Abstract
Brown rust, caused by Puccinia melanocephala, has had devastating effects on sugarcane (Saccharum spp.) breeding programs and commercial production. The discovery of Bru1, a major gene conferring resistance to brown rust, represented a substantial breakthrough. Markers for Bru1 are the first available for sugarcane molecular breeding. The contribution of Bru1 towards brown rust resistance in the Canal Point (CP) sugarcane breeding program was determined as a means of directing future breeding strategies. Bru1 was detected in 285 of 1,072 (27 %) clones used for crossing; this germplasm represents the genetic base for cultivar development in Florida. The frequency of Bru1 was greatest in CP clones (42 %) and lowest among Louisiana clones (6 %). Bru1 was not detected in clones with year assignments before 1953. However, Bru1 frequency increased from 15 % (assignments 1975–1985) to 47 % in the current decade. The increase coincided with the introduction of brown rust to Florida. Bru1 was detected in 155 (32 %) of 485 parental clones tested for brown rust susceptibility at two field locations. Of clones classed resistant to brown rust, 154 (59 %) contained Bru1, yet none of 100 susceptible clones contained the gene. Bru1 was detected in 667 (44 %) clones in the second clonal stage of selection, 87 % of which were free of brown rust symptoms. Bru1 is the predominant source of resistance in the Florida sugarcane genetic base. Efforts to identify and integrate new brown rust resistance genes must be pursued to minimize risks associated with a future breakdown in major gene resistance provided by Bru1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown rust of sugarcane, caused by the biotrophic fungal pathogen Puccinia melanocephala, is an economically important pathogen of sugarcane worldwide. Brown rust symptoms occur mainly on the leaves and in severe infections can cause leaf necrosis and premature death of even young leaves (Raid and Comstock 2006). Severe infections have caused reductions in both stalk mass and stalk numbers, thereby reducing cane tonnage (Comstock et al. 1992; Hoy and Hollier 2009). The disease was first reported in the Western Hemisphere in the Dominican Republic in 1978 and subsequently proliferated rapidly through the Americas, becoming prevalent in almost all sugarcane industries in the Americas within 1 year (Purdy et al. 1983). The source of introduction was subsequently identified as the sub-Saharan region of West Africa, facilitated by conducive wind currents (Purdy et al. 1985).
Although control of brown rust of sugarcane is possible using fungicide applications and the effects of disease epidemics can be limited through cultural practices (Hoy and Hollier 2009), control is most efficiently achieved through host resistance. Since the mid-1980s, almost all sugarcane breeding programs in the Western Hemisphere have used brown rust resistance (determined by the absence of visible symptoms) as a major breeding and selection criterion.
Sugarcane breeding programs release brown rust-resistant cultivars; however, breakdown of resistance is one of the major contributing factors to the withdrawal from use of otherwise productive sugarcane cultivars in many sugarcane industries. In addition to losses due to brown rust of sugarcane cultivars in commercial production, the loss due to brown rust of otherwise potentially extremely successful genotypes in sugarcane breeding programs has been substantial. During a 5-year period, 15 genotypes on the verge of being released from the CP program which were apparently rust resistant were not released due to rust infections in the year prior to release (unpublished data). The CP program responded to increase the emphasis on only advancing rust-free clones from the early stages.
The breakdown of host resistance to rusts can be rapid, as the virulent forms of the pathogen proliferate due to the development of pathogenic races. A genetic basis for races is lacking for P. melanocephala; however, apparent pathogenic races have been reported in India (Srinivasan and Muthaiyan 1965) and in the USA (Comstock 1987; Dean and Purdy 1984; Shine et al. 2005). Notable examples include the major sugarcane cultivar LCP 85-384, which was expanded up to an acreage of 91 % of the Louisiana sugarcane industry in 2004 (Legendre and Gravois 2005). Following the breakdown of resistance in this cultivar, the acreage of this cultivar was reduced rapidly (Hoy and Hollier 2009). Knowledge of the underlying genetic basis of resistance is needed in order to develop sugarcane cultivars with durable resistance to brown rust and reduce the number of genotypes lost from the selection stage of sugarcane breeding programs due to rust susceptibility.
Traditional breeding approaches for brown rust resistance have involved understanding inheritance of resistance in seedling populations (Hogarth et al. 1993; Ramdoyal et al. 2000) and selection and advancement of genotypes that are free from visual disease symptoms. Molecular genetic approaches towards understanding the genetic basis of brown rust resistance led to the identification of Bru1, a major gene for brown rust resistance (Asnaghi et al. 2004; Daugrois et al. 1996) in the cultivar R570 from the Reunion breeding program. This gene was shown to provide broad-spectrum resistance against isolates from Brazil, Colombia, Reunion Island, Guadeloupe, and Florida (three isolates) (Asnaghi et al. 2001). Targeted mapping of Bru1 confirmed its status as a major gene and identified markers 2.2 and 1.9 cM either side of the gene (Asnaghi et al. 2004). Further exploration of the Bru1 loci in R570 located the gene within a 0.42-cM region of sugarcane CG VII syntenic to an approximately 225-kb region of Sorghum bicolor chromosome 4 and orthologous to an approximately 600-kb region on the short arm of chromosome 2 of rice (Le Cunff et al. 2008). These studies, which consisted of the first map-based cloning approach of any gene attempted in sugarcane, led to the identification of molecular diagnostic markers for the detection of Bru1 (Costet et al. 2012). Bru1 was recently found to be prevalent in 86 % of brown rust-resistant clones in a sample of 380 modern cultivars and breeding materials covering the worldwide diversity (Costet et al. 2012). The opportunity therefore exists to utilize Bru1 in marker-assisted breeding and selection in order to improve brown rust resistance in sugarcane.
The objectives of this study are to determine the contribution of Bru1 towards brown rust resistance in the Canal Point sugarcane breeding program as a means of supporting and directing future strategies for breeding for brown rust resistance in Florida.
Materials and methods
Plant material and field experiments
One source of sugarcane genotypes selected for these studies was the breeding nursery at the USDA-ARS sugarcane breeding station, Canal Point, FL. This collection of germplasm is maintained to support the sugarcane crossing program and represents the genetic base for sugarcane cultivar development in Florida. The clones are from a number of sources, including current and historical domestic USA commercial cultivars, exotic cultivars, and advanced selections, not commercially released but used for crossing. A total of 1,072 genotypes were recovered from the nursery plots and leaf pieces sampled for DNA extraction. The sources of clones represented are given in Table 1 of supplementary material. A representative selection totaling 485 genotypes was chosen from this parental pool for field testing (Table 1 of supplementary material). Clones were chosen on the basis of having previously been used in crosses at the station and/or representing diverse sources. All genotypes were planted in a randomized complete block design with three replications in February 2010 at Canal Point, FL. One stalk of each genotype was placed in a furrowed field plot, approximately 2.6 m in length (one plot per replicate). This “seed cane” was chopped into sections approximately 0.5 m in length and moved into a parallel orientation such that approximately 1.3 m of the plot was covered with seed cane. Identical procedures were used to repeat the planting in October 2010 in a commercial field at the Townsite Farm of the United States Sugar Corporation (USSC), approximately 5 km west of Clewiston, FL. Field plots were maintained according to standard sugarcane production practices employed in the area.
Data were also generated from 1,527 genotypes in the third stage (stage 2) of the six-stage Canal Point sugarcane breeding and selection program (CP program). These genotypes are herein referred to as the CP 10 series. Planting of the CP 10 series in stage 2 occurred in November 2010 by planting eight stalks of each of the CP 10 genotypes in the field at the Sugarcane Field Station, Canal Point, FL in a two-row plot in which each row was 4.6 m long, separated by 1.6 m. The field was surrounded by border-row plots 4.6 m in length of genotypes known to be highly susceptible to brown rust in order to increase the disease pressure in the field. Plots were maintained according to standard field practices used in the area.
Disease ratings
Field plots were monitored regularly for evidence of the onset of brown rust symptoms in order to capture the peak disease epidemic. Disease ratings in the field were conducted by visual inspection of the plots using standardized procedures. Complete plots were visually inspected with attention given to symptoms occurring on fully expanded, mature leaves. A scale of 0–4 was used to assign a severity rating to each plot based on observed symptoms (Table 2 of supplementary material). Symptoms of brown rust of sugarcane can be variable between genotypes, locations, infection stages, and plant ages. When necessary, brown rust symptoms were confirmed by examination under a 10× handheld lens. Visual ratings for the parental field tests were performed in June 2010 at Canal Point and June 2011 at Townsite. For the stage 2 test, visual ratings were performed in June 2011. These periods represented the expected peak epidemic at each location.
Detection of Bru1
Leaf samples were excised from plants in each of the sources listed above. Leaf sampling was standardized by sampling the first, fully expanded leaf. Eight 6-mm-diameter leaf discs were sampled per leaf using a 6-mm Harris punch (Qiagen, USA). DNA was extracted from discs using a BioSprint kit (Qiagen), according to the manufacturer’s instructions. Detection of Bru1 in sugarcane DNA was achieved through polymerase chain reaction (PCR) using the methods and two pairs of primers (9O20-F4 and R12H16) and reaction conditions described in Costet et al. (2012) with the following exceptions: The concentration of MgCl2 for both reactions was adjusted to 1.5 mM, 5 ng of DNA was used, and reaction volumes were 10 μl for primers 9O20-F4 and 20 μl for primers R12H16. Thermocycling consisted of denaturation initially for 5 min and, during cycling for 30 s and extension was performed for 72 s during cycling, and final extension of 8 min. Determination of a positive result using the primer set 9O20-F4 requires resolution of fragments following digestion of the amplified product with the restriction enzyme RsaI. Digestion was performed as described in Costet et al. (2012) with the exception that 2.4 units of RsaI was added to 15 μl amplified product. Products were resolved via electrophoresis through a 2 % agarose gel. A real-time PCR protocol was developed for the detection of the single amplicon resulting from primers R12H16. Reactions consisted of 0.4 μM of each primer, 10 μl of iQ SYBR® Green Supermix (BioRad), and molecular biology-grade water to final reaction volume of 20 μl. Thermocycling was performed on a BioRad Chromo 4 real-time PCR machine and was identical to the conventional PCR protocol with the exception that a melt-curve analysis (0.2 °C, 2 s hold) was performed following thermocycling. The positive control in batch testing of the samples was DNA from the sugarcane cultivar R570, which is the genotype in which Bru1 was originally discovered.
The parental clones were separated by source (USSC, Canal Point, Exotic, Historical, Louisiana, and Texas) and, for the clones from the Florida breeding programs, also according to the decade in which their year assignment occurred (1926–1935, 1936–1945, 1946–1955, 1966–1975, 1976–1985, 1986–1995, 1996–2005, and 2006–2012), thereby allowing the frequency of Bru1 among the parental pool over time to be examined.
Results
Bru1 in parental clones
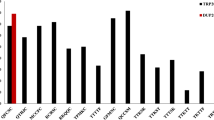
Bru1 was detected in 285 (27 %) of the 1,072 clones tested from the sugarcane parental pool. Fewer clones had Bru1 than did not have Bru1 in all six cohorts of sources (Fig. 1). The proportion of Bru1-positive clones was greatest among clones from the Canal Point program (41 %). The Exotic (33 %) and USSC sources (28 %) had similar proportions of clones with the Bru1 gene. Lower frequencies of Bru1-positive clones were observed among the Historical (10 %), Louisiana (7 %), and Texas (16 %) clones (Fig. 1). A long-term trend for increasing frequency of Bru1 was evident among the Florida clones separated by the decade in which they were named (Fig. 2). Bru1 was not detected in any of the 15 clones tested with years between 1925 and 1945. The earliest clone in which Bru1 was detected was assigned its cultivar year in 1953 (CP 53-0018). This was the only clone of 18 clones tested with years between 1926 and 1955 in which Bru1 was detected. Bru1 was later detected in 2 of 29 (1956 and 1965), 6 of 39 (1966 and 1975), 9 of 59 (1976 and 1985), 19 of 83 (1986 and 1995), 116 of 284 (1996 and 2005), and 35 of 74 (2006 to present) clones (Fig. 2).
Rust reactions of parental material
The mean brown rust severity ratings for the two locations across replicates and genotypes were 0.47 for Canal Point and 0.59 for Townsite. Regression analysis of rust ratings observed at Canal Point and Townsite showed a positive correlation of 0.78 (R 2 = 0.61) (Fig. 3, S1). Comparison of the rust resistance category assigned to each clone at each site based on the mean rust rating observed showed that the majority of genotypes (331, 68 %) were in the same resistance category at each location, 130 (27 %) changed one resistance category between locations, 22 (5 %) changed two categories, and 1 genotype (0.2 %) changed three rating categories (Table 1).
Comparison of the mean brown rust ratings among the 484 parental clones with and without Bru1 separated by source showed that the majority of genotypes with Bru1 showed no symptoms of brown rust (0 rating). The maximum rating observed in Bru1-positive clones was 1 across all genotype sources. For clones without Bru1, a normal distribution of rust ratings was observed in each clone source, the exception being those classified as Historical clones. The largest category of ratings was 1 in each source, again the exception being those within the Historical cohort (Fig. 3).
CP 10 series stage 2
The full range (0–4) of brown rust disease severity ratings were observed in stage 2 at Canal Point in the CP 10 series (Fig. 4). Although none of the test genotypes were replicated, rust was observed on all of the plots of the commercial check that had satisfactory stands of brown rust-susceptible genotype CP 78-1628. The mean brown rust severity rating on CP 78-1628 was 2.0 (max = 2.5, min = 1.0, SEM = 0.1), indicating that the brown rust disease epidemic was spread throughout the test field. Bru1 was detected in 667 (44 %) of the 1,521 genotypes tested. Brown rust was observed on 86 (13 %) of genotypes that tested positive for Bru1. No rust was observed on 591 (87 %) of clones with Bru1, whereas no rust was observed on 119 (14 %) of the 854 genotypes that did not test positive for Bru1. The mean brown rust rating for clones without Bru1 was 1.74, whereas for clones with Bru1 it was 0.18. The maximum brown rust rating for clones which were positive for Bru1 was 3 (2 clones), whereas the maximum brown rust rating for clones which did not test positive for Bru1 was 4 (11 clones).
Discussion
Brown rust has had a devastating impact on sugarcane production in Florida, where otherwise outstanding commercial cultivars have been withdrawn from production due to the development of virulence in the pathogen population and the breakdown of resistance. The disease has also had a major impact on sugarcane breeding programs both in Florida and worldwide, with numerous genotypes lost during the selection stages due to brown rust susceptibility. This has led to a reduced number of genotypes for selection of high-yielding cultivars. The first objective of this study is to determine the prevalence of Bru1, a major gene for resistance to brown rust of sugarcane, within the genetic base of a sugarcane breeding program. This was achieved by determining the frequency of Bru1 among the various sources of genetic material used for crossing and also among clones within the second clonal selection stage of the program. This information is important in order to determine the status, needs, and future directions for breeding for resistance against brown rust in sugarcane.
Understanding the frequency of Bru1 related to the brown rust susceptibility status provides important information for utilizing Bru1 to control brown rust of sugarcane caused by P. melanocephala. Bru1 was highly prevalent among brown rust-resistant genotypes in the second clonal stage of selection in the breeding program; the majority of clones on which no visual symptoms of brown rust were observed contained Bru1. However, a number of clones with Bru1 were moderately susceptible to brown rust in stage 2. This could suggest the initiation of selection of virulence towards Bru1. However, subsequent field observations made on these plots resulted in some cases where only orange rust was identified (suggesting that the original record of brown rust was a misidentification of orange rust). Where symptoms of brown rust were still observed, their severity was much reduced compared with the original rating (data not shown). These results emphasize both the difficulty in differentiating between the symptoms of orange rust and brown rust in the field and also temporal changes in brown rust symptom severity that occur in the field during the course of the growing season. The two pathogens can be positively identified using PCR testing (Glynn et al. 2010). However, because PCR testing is expensive to apply on a large scale, performing disease ratings during the optimum epidemic conditions for disease is a more practical solution.
An important need for Bru1 testing and the advancement of genotypes with Bru1 is in growing seasons in which natural brown rust epidemics are not severe. In this case, absence of disease would not necessarily be an indicator of brown rust resistance, since exposure to the pathogen was not adequate and therefore Bru1 presence could provide a useful additional criterion for selection. This is also a problem in the seedling selection stage when the plants are transplanted to the field after the peak brown rust development.
A noteworthy finding is the increase in frequency of Bru1 over time. Bru1 was detected in genotypes that predated the introduction of brown rust to the Western Hemisphere, confirming its presence as a native trait among sugarcane germplasm. However, a large increase in Bru1 frequency occurred following the discovery of brown rust in Florida in the late 1970 s (Purdy et al. 1985). This indicates that the selection by Canal Point breeders for rust-free genotypes was also a selection predominantly of Bru1. The prevalence of Bru1 among the sugarcane parental pool available for crossing when brown rust was discovered in Florida in the 1970 s was important as it provided a durable source of resistance, which in the selection of rust-free genotypes was increased in frequency among genotypes selected and released for Florida sugarcane growers. The lower frequency of Bru1 among both Louisiana and Texas germplasm suggests that another source(s) of resistance is (are) prevalent in cultivars released for these industries or that Bru1 can be transmitted by another haplotype than the one bearing the PCR diagnostic markers used in this study. A possible explanation for this reduced frequency is that the Bru1 loci are in linkage disequilibrium with a trait which is selected against among genotypes developed for these environments.
The rust reactions observed at the two experimental sites were in generally good correlation. Although notable exceptions were evident, the majority of clones were within one resistance rating category at each location. The differences observed could be due to different soil types, epidemics between growing seasons, or different pathogen populations. Although some differences in pathogenicity between isolates have been reported in P. melanocephala (Shine et al. 2005), a formal genetic race population structure has not been reported for this pathogen. Differences in the severity of brown rust symptoms, associated with excess nutrient availability, have also been reported (Johnson et al. 2007; McFarlane et al. 2008) and could in part explain the differences between locations in this study. The soil at the Canal Point field location is a Torry muck, noted for a high organic matter content, whereas the soil type at the Townsite field location is a mineral soil with extremely low organic matter content.
Overuse of resistance genes accompanied by a narrow genetic base can lead to a situation known as a “boom-and-bust” cycle (Priestly 1978). The “boom” occurs when resistant cultivars are expanded in acreage as growers take advantage of resistant cultivars and the resulting improved yields. Likewise, breeders use the resistant clones as parents for the development of new cultivars, thereby narrowing the genetic base. The expanded acreage of the cultivars and hence the resistance gene(s) provides the pathogen population an ever-increasing opportunity to place more extreme selection for virulent races among the pathogen population. Eventually resistance breaks down, and since there are no pathogen races with which to compete, the virulent races rapidly proliferate among the large acreages, and the “bust” occurs. Boom-and-bust cycles have been most comprehensively documented in cereals for rust (Kolmer 1996) and powdery mildews (Wolfe and McDermott 1994). This boom-and-bust cycle likely explains the situation in the Louisiana sugarcane industry where the sugarcane cultivar LCP 85-384 at one point occupied close to 90 % of the acreage. Although this cultivar was resistant to brown rust for a number of years, resistance was finally overcome by the pathogen population, and substantial yield losses resulted due to brown rust. Bru1 was not detected in LCP 85-384. The clone was, however, used extensively by Louisiana breeders. This implies that the resistance genes provided by LCP 85-384, which are now ineffective against the brown rust strains prevalent in Louisiana, are likely highly represented among the selection stages of the Louisiana sugarcane breeding programs.
Disease resistance in crops resulting from a major, single gene is known as vertical resistance, whereas resistance controlled by multiple genes is known as horizontal resistance (Van der Plank 1963). The most devastating effects of boom-and-bust cycles in crop production occur when vertical resistance breaks down. The data presented in this study show a continual increase in the frequency of Bru1 in the parental material used for cultivar development in Florida and also that Bru1 was the predominant source of brown rust resistance in a selection stage within the CP program. Although the genotypes tested within the selection program were from only one series, since similar parents are used in crosses each year, the data are likely representative of the recent and near-term future series in the selection stages of the program.
Data from the 2010 Florida sugarcane variety census show that over 80 % of the Florida sugarcane acreage for which cultivars are identified is planted to a cultivar with Bru1 (Rice et al. 2011). This has been the case for the past several years; the current three leading cultivars (CP 89-2143, CP 88-1762, and CP 80-1743) all tested positive for Bru1 and have accounted for 60–70 % of the Florida acreage since 2004. Of the commercially produced sugarcane cultivars that make up the remaining 20 % of the Florida acreage, none are resistant to brown rust. Taken together, these data indicate that brown rust resistance in sugarcane is in a “boom” phase, and as such extreme vulnerability exists due to the potential of a breakdown in resistance to Bru1 due to P. melanocephala race evolution. Efforts should be made to diversify the on-farm sources of resistance by planting cultivars that are resistant to brown rust with their resistance due to either Bru1 or some other mechanism. Following such a plan would be an attempt to preserve the useful life of Bru1 for controlling brown rust. Breeders should also increase efforts to identify more sources of brown rust resistance so that sugarcane growers can move more from the apparent vertical resistance (notwithstanding additional sources of resistance among clones which also have Bru1) they are now using that depends on Bru1 towards a more horizontal-based resistance. Knowledge of the frequency of Bru1 among the sugarcane breeding genetic base will allow breeders to focus on those clones exhibiting resistance which do not have Bru1. A coordinated effort integrating the testing of Bru1 in parental material and progeny and the identification and integration of additional genetic sources of brown rust resistance is required to broaden the brown rust resistance genetic base. The identification and functional characterization of the Bru1 gene(s) and additional genes responsible for brown rust resistance combined with the development of additional markers would help considerably to combine those different sources through breeding.
Abbreviations
- CP:
-
Canal Point
- CP program:
-
Canal Point sugarcane cultivar breeding and selection program
- USSC:
-
United States Sugar Corporation
References
Asnaghi C, D’hont A, Glaszmann J, Rott P (2001) Resistance of sugarcane cultivar R 570 to Puccinia melanocephala isolates from different geographic locations. Plant Dis 85:282–286
Asnaghi C, Roques D, Ruffel S, Kaye C, Hoarau JY, Telismart H, Girard J, Raboin L, Risterucci A, Grivet L (2004) Targeted mapping of a sugarcane rust resistance gene (Bru1) using bulked segregant analysis and AFLP markers. Theor Appl Genet 108:759–764
Comstock JC (1987) Rust. Varietal differences in urediospore production. Hawaii. Sugar Plant Assoc Exp Stn Annu Rep 1986, p 31
Comstock JC, Shine J Jr, Raid RN (1992) Effect of rust on sugarcane growth and biomass. Plant Dis 76:175–177
Costet L, Le Cunff L, Royaert S, Raboin L-M, Hervouet C, Toubi L, Telismart H, Garsmeur O, Rousselle Y, Pauquet J, Nibouche S, Glaszmann J-C, Hoarau J-Y, D’Hont A (2012) Haplotype structure around Bru1 reveals a narrow genetic basis for brown rust resistance in modern sugarcane cultivars. Theor Appl Genet 125:825–836
Daugrois J, Grivet L, Roques D, Hoarau J, Lombard H, Glaszmann J, D’Hont A (1996) A putative major gene for rust resistance linked with a RFLP marker in sugarcane cultivar ‘R570’. Theor Appl Genet 92:1059–1064
Dean J, Purdy L (1984) Races of the sugar cane rust fungus, Puccinia melanocephala, found in Florida. Sugar Cane 1:15–16
Glynn NC, Dixon LJ, Castlebury LA, Szabo LJ, Comstock JC (2010) PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P. melanocephala and detection of a SNP associated with geographic distribution in P. kuehnii. Plant Path 59:703–711
Hogarth D, Ryan C, Taylor P (1993) Quantitative inheritance of rust resistance in sugarcane. Field Crops Res 34:187–193
Hoy J, Hollier C (2009) Effect of brown rust on yield of sugarcane in Louisiana. Plant Dis 93:1171–1174
Johnson RM, Grisham MP, Richard EP Jr (2007) Relationship between sugarcane rust severity and soil properties in Louisiana. Phytopathology 97:748–755
Kolmer J (1996) Genetics of resistance to wheat leaf rust 1. Annual Rev Phytopath 34:435–455
Le Cunff L, Garsmeur O, Raboin LM, Pauquet J, Telismart H, Selvi A, Grivet L, Philippe R, Begum D, Deu M (2008) Diploid/polyploid syntenic shuttle mapping and haplotype-specific chromosome walking toward a rust resistance gene (Bru1) in highly polyploid sugarcane (2n–12x ~ 115). Genetics 180:649–660
Legendre BL, Gravois KA (2005) The 2004 Louisiana sugarcane variety survey. Sugar Bull 89:15–21
McFarlane SA, Meyer JH, Cadet P, Rutherford RS (2008) Investigation into the effect of nutrition on brown rust development in sugarcane Investigation into the effect of nutrition on brown rust development in sugarcane. Proc S Afr Sug Technol Ass 81:388–392
Priestly R (1978) Detection of increased virulence in populations of wheat yellow rust. In: Scott PR, Bainbridge A (eds) Plant disease epidemiology. Oxford, Blackwell Scientific, pp 63–70
Purdy L, Liu LJ, Dean J (1983) Sugarcane rust, a newly important disease. Plant Dis 67:1292–1296
Purdy L, Krupa S, Dean J (1985) Introduction of sugarcane rust into the Americas and its spread to Florida. Plant Dis 69:689–693
Raid RN, Comstock JC (2006) Sugarcane rust disease (SS-AGR-207). In: Gilbert RA (ed) Florida sugarcane handbook, 1st Edition 1991, revised 2006 [Online]. Agronomy Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Available: http://edis.ifas.ufl.edu. 27 Aug 2007
Ramdoyal K, Sullivan S, Chong LLS, Badaloo G, Saumtally S, Domaingue R (2000) The genetics of rust resistance in sugar cane seedling populations. Theor Appl Genet 100:557–563
Rice RW, Baucum L, Glaz B (2011) Sugarcane variety census: Florida 2010. Sugar J 74:13–19
Shine JM Jr, Comstock JC, Dean JL (2005) Comparison of five isolates of sugarcane rust and differential reaction on six sugarcane clones. Proc Int Soc Sugar Cane Technol 25:638–647
Srinivasan KV, Muthaiyan MC (1965) A note on physiological races in Puccinia erianthi Padu & Khan affecting sugarcane varieties. Proc Int Soc Sugar Cane Technol 12:1126–1128
Van der Plank JE (1963) Plant diseases. Epidemics and control. Academic Press, New York
Wolfe M, McDermott J (1994) Population genetics of plant pathogen interactions: the example of the Erysiphe graminis–Hordeum vulgare pathosystem. Ann Rev Phytopath 32:89–113
Acknowledgments
The authors acknowledge technical support from Kay McCorkle and Moaiad Kaanan, USDA-ARS, Sugarcane Field Station and Paula Gadea, United States Sugar Corporation, Clewiston, FL.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Glynn, N.C., Laborde, C., Davidson, R.W. et al. Utilization of a major brown rust resistance gene in sugarcane breeding. Mol Breeding 31, 323–331 (2013). https://doi.org/10.1007/s11032-012-9792-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-012-9792-x