h to allow for MV diffusion, after which they were illuminated under continuous light (150 μmoL photon m−2 s−1) at 25–28 °C. The extent of cellular membrane damage was subsequently quantified by ion leakage from 0 to 72 h using an ion conductivity meter (model 455C; Isteck Co., Seoul Korea). At the end of the specified time-period, the samples were autoclaved for 15 min at 121 °C to release all of the solutes, after which the conductivity of the solution was measured and used to calculate ion leakage at different time points. The experiments measuring damage by MV application were repeated in triplicate.
Chlorophyll-fluorescence measurements under stress conditions
Chlorophyll-fluorescence was estimated by the photosynthetic activity of photochemical yield (Fv/Fm), which represents the maximum yield of the photochemical reaction of photosystem II. To accomplish this, ten leaf discs were immediately floated on 500 mM NaCl (24-well tissue culture test plate) in a 9.0-cm diameter Petri dish and incubated for 16 h at 25–28 °C under continuous light (150 μmoL photon m−2 s−1). The Fv/Fm values were then measured as previously described (Oh et al. 2005). A Handy Fluor Cam FC 1000-H (Photon Systems Instruments Ltd., Brno, Czech Republic) was used to measure the time-resolved chlorophyll-fluorescence images of the transgenic and WT rice leaves (Nedbal et al. 2000).
Seed germination assay
Fifty seeds of two independent transgenic and WT rice plants were selected for seed germination tests. Briefly, healthy rice seeds were sterilized with 70 % ethanol, rinsed thoroughly, and then soaked in deionized distilled water overnight. The rice seeds were placed in a Petri dish (9.0-cm diameter plate) and saturated with or without 100 mM NaCl solution. The seeds were then allowed to germinate for 3 days under 16-h light/8-h dark periods at 25–28 °C. Each test was replicated three times. At the end of the 3 days, the germination rate (%) was determined. The shoot lengths were determined after 6 days of incubation at 25–28 °C.
Grain yield analysis under paddy field conditions
To measure rice yield components, rice plants were cultivated for 30 days after germination in a glasshouse and randomly transplanted to 20 × 30 cm spacing with a single seedling per hill in a paddy field at the experimental field of Kyungpook National University in the independent (2010, T2 generation) and homozygous (2011, T3 generation) lines of the transgenic rice plants together with WT rice plants. At this time, WT rice plants were inserted into every plot for comparison. Transgenic and WT rice plants were then grown for approximately 6 months under normal conditions in paddy fields, after which they were harvested and threshed by hand to separate the seeds from the vegetative parts of the rice plants. Yield parameters were scored for 12 rice plants from each genotype (transgenic and WT). Rice plants located at the borders were excluded from data scoring. Unfilled and filled grains were then taken apart, independently counted, and weighed. The following agronomic traits were scored: total plant biomass fresh weight (g), culm weight (g), root fresh weight (g), number of panicles per hill, number of spikelets per panicle, grain filling rate (%), total grain weight (g), and weight of 1,000 grains (g). The results from two independent transgenic rice plants were analyzed separately and compared with those of the WT rice plants.
Statistical analysis
The statistical significance of differences between transgenic and WT rice plants in measured parameters was tested by Student’s t test using Microsoft Excel 2007. Means were considered to be significantly different when P values were <0.05. All experiments were carried out at least in triplicate, and the results are expressed as the mean ± standard deviation (SD).
Results
Integration and expression of BrECS in transgenic rice plants
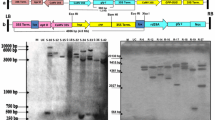
To develop BrECS-expressing transgenic rice plants, 78 independent transgenic event lines (T0 generation) were produced in the presence of the bar gene under control of the Cauliflower mosaic virus 35S promoter to enable herbicide-based rice plant selection (Fig. 1a). The 78 independent transgenic events (T0 generation) were cultivated under paddy field conditions, and their progeny (T1 seeds) were harvested. We selected 52 (T1 generation) independent event lines among 78 (T0 generation) independent event lines. The 52 independent transgenic event lines (T1 generation) were cultivated in a paddy field, and the progeny (T2 seeds) were harvested. First, we examined the salt stress response of transgenic rice lines. To accomplish this, a total of 5,200 (T2 generation) rice lines (52 independent rice lines × 100 per each subline seed) were separately grown in small pots and treated with 100 mM NaCl solution for 4 weeks. From these 52 (T2 generation) transgenic lines, we selected six independent homozygous salt-tolerant lines. These transgenic lines displayed very similar phenotypes in the presence of 100 mM NaCl (Supplementary Fig. S1). However, two transgenic rice plants (BrECS1 and BrECS2) revealed an improved phenotype in comparison to other transgenic rice plants (BrECS3 to BrECS6). Thus, these two independent transgenic lines (BrECS1 and BrECS2) were mainly used for our subsequent experiments. Subsequent genotype PCR analysis conducted using the Rab21-F and BrECS-R primers revealed a single band in samples of these six independent transgenic lines, whereas no signal was observed in WT plants, indicating that the construct containing the Rab21 promoter and BrECS gene had been inserted into the rice genome (Fig. 1b, and Fig. S3, Supplementary Information). To check whether the BrECS gene was properly expressed in the transgenic rice plants (BrECS1 and BrECS2), semi-quantitative RT-PCR analysis was performed using RNA extracted from the leaves of 4-week-old BrECS1 and BrECS2 rice plants treated with 100 mM NaCl. Transcriptional expression of BrECS increased gradually in BrECS1 and BrECS2 rice plants under salt stress conditions with time, while rice plants cultivated under normal conditions produced no signal. However, WT rice plants produced no signal in the presence and absence of 100 mM NaCl (Fig. 1c). Taken together, these results indicated that BrECS was integrated into the transgenic rice genome and effectively expressed in the presence of high salinity under control of the stress-inducible Rab21 promoter.
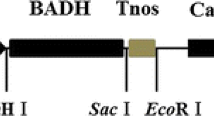
Components of Rab21::BrECS transgenic rice plants. a The Rab21::BrECS plasmids consisted of the Rab21 promoter, a stress-inducible promoter linked to the BrECS coding region, the 3′ region of the potato (Solanum tuberosum) proteinase inhibitor II gene (PinII), and a gene expression cassette that contains the 35S promoter, the bar coding region, and the 3′ region of the Tnos terminating sequence of the nopaline synthase gene. The entire expression cassette was flanked by the 5′ matrix attachment region (MAR) of the chicken lysozyme gene (Oh et al. 2005). LB left border; RB right border. b PCR analysis of genomic DNA from transgenic rice plants to monitor BrECS gene expression. Numbers (1–16) represent independent transgenic rice lines (BrECS1 and BrECS2); transgenic rice lines and WT represents control WT rice plants. c Semi-quantitative RT-PCR analysis of BrECS1, BrECS2, and control WT rice plants after 100 mM NaCl. BrECS1, BrECS2, and WT rice plants were subjected to treatment with 100 mM NaCl for 12 days. Tubulin was used as an internal control
Stress response and glutathione redox state in the presence of high salinity
To investigate whether BrECS overexpression was correlated with stress tolerance in rice plants, 4-week-old transgenic (BrECS1 and BrECS2) and WT rice seedlings were exposed to 100 mM NaCl for 30 days and then recovered for 14 days by irrigation with salt-free water. Under normal conditions, there were no differences in growth development between transgenic and WT rice plants (data not shown). However, BrECS1 and BrECS2 rice plants grew more rapidly under recovery conditions following salt stress than WT rice plants (Fig. 2a). WT rice plants also showed visual symptoms of salt-induced damage such as leaf wilting as a result of reduced chlorophyll synthesis capacity when compared to BrECS1 and BrECS2 rice plants (Fig. 2a). MV is a superoxide-generating agent that leads to oxidative stress similar to that seen in salt stress. The superoxide anion (O2 −) is rapidly converted into H2O2 by superoxide dismutase. Accordingly, H2O2 accumulation was measured in MV-treated whole stem tissues by the DAB staining method. Under normal conditions, there was no difference in DAB staining between transgenic and WT rice plants (data not shown). However, the stem tissues of WT rice plants displayed a reddish-brown coloration in the presence of MV when compared to transgenic rice plants (BrECS1 and BrECS2; Fig. 2b), indicating that WT rice plants accumulated higher levels of H2O2 than transgenic rice plants during treatment with 50 μM MV. To investigate whether lower cellular damage and higher stress tolerance following reduced H2O2 levels resulting from GSH content via BrECS overexpression, the GSH redox state was estimated in the absence or presence of 100 mM NaCl. No changes were observed between transgenic rice plants (BrECS1 and BrECS2) and WT rice plants under normal conditions. However, under salt stress, the GSH redox state (GSH/GSSG) increased by approximately 1.4-fold in BrECS1 and BrECS2 rice plants, although the GSH/GSSG ratio in WT rice plants was augmented under salt stress (Fig. 2c). Taken together, these results suggested that the enhanced GSH redox state through BrECS overexpression conferred intrinsic tolerance to ROS-induced oxidative stress by minimizing cellular damage via improved cellular redox homeostasis.
Salt and MV stress tolerance tests in transgenic rice plants. a BrECS1, BrECS2, and WT rice plants were grown in a glasshouse for 4 weeks and then treated for 30 days with 100 mM NaCl as a source of stress. b DAB staining of the leaves from BrECS1 and BrECS2 rice plants. The leaf damage that appeared on the leaves after spraying with 50 μM MV was evaluated after 12 h of treatment. c The GSH/GSSG ratio of BrECS1, BrECS2, and WT rice plants after treatment with 100 mM NaCl for 12 days. White bars correspond to before salt treatment (normal condition); black bars correspond to after salt treatment (stress condition). Data are expressed as the mean ± SD of at least three replications from three independent experiments. Bars labeled with asterisks differed significantly between transgenic and WT rice plants at P < 0.05 as determined by ANOVA
Enhanced tolerance of transgenic rice plants against MV-induced oxidative stresses
MV-mediated oxidative stress generates massive ROS bursts, which affect metabolic processes and membrane stability (Bowler et al. 1991). To evaluate the protective effects of BrECS overexpression, ten leaf discs from rice plants at the same stage of development were prepared and exposed to 10 μM MV solution for 12 h in dark conditions. There were no differences in chlorophyll contents under normal conditions. BrECS1 and BrECS2 rice plants showed higher visibility of chlorophyll content in the presence of MV than WT rice plants, even though chlorophyll contents decreased visibly in both transgenic and WT rice plants under the same conditions (Fig. 3a). BrECS1 and BrECS2 rice plants showed significantly lower ion leakage than WT rice plants under MV stress treatment. Although ion leakage increased in a time-dependent manner in the presence of MV, about 65 % of the ion content of WT rice plants was seen at 72 h after the initiation of MV treatment, while the ion contents of BrECS1 and BrECS2 rice plants was reduced by 45 % under the same conditions (Fig. 3b). These findings indicated that BrECS overexpression contributed to membrane stability under oxidative conditions. Based on the observed metabolic effects, photosynthetic capacity was also measured using chlorophyll-fluorescence parameters (Fv/Fm) under oxidative stress. BrECS1 and BrECS2 rice plants showed higher levels of photosynthetic efficiency than WT rice plants in the presence of 500 mM NaCl, even though the Fv/Fm values decreased in a time-dependent manner. The WT rice plants showed an approximately 50 % reduction in Fv/Fm values after treatment with 500 mM NaCl, while BrECS1 and BrECS2 rice plants showed an approximately 35 % decrease in photosynthetic efficiency under the same conditions (Fig. 3c). Furthermore, BrECS1 and BrECS2 rice plants showed much better survival than WT rice plants when 4-week-old seedlings were exposed to 50 mM H2O2 for 30 days (Fig. 4). Therefore, our results showed that BrECS1 and BrECS2 rice plants exhibited improved photosynthetic activity and ionic homeostasis, leading to enhanced stress tolerance against ROS-induced oxidative conditions.
Analysis of cellular damage in MV- and salt-treated leaf discs of transgenic rice plants overexpressing BrECS under control of the stress-inducible Rab21 promoter. a Relative ion leakage from the leaf discs of transgenic and WT rice plants floated on 10 μM MV solution was determined based on measurement of electrolyte leakage over time post-treatment. b The electrical conductivity of the MV solution was compared with the total conductivity of the solution following tissue destruction. c Changes in chlorophyll-fluorescence (Fv/Fm) in BrECS1, BrECS2, and WT rice plants under 500 mM NaCl stress conditions. All experiments were conducted under continuous light at 150 μmoL m−2 s−1. Data are expressed as the mean ± SD of at least three replication experiments, with each replicate consisting of ten leaf discs. Bars labeled with asterisks indicate significant differences between transgenic and WT rice plants at P < 0.05 as determined by ANOVA
Effects of H2O2 stress on BrECS1, BrECS2, and WT rice plants. Three-week-old BrECS1, BrECS2, and WT rice plants suffered visible differential damage to leaves after treatment with 50 mM H2O2 in test tubes at 25–28 °C for 30 days. WT wild-type rice plants; BrECS1 and BrECS2 independent transgenic rice plants. Six independent experiments were carried out with every replication using five rice plants of the same line in one tube
Germination effects of BrECS overexpression under salt stress
To determine whether BrECS overexpression affects seed germination, germination tests were performed using BrECS1, BrECS2, and WT rice seeds in the absence or presence of 100 mM NaCl. When seeds were incubated at 25–28 °C for 3 days under normal conditions, there was no difference in germination rate between BrECS1, BrECS2, and WT rice seeds. However, BrECS1 and BrECS2 rice seeds were rapidly germinated in the presence of 100 mM NaCl as compared to WT rice seeds (Fig. 5a), leading to a 25 % increase in germination rate under the same conditions (Fig. 5b). Post-germination growth was also examined by measuring shoot length under salt stress conditions. No changes in shoot length were observed between transgenic and WT rice seeds under normal conditions. However, the shoot lengths of BrECS1 and BrECS2 rice seeds grown under 100 mM NaCl conditions for 6 days were approximately 1.2-fold greater than those of WT rice plants (Fig. 5c). These results indicated that BrECS overexpression enhanced germination ability and growth development in the presence of NaCl, but not under optimal conditions.
Seed germination rate of BrECS1, BrECS2, and WT rice seeds exposed to normal and salt conditions. a Rice seeds were germinated on Petri dishes containing 0 or 100 mM NaCl solution. BrECS1, BrECS2, and WT rice seeds showed different germination rates on Petri dishes containing 0 or 100 mM NaCl solution. b Effects of salt on seed germination and the subsequent growth of the seedlings. Rice seeds were germinated on Petri dishes containing 0 or 100 mM NaCl solution. c Shoot lengths of BrECS1, BrECS2, and WT rice plants were measured at 6 days. Data are expressed as the mean ± SD of at least three replication experiments, with each replicate consisting of 50 seeds. Bars labeled with asterisks show significant differences between transgenic and WT rice plants at P < 0.05 as determined by ANOVA
Grain yield analysis in paddy field conditions
We investigated rice yield parameters in the vegetative and reproductive stages of entire rice plants under normal paddy field conditions. Paddy field environments during two seasons exhibited a significant difference in mean temperature, total precipitation, and total sunshine over the 2 years of the study period (means for 2010 and 2011; Table S1, Supplementary Information). Despite these significant environmental differences in the paddy field, yield parameters in the transgenic rice plants (BrECS1–BrECS6) increased over the 2-year study period (mean 2010 and 2011) as compared to WT rice plants. These results indicated that the increase in rice yield was attributable to the performance of the transgene and the Rab21 promoter. Rice yield analysis was conducted at Kyungpook National University (Gunwi campus) in South Korea for the two study seasons (2010 and 2011), and the following yield factors were analyzed: total plant biomass fresh weight (TPW), culm weight (CW), root fresh weight (RW), number of panicles per hill (NP), number of spikelets per panicle (NSP), grain filling rate (FR), total grain weight (TGW), and weight of 1,000 grains (1,000 GW). Overall, rice yield parameters of the BrECS1 and BrECS2 rice plants increased moderately when compared to WT rice plants under normal paddy field conditions (Fig. 6a). The results from a trial conducted under normal paddy field conditions in 2010 showed that most yield parameters of the BrECS1 and BrECS2 rice plants increased by 27, 12.5, 13, 10, 16, and 18 % for TPW, RW, CW, NSP, TGW, and NP, respectively, when compared with control WT rice plants. The TGW of BrECS1 and BrECS2 increased by about 18 and 15 %, respectively (Fig. 6b and Fig. S2, Supplementary Information). We also obtained similar results from our 2011 field trial (Fig. 6c, d, and Fig. S2, Supplementary Information). Taken together, these results showed that BrECS overexpression improved rice grain yield and total biomass through an increase in TGW following enhanced TPW and NP in transgenic rice plants (BrECS1 and BrECS2) under natural paddy field conditions.
Comparison of the agronomic traits of transgenic and WT rice plants under normal paddy field conditions. Appearance of the BrECS1 and BrECS2 rice plants under normal paddy field conditions in 2010 (a) and 2011 (c). Homozygous (T2) or (T3) lines of BrECS1, BrECS2, and WT rice plants grown under paddy field conditions for over 6 months. Spider plots of the agronomic traits of BrECS1 (red), BrECS2 (yellow), and corresponding WT (blue) rice plants under normal paddy field conditions were drawn using Microsoft Excel for study years 2010 (b) and 2011 (d). Each data point represents a percentage of the mean values (n = 12). The mean measurements from WT rice plants were assigned to 100 % as a reference value. TPW total plant biomass fresh weight, CW culm weight, RW root fresh weight, NP number of panicles per hill, NSP number of spikelets per panicle, FR grain filling rate, TGW total grain weight, 1,000 GW 1,000 grain weight
Discussion
Investigations of the salinity tolerance of crop plants such as rice are urgently needed because salinity is increasing globally at an alarming rate, threatening agricultural production. When plants are exposed to environmental stresses including salt, ROS accumulate in plant cells, where they affect cellular macromolecules such as lipids, proteins, DNA, and carbohydrates. ROS have the ability to oxidize redox-sensitive proteins directly or indirectly through the use of molecules such as GSH (Pasqualini et al. 2001). GSH is a major non-enzymatic scavenger of ROS due to its unique structural properties, broad redox potential, abundance, and widespread distribution in plants (Rahman et al. 2006). GSH is catalyzed by γ-ECS and GS (Noctor et al. 1998, 2002), primarily in the chloroplasts and cytosol of plant cells. Therefore, we developed transgenic plants bearing chloroplastic BrECS (Fig. 1a). The BrECS gene was stably integrated into the transgenic rice genome (Fig. 1b) and was strongly expressed under the regulation of a stress-inducible Rab21 promoter in the presence of salt stress, but not under normal conditions (Fig. 1c). Accordingly, Rab21 activity increased rapidly and steadily in response to drought and salt stress in transgenic rice plants (Huang et al. 2009; Tang et al. 2012), suggesting that the most important factor controlling plant GSH is the activity of ECS.
BrECS overexpression in transgenic rice plants (BrECS1 and BrECS2) increased acquired tolerance by reducing the accumulation of toxic ROS through improvement of the GSH redox state (GSH/GSSG; Fig. 2) when compared to WT rice plants after exposure of rice plants to high salinity stress. Moreover, BrECS1 and BrECS2 rice plants enhanced membrane stability by minimizing ion leakage and photosynthetic capacity following improved Fv/Fm values, maintaining higher chlorophyll content and leading to stress tolerance in the presence of MV (Fig. 3). However, there were no physiological changes between transgenic and WT rice plants under normal conditions. Generally, overexpression of ECS, not GS, in plants raises GSH content by increasing flux through the pathway. Feedback mechanisms through which excessive accumulation of GSH inhibits its de-novo biosynthesis have been proposed (Galant et al. 2011); however, our studies suggested that feedback inhibition may not be a major control feature because the GSH/GSSG ratio in transgenic rice plants with the stress-responsive Rab21 promoter was similar to that of WT rice plants. Conversely, salt stress reduces gas exchange, limiting CO2 supply to the leaf (Asada 1999) and causing over-reduction of the photosynthetic electron transport chain (Asencio et al. 2003), thereby producing ROS (Asada 1999). During the oxidative stress response, GSH participates in the control of H2O2 levels. The change in redox state (GSH/GSSG), an indicator of cellular redox balance, during the neutralization of H2O2 is very important in ROS sensing events. GSH acts as an antioxidant and is directly involved in the reduction of most active ROS generated due to stress (Foyer and Halliwell 1976; Romero et al. 2001). In transgenic plants, GSH, an antioxidant, helped to withstand oxidative stress induced by salt, drought, high light, extreme temperatures, ozone, and paraquat (MV) (Pastori and Trippi 1992; Gueta-Dahan et al. 1997; Kocsy et al. 2001). Moreover, transgenic plants with a high level of GSH exhibited stress resistance by protecting NaCl-induced oxidative damage (Yadav et al. 2005; Huang et al. 2006). In the roots of salt-sensitive species of tomato, decreased activities of antioxidant enzymes along with lower GSH content and redox states were observed, while the reverse was true for salt-tolerant species (Shalata et al. 2001). Exogenous GSH injection partially attenuated the harmful effects of salt stress at an early stage as well as during the fruiting stage (Foyer et al. 1997; Romero et al. 2001; Kwon et al. 2003). In this regard, GSH is an attractive target of plant defenses against abiotic stresses. Therefore, our results showed that improved GSH (GSH/GSSG) homeostasis via BrECS overexpression in the regulation of the Rab21 promoter provides a useful link between environmental factors (salt, MV, and H2O2) and the mechanism involved in physiological and molecular adaptation processes in transgenic rice plants, suggesting a definite role of GSH in salinity tolerance.
When rice seeds were exposed to 100 mM NaCl, germination rates of BrECS1 and BrECS2 rice seeds were approximately 25 % higher than those of WT rice seeds in the presence of salt (Fig. 5a, b), leading to better development under the same conditions (Fig. 5c). In contrast, there were no differences in germination rate or shoot length between transgenic and WT rice plants under salt-free conditions. ROS are also generated during seed germination. To detoxify unfavorable ROS at this stage, plant seeds rapidly accumulate a wide range of antioxidant molecules such as GSH, ascorbate, and tocopherols within 24 h of the initiation of water uptake (Tommasi et al. 2001; Simontacchi et al. 2003). For example, Chenopodium rubrum L. seeds showed increased GSH contents that coincided with radicle protrusion during seed germination (Dučić et al. 2003). Additionally, transgenic tobacco plants overexpressing GSH-S-transferase (GST) and GSH peroxidase (GPX) exhibited improved seed germination and seedling growth in the presence of salt associated with higher levels of GSH when compared to WT seedlings (Roxas et al. 2000). GSH1-deficient Arabidopsis resulted in a recessive embryo-lethal phenotype, indicating that GSH plays an important role in seed germination (Rausch et al. 2007). Therefore, our results suggested that high GSH content led to improved seed germination and development in transgenic rice plants under salt stress and contributed to increased salt tolerance.
Interestingly, BrECS-expressing transgenic rice plants (BrECS1 and BrECS2) exhibited enhanced vegetative and reproductive growth under natural field conditions when compared to WT rice plants (Fig. 6a), leading to increased rice yield (Fig. 6b). BrECS1 and BrECS2 rice plants was showed to increase TPW, CW, RW, NP, and NSP following the development of shoots and roots in the vegetative stages, leading to increased TGW at the reproductive stage when compared with control WT rice plants (Fig. 6b). Increased rice yield of transgenic rice plants is likely a result of increased TPW and NP because no changes in 1,000 GW were observed in the transgenic rice plants. Moreover, we conducted a comparison of the agronomic traits of the BrECS1–BrECS6 transgenic and WT rice plants under normal paddy field conditions. Homozygous 2010 (T2 generation) or 2011 (T3 generation) independent transgenic lines (n = 6) and WT rice plants were grown under paddy field conditions for 6 months. Transgenic rice plants (BrECS1 to BrECS6) exhibited enhanced vegetative and reproductive growth under natural field conditions when compared to WT rice plants (Fig. S2a, Supplementary Information). The results of a normal paddy field trial in crop years (2010 and 2011) showed that the average yield parameters for 2010 and 2011 from BrECS1–BrECS6 rice plants were increased by total biomass and grain yield when compared with control WT rice plants (Fig. S2b, Supplementary Information). Currently, many studies are being conducted in an attempt to improve rice yields in transgenic rice plants using genes involved in stress signaling, transcriptional regulation, redox regulation, osmotic regulation, ionic balance, and cellular protection (Wang et al. 2003; Gao et al. 2007; Ismail et al. 2007; Rao et al. 2008). However, there is little information available on the response of rice plants to abiotic stresses because environmental stresses are multigenic as well as quantitative in nature. Accordingly, transgenic plants with improved performance in the presence of abiotic stresses have not yet been used in the field. In addition, although many investigations of the response of GSH to environmental stresses have been conducted, there is still no firm explanation for these responses. Our results showed that physiological, biochemical, and molecular approaches to investigations of BrECS-overexpressing transgenic rice plants would provide some insight into the precise role and mechanism of GSH involved in resistance to abiotic stresses and rice yield under paddy field conditions.
With the development of high-efficiency Agrobacterium-mediated transformation of rice, T-DNA has become a major method. Generally, T-DNA can be randomly and stably inserted into the plant genome, which has made the generation of a population saturated with insertions possible. After T-DNA insertion, various phenotypes have been observed, including changed growth rates, different plant statues, pollen and seed fertility, and so on. The most common characteristics are the dwarf phenotype and leaf-pigment mutations such as albino, pale green, chlorine, striped, or zebra (transverse green and chlorotic bands). Spotted leaves, leaf-morphology mutations, and seed mortality are also found. The T-DNA-tagged populations also carry a low frequency of mutations in the reproductive organs, e.g., depressed paleae, filamentous flowers, extra glumens, and long sterile glumens, as well as flowering-time mutations, including early- and late-flowering phenotypes (Jeon and An 2001; Jiang and Ramachandran 2010). However, these phenotypes (dwarf phenotype and leaf-pigment mutations) induced by T-DNA tagging, including somatic mutations, have not been observed in BrECS-expressing transgenic rice plants. Therefore, our results showed that these similar phenotypes and rice yield increases in six homozygous independent transgenic rice plants could be significantly attributed to the overexpression of the BrECS gene rather than the effect of random somatic mutations. In addition, various types of abiotic stresses that plants encounter between seedling and harvest stages are as follows: unseasonal rain (drought and flood), abundant and copious soil moisture or its quick retreat, soil salinity, micronutrient (soil nutrition) shortage in the root zone, global changes in weather patterns (low and high temperatures, light intensity, and UV), economic and political uncertainty, disease damage, insect or nematode damage, and invasive threats. The Rab21 promoter used in this study is responsive to abscisic acid (ABA) and drought. ABA accumulates significantly under abiotic and biotic stresses including cold, osmotic stress (drought and NaCl), heat, pathogen exposure, UV radiation, and excess water generated by natural paddy field conditions (Ben-Ari 2012; Sreenivasulu et al. 2012). Based on these results, the ABA-responsive Rab21 promoter can effectively regulate expression of the BrECS gene throughout all stages of plant growth, from dry seeds to vegetative tissues to flowers under paddy field conditions.
In conclusion, our results showed that transgenic rice plants overexpressing BrECS under control of the stress-inducible Rab21 promoter showed increased intrinsic tolerance to abiotic stresses (salt, MV, and H2O2) and improved rice grain yield and biomass under natural paddy field conditions, thereby providing a new possibility for understanding the genetics associated with stress-resistance genes and their contribution to plant biotechnology. Further studies are needed to determine how regulatory networks are correlated with physiological and molecular mechanisms against abiotic stresses and environmental conditions in transgenic rice plants.
Abbreviations
- Br:
-
Brassica juncea L.
- BrECS:
-
BrECS-overexpressing transgenic
- ECS:
-
Glutamylcysteine synthetase
- Fv/Fm:
-
Maximum quantum yield of PSII
- GS:
-
Glutathione synthetase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- H2O2 :
-
Hydrogen peroxide
- MV:
-
Methyl viologen
- ROS:
-
Reactive oxygen species
- WT:
-
Wild type
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aravind P, Prasad MN (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asencio C, Rodriguez-Aguilera JC, Ruiz-Ferrer M, Vela J, Navas P (2003) Silencing of ubiquinone biosynthesis genes extends life span in Caenorhabditis elegans. FASEB J 17:1135–1137
Ben-Ari G (2012) The ABA signal transduction mechanism in commercial crops: learning from Arabidopsis. Plant Cell Rep 31:1357–1369
Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10:1723–1732
Chen J, Goldsbrough PB (1994) Increased activity of γ-glutamylcysteine synthetase in tomato cells selected for cadmium tolerance. Plant Physiol 106:233–239
Dučić T, Lirić-rajlić I, Mitrović A, Radotić K (2003) Activities of antioxidant systems during germination of Chenopodium rubrum seeds. Biol Plant 47:527–533
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Descourvières P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant, Cell Environ 17:507–523
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant 100:241–254
Francisco M, Cartea ME, Butrón AM, Sotelo T, Velasco P (2012) Environmental and genetic effects on yield and secondary metabolite production in Brassica rapa crops. J Agric Food Chem 60:5507–5514
Galant A, Preuss ML, Cameron JC, Jez JM (2011) Plant glutathione biosynthesis: diversity in biochemical regulation and reaction products. Front Plant Sci 2:45
Gao JP, Chao DY, Lin HX (2007) Understanding abiotic stress tolerance mechanism: recent studies on stress response in rice. J Integr Plant Biol 49:742–750
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Goraj S, Libik-Konieczny M, Surówka E, Rozpądek P, Kalisz A, Libik A, Nosek M, Waligórski P, Miszalski Z (2012) Differences in the activity and concentration of elements of the antioxidant system in different layers of Brassica pekinensis head. J Plant Physiol 169:1158–1164
Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G (1997) Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta 203:460–469
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of the (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Huang YZ, Zhang GP, Wu FB, Chen JX, Zhou MX (2006) Difference in physiological traits among salt stressed barley genotype. Commun Soil Sci Plant Anal 37:557–570
Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23:1805–1817
Ismail AM, Heuer S, Thomson MJ, Wissuwa M (2007) Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol Biol 65:547–570
Jeon JS, An G (2001) Gene tagging in rice: a high throughput system for functional genomics. Plant Sci 161:211–219
Jiang SY, Ramachandran S (2010) Natural and artificial mutants as valuable resources for functional genomics and molecular breeding. Int J Biol Sci 6:228–251
Kang HG, Jeon JS, Lee S, An G (1998) Identification of class B and class C floral organ identity genes from rice plants. Plant Mol Biol 38:1021–1029
Kocsy G, Galiba G, Brunold C (2001) Role of glutathione in adaptation and signaling during chilling and cold acclimation in plants. Physiol Plant 113:158–164
Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS (2002) Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant, Cell Environ 25:873–882
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mullineaux PM, Rausch T (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Phytosynth Res 86:459–474
Nedbal L, Soukupová J, Kaftan D, Whitmarsh J, Trtílek M (2000) Kinetic imaging of chlorophyll fluorescence using modulated light. Photosynth Res 66:3–12
Nesi N, Delourme R, Brégeon M, Falentin C, Renard M (2008) Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed. C R Biol 331:763–771
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Arisi AM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53:1283–1304
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Pasqualini S, Batini P, Ederli L, Porceddu A, Piccioni C, De Marchis F, Antonielli M (2001) Effects of short-term ozone fumigation on tobacco plants: response of the scavenging system and expression of the glutathione reductase. Plant, Cell Environ 24:245–252
Pastori GM, Trippi VS (1992) Oxidative stress induces high rate of glutathione reductase synthesis in a drought-resistant maize strain. Plant Cell Physiol 33:957–961
Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Rao PS, Mishra B, Gupta SR, Rathore A (2008) Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed 127:256–261
Rausch T, Gromes R, Liedschulte V, Müller I, Bogs J, Galovic V, Wachter A (2007) Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol (Stuttg) 9:565–572
Rennenberg H, Stcitikamp R, Kesselmeier J (1981) 5-oxo-prolinase in Nicotiana tabacum: catalytic properties and subcellular localization. Physiol Plant 52:211–214
Romero LC, Dominguez-Solis JR, Gutierrez-Alcala G, Gotor C (2001) Salt regulation of O-acetylserine(thiol)lyase in Arabidopsis thaliana and increased tolerance in yeast. Plant Physiol Biochem 39:643–647
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the antioxidative system. Physiol Plant 112:487–494
Simontacchi M, Sadovsky L, Puntarulo S (2003) Profile of antioxidant content upon developing of Sorghum bicolor seeds. Plant Sci 164:709–715
Singh LR, Poddar NK, Dar TA, Rahman S, Kumar R, Ahmad F (2011) Forty years of research on osmolyte-induced protein folding and stability. J Iran Chem Soc 8:1–23
Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506:265–273
Sung CH, Hong JK (2010) Sodium nitroprusside mediates seedling development and attenuation of oxidative stresses in Chinese cabbage. Plant Biotechnol Rep 4:243–251
Szarka A, Tomasskovics B, Bánhegyi G (2012) The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci 13:4458–4483
Tang N, Zhang H, Li X, Xiao J, Xiong L (2012) Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol 158:1755–1768
Tommasi F, Paciolla C, de Pinto MC, De Gara L (2001) A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J Exp Bot 52:1647–1654
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen21 Program (PJ008115012012), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mi-Jung Bae and Young-Saeng Kim contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bae, MJ., Kim, YS., Kim, IS. et al. Transgenic rice overexpressing the Brassica juncea gamma-glutamylcysteine synthetase gene enhances tolerance to abiotic stress and improves grain yield under paddy field conditions. Mol Breeding 31, 931–945 (2013). https://doi.org/10.1007/s11032-013-9846-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-9846-8