Abstract
Fusarium head blight (FHB) is a destructive disease of wheat and barley. In wheat it is mainly caused by the fungal pathogens Fusarium graminearum and Fusarium culmorum. We report the identification and evaluation of candidate genes for quantitative FHB resistance. These genes showed altered expression levels in the moderately resistant winter wheat genotypes Capo and SVP72017 after inoculation with F. graminearum. Amongst others, a NPR1-like gene was identified. Sequence analysis of this gene fragment revealed a high level of variation between the parents of a doubled haploid population. Single nucleotide polymorphism and polymerase chain reaction markers were developed and two homoeologous genes were mapped on the long arms of chromosomes 2A and 2D, respectively. Markers for both genes had significant effects on FHB resistance in a diverse collection of 178 European winter wheat cultivars evaluated in multi-environmental field trials after spray inoculation with F. culmorum. These results revealed that allelic variation in two homoeologous NPR1-like genes is associated with FHB resistance in European winter wheat. Markers for these genes might therefore be used for marker-assisted breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB) is a destructive disease of wheat and barley worldwide. In Central Europe, the disease is mainly caused by the trichothecene mycotoxin-producing species Fusarium graminearum Schwabe and Fusarium culmorum Saccardo. The contamination of the grain by mycotoxins like deoxynivalenol (DON) is the most serious problem caused by FHB. No complete resistance has been described in wheat. However, a wide range of quantitative resistance has been found in the wheat gene pool. To date, numerous FHB resistance quantitative trait loci (QTL) have been identified in spring as well as in winter wheat (for review see Buerstmayr et al. 2009).
Some of the genes underlying QTL for quantitative disease resistance are believed to be involved in hormone-mediated signal transduction (Poland et al. 2009). Phytohormone-dependent signal transduction pathways mediate defence responses in plants against pathogens with various lifestyles. These pathways can act synergistically or antagonistically, depending on the phytohormones involved. Generally, salicylic acid (SA) mediates resistance against biotrophic pathogens whereas jasmonate (JA) and ethylene mediate defense mechanisms leading to effective defense against necrotrophs. Nevertheless, there are many exceptions to this rule (Glazebrook 2005). NPR1 (non-expresser of pathogenesis related protein 1) is one of the key regulators in the cross-talk between different hormone-dependent pathways. Activation of NPR1 boosts SA-mediated signal transduction and leads to systemic acquired resistance (SAR). At the same time, NPR1 influences both JA biosynthesis and signal transduction (Beckers and Spoel 2006). F. graminearum and F. culmorum are generally believed to have a necrotrophic lifestyle even though they are able to grow on the plant without causing cell death in the first 36–48 h after inoculation (Kang and Buchenauer 1999; 2000). The role of SA and JA signaling in the resistance of wheat against FHB is not completely understood. Recent studies indicate a positive effect of SA signaling on basal resistance during the early stages of the infection and of JA signal transduction during the later stages (Ding et al. 2011; Makandar et al. 2012; Gottwald et al. 2012; Gunnaiah et al. 2012; Xiao et al. 2013). Additionally, SA was shown to have a direct inhibitory effect on F. graminearum mycelial growth and conidia germination, at least under acidic conditions (Qi et al. 2012).
Candidate genes for FHB resistance have been mainly identified for Fhb1, a QTL on wheat chromosome 3B that confers a strong resistance against fungal spread through the rachis and originates from Asian spring wheat genotypes. Fhb1 is not common in the European winter wheat genepool. Lines carrying Fhb1 have been shown to be able to detoxify DON to DON-3-glycoside, a reaction catalyzed by uridine diphosphate (UDP)-glycosyltransferases (Poppenberger et al. 2003; Lemmens et al. 2005; Schweiger et al. 2010). The wheat UDP-glycosyltransferase TaUGT12887 was upregulated to a higher extent after the inoculation with F. graminearum in a Fhb1 background. However, this protein only had a weak activity against DON in a yeast two-hybrid assay (Schweiger et al. 2013). A gene with similarity to an Arabidopsis thaliana pectin methyl esterase inhibitor is located in the Fhb1 region and shows a differential expression between lines with and without Fhb1 (Zhuang et al. 2013). Recently, a lipid transfer protein was identified that has a higher expression upon F. graminearum inoculation in lines carrying a QTL on chromosome 5A (Schweiger et al. 2013). In European winter wheat, two wheat-induced resistance (WIR1) genes that were upregulated after the inoculation with F. graminearum have been shown to co-locate with a QTL on chromosome 7B (Diethelm et al. 2012).
Many studies have investigated changes in global gene expression in Fusarium- or DON-challenged wheat tissue, shedding light on the complicated host–pathogen interactions (reviewed by Walter et al. 2010), while in numerous QTL studies genomic regions contributing to quantitative host resistance were identified (reviewed by Buerstmayr et al. 2009). In this study, a combination of expression analysis and association study was applied in order to identify candidate genes for FHB resistance in winter wheat and to develop markers for marker-assisted selection: a limited number of gene fragments differentially expressed upon F. graminearum inoculation in the floret tissue of the two moderately resistant winter wheat genotypes Capo and SVP72017 was screened for genetic variation. Markers for these genes were developed, integrated in genetic maps, and verified by an association study with phenotypic data of 178 winter wheat genotypes which were phenotyped for FHB resistance in 12 environments.
Materials and methods
Plant material
The European winter wheat genotypes Capo (Martin/Pokal) and SVP72017 (Marzotto//Dippes Triumph/Miranovskaja 808) were used for expression analysis. SVP72017 was developed by CPRO-DLO in Wageningen, Netherlands and has a high level of quantitative resistance to FHB (Buerstmayr et al. 2000). Capo, a high baking quality wheat variety from the Probstdorfer Saatzucht in Austria, is moderately resistant to FHB. Plants were vernalized at 4 °C for 8 weeks and then grown in the greenhouse with a 16-h light period and 22/18 °C day/night temperatures.
For the development of the mapping population, the susceptible winter wheat breeding line Sngh3559 was crossed with the recombinant inbred line (RIL) A40-22-1-2 (Capo/SVP72017), kindly provided by H. Buerstmayr (Department for Agrobiotechnology, Tulln, Austria). The mapping population consisted of 81 doubled haploid (DH) lines. The population History/Rubens (Holzapfel et al. 2008) consisting of 103 RILs was used for validation of marker positions.
A diverse set consisting of 178 European winter wheat cultivars (Online Resource 5) was used to evaluate the effect of markers developed from the differentially expressed gene fragments on FHB resistance.
Inoculation and FHB field trials
Before expression analysis, a single floret inoculation of the winter wheat genotypes Capo and SVP72017 was carried out with approximately 500 macroconidias per floret of the F. graminearum strain IFA 65 (Department for Agrobiotechnology, Tulln, Austria). The experiment was performed in the greenhouse according to Steiner et al. (2009) with modifications described in Diethelm et al. (2012).
A diverse set consisting of 178 European winter wheat cultivars was tested in 11 environments (Roggenstein 2007, 2008; Leopoldshöhe 2007; Herzogenaurach 2007, 2008; Leutewitz 2007; Leopoldshöhe 2008; Herzogenaurach 2008; Leutewitz 2008, two locations near Freising 2009, Tulln, Austria 2009). Inoculation of the plants was performed during flowering time using a highly aggressive F. culmorum isolate (FC33), which was produced as described by Miedaner et al. (1996). The spore suspension had a concentration of 3 × 105 spores ml−1 and was sprayed onto the plants with an automatic small plot sprayer (100 ml m−2). Due to differences in flowering date, all genotypes were spray-inoculated two to three times within a period of 10 days to inoculate all plants at least once at mid-anthesis. FHB severity was scored visually as the percentage of infected spikelets per plot and started with the first visible symptoms 9–10 days after the last inoculation and was repeated two to three times at intervals of 3–5 days. At one additional location (Tulln, Austria 2009), inoculation was provoked by infected maize stubble spread on the ground. FHB severity was scored visually as percentage of infected spikelets per plot as described above. The panel of cultivars was split into an early and a late flowering set because of the variation in heading time and treated as independent trials. The experimental layout for all field trials was a lattice design with two replications. Plot size at the different locations varied between 0.6 m2 and 3 m2. For further statistical analyses of the phenotypic data, adjusted values for each replication were calculated based on the lattice design.
cDNA-AFLP analysis and qRT-PCR
RNA extraction, cDNA synthesis, cDNA-amplified fragment length polymorphism (ALFP) and BLAST analysis of differentially expressed transcript-derived fragments (TDF) were carried out as described in Diethelm et al. (2012) with the following modifications: for cDNA-AFLP, 300 ng ds-cDNA were digested with 5.0 U MseI and 2.5 U PstI. The fragments were ligated with 1 U T4-ligase to 25 pmol MseI and 2.5 pmol PstI at 37 °C for 3 h. Samples of F. graminearum-inoculated tissues of SVP72017 and Capo taken 0, 8, 24, 32, 48, 72 and 96 h after inoculation (hai) as well as the respective mock-treated controls were analyzed by cDNA-AFLP. TDFs were consecutively numbered (e.g. TDF_032).
qRT-PCR for the differentially expressed TDFs TDF_032, TDF_102 and TDF_076 was performed with samples taken 8, 24, 48, 72 and 96 hai in order to validate the results of the cDNA-AFLP analysis. qRT-PCR was carried out as described in Diethelm et al. (2012). Ubiquitin (X56601) was used as an internal control. Primer sequences, slopes of the standard curves and primer efficiencies are given in Online Resource 1.
Detection of sequence polymorphisms in cDNA-AFLP fragments and marker development
Primers were designed with Primer3 software (Rozen and Skaletsky 2000) for the sequences of differentially displayed transcript-derived cDNA-AFLP fragments or corresponding ESTs, and PCR was carried out by using these specific primers and genomic DNA of SVP72017 and Capo. PCR products were sequenced by MWG (Ebersberg, Germany). PCR markers were developed for the polymorphic sequences of TDF_032 and TDF_102 (Online Resource 1).
In order to obtain a longer PCR product for TDF_076, the primer pair TDF_076_3 was developed from the respective sequences of the original TDF and TA100375_4565, a sequence from the TIGR-database (http://plantta.jcvi.org) with 99 % identity to TDF_076. The PCR conditions were as follows: initial denaturation for 2 min at 94 °C, 35 cycles of 30 s at 94 C, 30 s at 55 °C and 50 s at 72 °C, and 15 min final elongation at 72 °C. PCR products obtained with genomic DNA of SVP72017, Capo and Sngh3559 and cDNA of SVP72017 and Capo were purified with the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). 35 ng of purified PCR product were used to ligate the fragment into the pDrive cloning vector using Qiagen PCR Cloning Kit (Qiagen, Hilden, Germany). Qiagen EZ competent Escherichia coli cells were used for transformation. Miniprep was conducted using the Nucleo Spin Plasmid Kit from Macherey and Nagel (Düren, Germany). Plasmid DNA of at least six colonies per genotype was sequenced by MWG. Sequences were aligned with the CLC-Bio DNA-Workbench Software (CLC-Bio, Aarhus, Sweden).
Markers for pyroseqencing (Pyrosequencing Assay Design software, Qiagen) were developed for the single nucleotide polymorphisms (SNPs), distinguishing between the two homoeologous genes as well as different alleles of each gene (Online Resource 1). Pyrosequencing was carried out as described in Diethelm et al. (2012). PCR markers for insertion–deletion polymorphisms were developed and visualized on 2 % agarose gels.
Primer sequences and annealing temperatures of all markers are specified in Online Resource 1.
Genotyping of the DH population and the set of 178 winter wheat genotypes
Genomic DNA was isolated from leaves using the CTAB method (Saghai-Maroof et al. 1984). The DH lines as well as the parents of the mapping population were genotyped with AFLP and simple sequence repeat (SSR) markers. In total, 29 PstI/MseI AFLP primer combinations were applied according to the procedure of Hartl et al. (1999) and Schmolke et al. (2005). The name of the AFLP markers consisted of the applied primer combination followed by the estimated fragment size in base pairs.
The parents of the mapping population were screened with 126 SSR markers (barc, cfd, gwm, wmc) based on the marker information published in the GrainGenes database (http://wheat.pw.usda.gov/GG2/index.shtml). In the case of polymorphism, the SSR markers were used to genotype the DH lines. The diverse set of 178 winter wheat genotypes was genotyped with 37 SSR markers. In general, the amplification of the SSR markers was performed according to Roeder et al. (1998).
Markers for the differentially expressed gene fragments TDF_032 (XTDF_032), TDF_076 (XTDF_076_SNP1, XTDF_076_SNP12, XTDF_076_INDEL1, XTDF_076_INDEL2, XTDF_076_INDEL3) and TDF_102 (XTDF_102) were developed according to sequence polymorphisms between Capo, SVP72017 and Sngh3559 and used to genotype the mapping population. Additionally, these markers were mapped in the population History/Rubens (Holzapfel et al. 2008). The diverse set of 178 winter wheat genotypes was genotyped with the same marker set. Nulli-tetrasomic lines of Chinese Spring (Sears 1966) were used to assign AFLP markers and markers for differentially expressed TDFs to specific wheat chromosomes. The nulli-tetrasomic lines were kindly provided by Prof. Zeller (Technische Universität München, Germany).
DNA samples of the set of 178 winter wheat genotypes were sent to Diversity Arrays Technology Pty Ltd (DArT; Canberra, Australia) and genotyped with a custom-built wheat DArT array version Wheat PstI (TaqI) 2.3_D, as described in Akbari et al. (2006).
Construction of the genetic map
The genetic map for segregating marker data was constructed with JoinMap® version 3.0 (van Ooijen and Voorrips 2001). The calculation of the marker distances was performed using the Kosambi transformation (Kosambi 1944). For assigning the molecular markers to linkage groups, a minimum LOD of 3.0 was applied.
Association study
The population structure of the 178 winter wheat genotypes was determined by STRUCTURE 2.2 (Pritchard et al. 2000) as described by Mohler et al. (2012), based on 37 unlinked SSR markers and 62 DArT markers distributed across the wheat genome.
Marker–trait associations for the candidate gene loci with FHB severity (=% of infected spikelets) in 12 environments are based on adjusted values for each replication using the following linear mixed-effects model:
where y ijln was the phenotypic observation for the ith wheat cultivar (i = 1,…,I with I being the number of genotypes) at the jth environment in the nth replication carrying the lth allele of the marker, μ was an intercept term, g i was the random effect of the ith genotype, l j was the random effect of the jth environment, α l was the fixed effect of the lth marker genotype, β m was the fixed effect of the mth column of the subpopulation matrix D (gl) ij was the random effect of the interaction between the ith genotype and the jth environment, and e ijlmn was the residual. The random effect vectors of genotypes, locations, genotype × location interactions and residuals are assumed to be independent and normally distributed with mean 0 and variances \( \sigma_{g}^{2} ,\sigma_{l}^{2} ,\sigma_{g \times l}^{2} \,{\text{and}}\,\sigma^{2} , \) respectively.
The lme() function of the software R (R Development Core Team 2010) was used to fit the model through restricted maximum likelihood. Based on the Wald F statistic, tests for the presence of marker–phenotype associations with a significant (P < 0.05) effect on the trait of interest were performed applying the Bonferroni–Holm procedure for multiple tests (Holm 1979).
Results
cDNA-AFLP analysis
Samples from floret tissue, taken 0, 8, 24, 32, 48, 72 and 96 h after treatment with macroconidia of F. graminearum or water, respectively, were analyzed by cDNA-AFLP. TDF, displayed by cDNA-AFLP analysis, ranged from 80 to 800 bp in length. An average of 85 AFLP bands per primer combination was obtained, resulting in approximately 2,500 analyzed bands in total. Forty-five cDNA-AFLP fragments which exhibited a clear pattern of differential expression between F. graminearum-inoculated samples and water-treated controls were recovered from the gels, re-amplified, cloned and sequenced. Differentially expressed TDFs and corresponding BLASTN results (Genbank no. and EST database), TIGR Plant TA Triticum aestivum release 2 (http://plantta.jcvi.org) and Fungal Genome Initiative of the Broad Institute are summarized in Online Resource 2. Primers were generated according to the sequence of the differentially expressed TDFs or corresponding ESTs to amplify genomic DNA of SVP72017 and Capo. The resulting PCR products were sequenced. Three PCR products contained polymorphisms in the genomic sequences of Capo versus SVP72017: TDF_032 (BLAST result: CJ595840, T. aestivum, EST; E value 6.0 e−37), TDF_076 (BLAST result: TA100375_4565, T. aestivum, NPR1-like protein; E value 2.2 e−48) and TDF_102 (BLAST result: CD808864, Leymus chinensis, putative cytochrome P450; E value 3.0 e−50). Genetic markers for these TDFs were also polymorphic between the parents of the Sngh3559//Capo/SVP72017 and the History/Rubens (Holzapfel et al. 2008) populations and, therefore, integrated in the genetic maps.
The results of the expression analysis by cDNA-AFLP for the three polymorphic TDFs were validated by qRT-PCR (Online Resource 3). TDF_032 was predicted to be upregulated at 8 hai in SVP72017 by cDNA-AFLP analysis. This result was confirmed by qRT-PCR where TDF_032 was highly upregulated at 8 hai in F. gramineaum-inoculated spike tissue in SVP72017, but was not upregulated in Capo. TDF102 was induced in cDNA-AFLPs of Fusarium-treated spikes of the cultivar Capo between 24 and 72 hai. qRT-PCR showed an upregulation of TDF_102 between 8 and 96 hai in Capo. In SVP72017 TDF102 was not induced. TDF_076 was predicted to be upregulated in Capo by cDNA-AFLPs. qRT-PCR showed that TDF_076 was slightly induced in F. graminearum-treated tissue of Capo and SVP72017 between 8 and 72 hai.
Genetic mapping of candidate genes
The genetic map consisted of 46 linkage groups and comprised in total a length of approx. 1,000 cM. Except for three linkage groups, all other groups could be assigned to specific genome regions. All chromosomes were represented by at least one linkage group. In total, 178 AFLP and 32 SSR markers were used for the construction of the genetic map. Genetic markers for TDF_032, TDF_076 and TDF_102, which were designed on the basis of differentially expressed sequences as described above, were polymorphic between the parents of the Sngh3559//Capo/SVP72017 and the History/Rubens (Holzapfel et al. 2008) populations. They were integrated in the genetic maps. Markers for TDF_TDF_076 (NPR1-like sequence) mapped to the long arm of chromosome 2A in both populations and on chromosome 2D in Sngh3559//Capo/SVP72017 as History and Rubens are not polymorphic for the gene on chromosome 2D (Fig. 1). TDF_102 (putative cytochrome P450) mapped to the short arm of chromosome 2B and TDF_032 (unknown function) to chromosome 5A in both populations.
Sequence analysis of TDF_076
The primer pair TDF_076_3 was generated according to the sequences of TDF_076 and TA100375_4565 (mRNA NPR1-like protein; E value 5.1 e−49) and used for amplification of genomic DNA of SVP72017, Capo and Sngh3559. The resulting PCR products were cloned and six colonies per genotype were sequenced. In SVP72017 and Sngh3559 two different sequences differing by 13 SNPs and 3 INDELs (SVP7217) or 22 SNPs and two INDELs (Sngh3559), respectively, were obtained. In Capo, all clones contained identical sequences. An alignment of all five different sequences is shown in Online Resource 4. Markers for SNP1, SNP11, SNP12 and SNP13 as well as for the insertion/deletion polymorphisms INDEL1, INDEL2 and INDEL3 were developed. They were used for the genotyping of the DHs of the populations Sngh3559//Capo/SVP72017 and RILs of History/Rubens and of nulli-tetrasomic lines of Chinese Spring. Genetic mapping of these markers showed that TDF_076_INDEL1, TDF_076_INDEL2, TDF_076_SNP1 and TDF_076_SNP12 (T = allele 2) mapped to the long arm of chromosome 2A while TDF_076_INDEL3, TDF_076_SNP13 and TDF_076_SNP12 (A = allele 2) mapped on the long arm of chromosome 2D (Fig. 1). These results indicate that there are at least two homoeologous genes in hexaploid wheat and that different alleles of both genes do exist. Whether Capo has two identical genes on chromosome 2A and 2D or only one copy could not be determined. Genotyping of nulli-tetrasomic lines of Chinese Spring with markers distinguishing between the two homoeologous genes (TDF_076_SNP11, TDF_076_SNP13) confirm the existence of near identical genes on homoeologous chromosomes. Sequence analysis of the homoeologous NPR1-like gene fragments showed that each sequence contains two open reading frames (ORFs) (marked in Online Resource 4). These are most probably two exons. Alignment of the translated exons showed high sequence consensus with the translated sequence of AM050559 (NPR1-like protein barley) (Fig. 3). Sixteen SNPs in the DNA sequence are predicted to lead to amino acid exchanges in the protein sequence. They are highlighted in the alignment in Online Resource 4.
Effect of TDF_076, TDF_032 and TDF_102 in a diverse collection of European winter wheat cultivars
The collection of 178 European winter wheat cultivars was analyzed for population structure using STRUCTURE. The analysis showed that two subpopulations (German and British gene pools) exist in this population (Online Resource 6). All cultivars were genotyped with PCR markers for TDF_032 (unknown function), TDF_076 (NPR1-like genes) and TDF_102 (putative cytochrome P450). Allele frequencies of all markers for differentially expressed TDFs were higher that 10 %. Marker patterns for the different alleles of both homoeologous NPR1-like genes are summarized in Table 1.
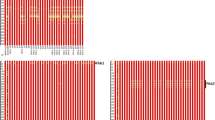
Based on a linear mixed model, markers for both NPR1-like genes showed significant associations (P < 0.05) with FHB severity. P values after 1,000 permutations were 0.036 for the gene on chromosome 2A and 0.00001 for the gene on chromosome 2D. The linear mixed model used for calculation of the association between NPR1-like genes and FHB severity took into account the population structure of the two subpopulations. Cultivars with haplotype 2 of TDF_076_2A (Table 1) showed significantly less FHB symptoms than those with haplotype 1, while cultivars with haplotype 1 of TDF_076_2D were significantly more susceptible to FHB than those with haplotype 2. The moderately resistant breeding line SVP72017 had haplotype 2 both on chromosome 2A and 2D and the moderately resistant Capo had haplotype 1 of TDF_076_2A and haplotype 2 of TDF_076_2D. The susceptible breeding line Sngh3559 had haplotype 1 of the genes on both chromosomes. Boxplot distributions showing FHB severity after spray inoculation with F. culmorum of cultivars with different haplotypes of TDF_076_2A and TDF_076_2D are shown in Fig. 2. The difference in the mean value of infected spikelets between the groups with different haplotypes of TDF_076_2D is 14.8 and 6.2 % for different haplotypes of TDF_076_2A. Eighty-seven percent of the genotypes with haplotype 1 of TDF_076_2D show an above-average percentage of infected spikelets in the mean over 12 environments. In plants with haplotype 2 of both homoeologous genes, there were 17.2 % fewer infected spikelets than in plants with haplotype 1 of both genes. Genotypes with a combination of haplotype 1 of TDF_076_2A and haplotype 2 of TDF_076_2A had 14.2 % fewer infected spikelets than genotypes with the negative allele 1 and 3 % more infected spikelets than genotypes with the positive allele 2 of both homoeologous genes. None of the 178 winter wheat accessions tested showed a combination of haplotype 2 of TDF_076_2A and haplotype 1 of TDF_076_2D.
Boxplot distributions of 178 winter wheat cultivars after spray inoculation with F. culmorum—differentiated by the two marker alleles of TDF_076_2A (a) and TDF_076_2D (b). c The effects of combinations of the different alleles of both homoeologous genes. TDF_076 is a fragment of a NPR1-like gene identified in the expression analysis described above. Data are based on means averaged across 12 environments. Boxes represent the 25th and 75th ‰, respectively, vertical lines the 5th and 95th ‰, respectively. The horizontal line within the boxes refers to the median and + to the mean. Outliers are indicated by dots
Alleles of TDF_076_2A and TDF_076_2D were not equally distributed between the two subpopulations. In the German gene pool 80.2 % of the genotypes had haplotype 1 and 19.8 % had haplotype 2 of TDF_076_2A. Only 2 % of the wheat accessions in the British gene pool showed haplotype 2 of this gene. 90 % of the genotypes in the German gene pool had allele 2 of and 10 % had allele 1 of TDF_076_2D. In contrast, the distribution of allele 1 (46 %) and allele 2 (54 %) of TDF_076_2D in the British gene pool was nearly equal.
The PCR markers for TDF_032 (chromosome 5A) and TDF_102 (chromosome 2B) produced two and three alleles, respectively. Neither TDF_032 nor TDF_102 had a significant effect on FHB severity in the set of winter wheat cultivars.
Discussion
Samples for cDNA-AFLP analysis were collected at seven dates starting immediately after the treatment with macroconidia of F. graminearum or water, respectively, until 4 days after the inoculation. Samples included tissue of the treated florets and the adjacent parts of the rachis. In order to identify crucial defense genes expressed during various stages of the infection process, the sampling dates were chosen to cover the most important initial steps of fungal development in the wheat florets. Kang and Buchenauer (1999, 2000) showed that the macroconidia germinate about 6 h after the inoculation (hai). Subsequently, the fungal hyphae grow on the surface of the floret cells until they start to penetrate the cell wall about 36 hai. At 48 hai, the mycotoxin DON can be detected and the fungus starts to colonize the host tissue. In susceptible wheat genotypes, the fungus enters the rachis after about 3 days and then starts spreading throughout the whole spike.
The average number of 85 cDNA-AFLP bands per primer combination and fragment sizes of 80–800 bp was comparable with other cDNA-AFLP studies in wheat (Steiner et al. 2009; Wang et al. 2009; 2010). 45 of the 2,500 cDNA-AFLP fragments analyzed showed a clear pattern of altered expression in F. graminearum-inoculated compared to control samples, accounting for 1.8 % of all displayed fragments. This proportion of differentially expressed fragments is in the range of what can be expected in wheat after inoculation with a fungal pathogen. Chapman et al. (2009) reported 0.72 % of differentially expressed genes in a cDNA-AFLP experiment of wheat inoculated with Oculimacula acuformis while Wang et al. (2009) observed a percentage of 4.2 % after inoculation with Puccinia striiformis.
In the present study, we carried out expression analysis in order to identify genes that are regulated in tissues of the two resistant winter wheat genotypes in response to Fusarium attack and that can be used for a candidate gene approach. Three fragments contained sequence polymorphisms between SVP72017 and Capo. Somers et al. (2003) predicted an average SNP frequency of one SNP every 500 bp in wheat ESTs. Reasons for the rather low number of polymorphic fragments in our experiment might be the short sequence length of cDNA-AFLP fragments (~265 bp on average) as well as the uneven distribution of SNPs within the coding regions of the wheat genome (Somers et al. 2003).
TDF_032 has no sequence similarity to genes with known function. It was upregulated only in SVP72017 8 h after the inoculation with F. graminearum and might therefore be involved in an early response specific to SVP72017. A PCR marker was developed on the basis of an insertion–deletion polymorphism in the intron region of the gene. The fragment was mapped on chromosome 5A in the populations Sngh3559//Capo/SVP72017 and History/Rubens 10 cM distal to the microsatellite marker Xgwm617. The marker for TDF_032 did not coincide with any QTL for FHB resistance in either population.
For TDF_102 (BLAST result: CD808864, L. chinensis, putative cytochrome P450, E value 3 e−50), which was upregulated in Capo 24–72 h after inoculation with F. graminearum, a dominant STS marker was developed. Cytochrome P450 genes form the biggest gene family in plants and are involved in detoxification as well as in secondary metabolism (Werck-Reichhart and Feyereisen 2000). Members of this gene family were repeatedly found to be upregulated in wheat in response to F. graminearum inoculation (Bernardo et al. 2007; Hill-Ambroz et al. 2006; Kong et al. 2007; Steiner et al. 2009). TDF_102 mapped on the short arm of chromosome 2B in both mapping populations. In the population History/Rubens it co-locates with a QTL for FHB resistance on chromosome 2BS with a relative additive effect of 11 % (Holzapfel et al. 2008). However, neither markers for TDF_032 nor those for TDF_102 had a significant effect on FHB severity in the set of winter wheat cultivars in single environments as well as across all environments.
Sequence analysis of TDF_076 revealed that there are actually two copies of this gene in the moderately resistant line SVP72017 and the susceptible line Sngh3559. Only one copy, or two identical copies, were apparent in the moderately resistant line Capo. These copies differ in 32 SNPs and five INDELs. Sixteen of the SNPs within the two predicted exons lead to the exchange of amino acids in the protein sequence and might have an impact on the function of the protein. As all INDELs were within intron sequences, no deletions in the protein sequence or shifting of the ORFs leading to non-functional proteins can be expected. Markers developed from the polymorphisms mapped on chromosomes 2AL and 2DL. FHB resistance QTL in the same genomic region on chromosome 2AL have been detected in the wheat cultivars Arina, Renan and Stoa (Liu et al. 2009) while no QTL for FHB resistance have been described in a position as distal on chromosome 2DL. Genotyping of nulli-tetrasomic lines of Chinese Spring with markers distinguishing between the two homoeologous genes (TDF_076_SNP11, TDF_076_SNP13) confirm the existence of near identical genes on homoeologous chromosomes.
Allelic diversity in both homoeologous genes had statistically significant effects on FHB resistance in a collection of 178 winter wheat genotypes over twelve environments. Genotypes with haplotype 2 (like the resistant breeding line SVP72017) on either chromosome 2AL (TDF_076_2A) or 2DL (TDF_076_2D) show less FHB symptoms after artificial inoculation with F. culmorum than those with haplotype 1 on the respective chromosome. Allelic variation in TDF_076_2D, with a difference of 14.8 % in the mean value of the groups having haplotype 1 and haplotype 2, has a greater effect on the mean FHB severity over 12 environments than in TDF_076_2A, where the difference is 6.2 % (see Fig. 2). Eighty-seven percent of the genotypes with haplotype 1 of TDF_076_2D show above average FHB severity in the mean over 12 environments. Markers for this haplotype should therefore be particularly useful for a negative marker-assisted selection. Most genotypes tested (n = 115) showed a combination of haplotype 1 of TDF_076_2A and haplotype 2 of TDF_076_2A. On average, they had 14.2 % fewer infected spikelets than genotypes with the negative allele 2 of TDF_076_2D and 3 % more infected spikelets than genotypes with the positive allele 1 of both homoeologous genes. This result confirms the greater effect of TDF_076_2D on FHB severity in comparison to TDF_076_2A. Genotypes with a combination of haplotype 2 had 17.2 % less FHB symptoms than genotypes with haplotype 1 of both homoeologous genes. None of the tested genotypes showed a combination of allele 1 of TDF_076_2A and allele 2 of TDF_076_2D. This could be due to the rather low number of genotypes with allele 2 of TDF_076_2A (n = 37) and allele 1 of TDF_076_2D (n = 26). Another reason could be the unequal distribution of the alleles between the subpopulations. In the British gene pool, allele 1 of TDF_076_2D is rather common while allele 2 of TDF_076_2A is extremely rare (2 %). As population structure was taken into account in the linear mixed model used for the association study, the unequal distribution of the alleles between the subpopulations cannot be the reason for the effects of the markers for TDF_076 on FHB resistance. In contrast, P-values improved when a model including population structure was applied in comparison to a model that did not consider population structure (data not shown).
Comparison of TDF_076 with the NH1 gene (described as NPR1-like) of barley (accession no. AM050559) demonstrates that TDF_076 has sequence similarities with parts of exons 2, 3 and 4 of the barley gene while there is only slight sequence consensus between the intron sequences of the genes. Figure 3 shows an alignment of the translated sequences of exon 3 of barley with the translated exon 1 of TDF_076. To date, no functional wheat NPR1 homologue has been identified and no complete sequence of wheat NPR1 has been submitted to public databases. It can only be speculated whether TDF_076 is a fragment of a functional wheat NPR1 homologue. In A. thaliana, NPR1 is a well investigated key regulatory protein in signal transduction leading to systemic acquired resistance mediated by the phytohormone SA (Pieterse and Van Loon 2004). At the same time NPR1 influences JA biosynthesis and signal transduction (Beckers and Spoel 2006).
a Schematic structure of the NH1 gene of barley (NPR1-like) in comparison to the gene fragment TDF_076. All polymorphisms analyzed between the different wheat sequences are marked. b The translated sequence of exon 3 of the barley NH1 gene (AM050559) (=A) was aligned to the translated sequence of TDF_076 (cultivar Capo) (=B). Alignment was carried out with the CLC-Bio DNA workbench
TDF_076 is slightly upregulated in Capo and SVP72107 after inoculation with F. graminearum. RT-PCR and subsequent cloning of the PCR product showed that both copies on chromosome 2AL and 2DL are expressed in F. graminearum-inoculated floret tissue of SVP7217. Ding et al. (2011) report the marked upregulation of an NPR1-like EST in spikes of the resistant spring wheat Wangshuibai at 3 h after inoculation with F. graminearum, while it was upregulated with a peak 12 h after inoculation in the susceptible mutant Meh0106. During the later stages of the infection, NPR1-like genes were reported to be either downregulated (Bernardo et al. 2007) or to not show any differential expression after inoculation with Fusarium (Qi et al. 2012, Gottwald et al. 2012; Xiao et al. 2013). These results lead to the conclusion that NPR1-like genes are involved specifically in the early defense response of resistant wheat genotypes against F. graminearum (Ding et al. 2011). The over-expression of AtNPR1 from A. thaliana in the moderately susceptible cultivar Bobwhite led to a strong increase in resistance against FHB, proving the positive effect of NPR1-mediated signal transduction in wheat (Makandar et al. 2006, 2012). These results were recently confirmed by Gao et al. (2013) by overexpressing AtNPR1 in a different genetic background. Transgenic lines exhibited enhanced resistance to FHB, but not Fusarium seedling blight (FSB), after inoculation with F. asiaticum.
In this study, markers for candidate genes were developed that showed an altered expression pattern in floret tissue of the two resistant winter wheat genotypes Capo and SVP72017 after inoculation with F. graminearum. These markers were mapped and their impact on FHB resistance was analyzed by an association study with a population of 178 winter wheat genotypes. Two homoeologous NPR1-like genes could be mapped on chromosomes 2AL and 2DL, respectively. Markers for both NPR1-like genes had significant effects on FHB resistance in an assortment of 178 European winter wheat cultivars over 12 environments. SNP as well as simple PCR markers for both genes were developed and will be further tested in marker-assisted breeding programs.
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp P, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Beckers GJM, Spoel SH (2006) Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol 8:1–10
Bernardo A, Bai G, Guo P, Xiao K, Guenzi AC, Ayoubi P (2007) Fusarium graminearum-induced changes in gene expression between Fusarium head blight-resistant and susceptible wheat cultivars. Funct Integr Genomics 7:69–77
Buerstmayr H, Steiner B, Lemmens M, Ruckenbauer P (2000) Resistance to Fusarium head blight in winter wheat: heritability and trait associations. Crop Sci 40:1012–1018
Buerstmayr H, Ban T, Anderson JA (2009) QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed 128:1–26
Chapman NH, Burt C, Nicholson P (2009) The identification of candidate genes associated with Pch2 eyespot resistance in wheat using cDNA-AFLP. Theor Appl Genet 118:1045–1057
Diethelm M, Rhiel M, Wagner C, Mikolajewski S, Groth J, Hartl L, Friedt W, Schweizer G (2012) Gene expression analysis of four WIR1-like genes in floret tissues of European winter wheat after challenge with G. zeae. Euphytica 186:103–114
Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z (2011) Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS One 6:e19008
Gao CS, Kou X-J, Li HP, Zhang JB, Saad ASI, Liao XC (2013) Inverse effects of Arabidopsis NPR1 gene on fusarium seedling blight and fusarium head blight in transgenic wheat. Plant Pathol 62:383–392
Glazebrook J (2005) Contrasting mechanisms of defence against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Gottwald S, Samans B, Lück S, Fried W (2012) Jasmonate and ethylene dependent defence gene expression and suppression of fungal virulence factors: two essential mechanisms of Fusarium head blight resistance in wheat? BMC Genomics 13:369
Gunnaiah R, Kushalappa AC, Duggavathi R, Fox S, Somers DJ (2012) Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against F. graminearum. PLoS One 7:e40695
Hartl L, Mohler V, Zeller FJ, Hsam SLK, Schweizer G (1999) Identification of AFLP markers closely linked to the powdery mildew resistance genes Pm1c and Pm4a in common wheat. Genome 42:322–329
Hill-Ambroz K, Webb CA, Matthews AR, Li W, Gill BS, Fellers JP (2006) Expression analysis and physical mapping of a cDNA library of Fusarium head blight infected wheat spikes. Plant Genome 1:15–26
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Holzapfel J, Voss HH, Miedaner T, Korzun V, Häberle J, Schweizer G, Mohler V, Zimmermann G, Hartl L (2008) Inheritance of resistance to Fusarium head blight in three European winter wheat populations. Theor Appl Genet 117:1119–1128
Kang Z, Buchenauer H (1999) Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum. Physiol Mol Plant Pathol 55:275–288
Kang Z, Buchenauer H (2000) Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum. Mycol Res 104:1083–1093
Kong L, Ohm HW, Anderson JM (2007) Expression analysis of defense-related genes in wheat in response to infection by Fusarium graminearum. Genome 50:1038–1048
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lemmens M, Scholz U, Berthiller F, Dall’Asta C, Koutnik A, Schuhmacher R, Adam G, Buerstmayr H, Mesterházy A, Krska R, Ruckenbauer P (2005) The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol Plant Microbe Interact 18:1318–1324
Liu S, Hall MD, Griffey CA, McKendry AL (2009) Meta-analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci 49:1955–1968
Makandar R, Essig JS, Schapaugh MA, Trick HN, Shah J (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of A. thaliana NPR1. Mol Plant Microbe Interact 19:123–129
Makandar R, Nalam VJ, Lee H, Trick HN, Shah J (2012) Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact 25:431–439
Miedaner T, Gang G, Geiger HH (1996) Quantitative-genetic basis of aggressiveness of 42 isolates of Fusarium culmorum for winter rye head blight. Plant Dis 80:500–504
Mohler V, Schmolke M, Paladey E, Seling S, Hartl L (2012) Association analysis of puorindoline-D1 and of puorindoline b-2 loci with 13 quality traits in European winter wheat (Triticum aestivum L.). J Cereal Sci 56:623–628
Pieterse CMJ, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7:456–464
Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci 14:21–29
Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G (2003) Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem 27:47905–47914
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qi PF, Johnston A, Balcerzak M, Rocheleau H, Harris LJ, Long WYM, Zheng YL, Quellet T (2012) Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biol 116:413–426
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN: 3-900051-07-0. http://www.R-project.org/
Roeder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ, pp 365–386
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphism in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8019
Schmolke M, Zimmermann G, Buerstmayr H, Schweizer G, Miedaner T, Korzun V, Ebmeyer E, Hartl L (2005) Molecular mapping of Fusarium head blight resistance in the winter wheat population Dream/Lynx. Theor Appl Genet 111:747–756
Schweiger W, Boddu J, Shin S, Poppenberger B, Berthiller F, Lemmens M, Muehlbauer GJ, Adam G (2010) Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol Plant Microbe Interact 23:977–986
Schweiger W, Steiner B, Ametz C, Siegwart G, Wiesenberger G, Berthiller F, Lemmens M, Jia H, Adam G, Muehlbauer GJ, Kreil DP, Buerstmayr H (2013) Transcriptomic characterization of two major Fusarium resistance quantitative loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Mol Plant Pathol 14(8):772–785. doi:10.1111/mpp.12048
Sears ER (1966) Nulli-tetrasomic combinations in hexaploid wheat. In: Riley R, Lewis KR (eds) Chromosome manipulation and plant genetics, a suppl. to heredity. Oliver and Boyd, Edinburgh, pp 29–45
Somers DJ, Kirkpatrick R, Moniwa M, Walsh A (2003) Mining single-nucleotide polymorphisms from hexaploid wheat ESTs. Genome 46:431–437
Steiner B, Kurz H, Lemmens M, Buerstmayr H (2009) Differential gene expression of related wheat lines with contrasting levels of head blight resistance effect Fusarium graminearum inoculation. Theor Appl Genet 118:753–764
Van Ooijen JW, Voorrips RE (2001) Joinmap® 3.0. Software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Walter S, Nicholson P, Doohan F (2010) Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol 185:54–66
Wang X, Tang C, Li Y, Wang C, Liu B, Qu Z, Zhao J, Han Q, Huang L, Chen X, Kang Z (2009) cDNA-AFLP analysis reveals differential gene expression in compatible interaction of wheat challenged with Puccinia striiformis f. sp. tritici. BMC Genomics 10:289
Wang X, Liu W, Chen X, Tang C, Dong Y, Ma J, Huang X, Wei G, Han Q, Huang L, Kang Z (2010) Differential gene expression in compatible interaction between wheat and stripe rust fungus revealed by cDNA-AFLP and comparison to compatible interaction. BMC Plant Biol 10:9
Werck-Reichhart D, Feyereisen R (2000) Cytochromes P450: a success story. Genome Biol 1:30031–30039
Xiao J, Jin X, Jia X, Wang H, Cao A, Weiping Z, Pei H, Xue Z, He L, Chen Q, Wang X (2013) Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai. BMC Genomics 14:197
Zhuang Y, Gala A, Yen Y (2013) Identification of functional genetic components of major Fusarium head blight resistance quantitative trait loci in wheat cultivar Sumai3. Mol Plant Microbe Interact 26:442–450
Acknowledgments
We would like to thank S. Wüllner, S. Schmidt, J. Brandlmeier, A. Bund and the staff of the departments of Genome Analysis and Wheat Breeding of the Bavarian State Research Centre for excellent technical assistance. We thank V. Mohler, B. Büttner and T. Albrecht from the plant breeding department of the Bavarian State Research Centre for Agriculture for helpful discussions and critical reading of the manuscript. We are grateful to M. Lemmens and B. Steiner from the IFA Tulln, Austria for kindly providing the F. graminearum inoculum and for sharing their protocols and experience in inoculation and sampling technique. We acknowledge J. Schondelmaier from the Saaten-Union Biotec GmbH for performing the SSR marker analysis of the Sngh3559//Capo/SVP72017 mapping population. We also thank Saatzucht Breun GmbH & Co. KG (Herzogenaurach, Germany), Deutsche Saatveredelung AG (Leutewitz, Germany), Prof. H. Buerstmeyr from the Department for Agrobiotechnology Tulln (Austria) and W. von Borries-Eckendorf GmbH & Co. KG (Leopoldshöhe, Germany) for performing the field trials. Results presented here were obtained from a project funded by the Deutsche Forschungsgemeinschaft DFG (Schw 1201/1-2) and of the GABI-Canada (CGAT) cooperative project: Reducing Fusarium toxins in wheat through genomics (Subprojects A and C no. 0313711A and C), which was funded by the German Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diethelm, M., Schmolke, M., Groth, J. et al. Association of allelic variation in two NPR1-like genes with Fusarium head blight resistance in wheat. Mol Breeding 34, 31–43 (2014). https://doi.org/10.1007/s11032-013-0010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-013-0010-2