Abstract
A reported allele-specific dCAPS (derived cleaved amplified polymorphic sequence) marker, within the gene for the anthocyanin regulatory transcription factor MdMYB1, associated with apple fruit skin color, was tested in 17 elite breeding parents and two apple seedling progenies. In both progenies, the red skin color phenotype was usually associated with the MdMYB1-1 allele. This dCAPS marker provided approximately 80% predictability in a ‘Golden Delicious’ × ‘Arlet’ and a ‘Honeycrisp’ × ‘Cripps Pink’ progeny. Other potential genetic co-regulators may explain the less-than-perfect association. The specific dCAPS bands associated with red skin for the latter population were not the same as those identified in the former population or those reported in previous studies, indicating that skin color genotyping based on this marker will require prior association between specific marker alleles and color phenotypes for any given cross. The current form of this marker could be a useful tool for apple marker-assisted breeding, particularly in breeding programs in which ‘Golden Delicious’ is a parent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple (Malus × domestica) fruit skin or peel color is an important contributor to nutrition, consumer preference, and market value (King and Cliff 2002). Secondary metabolites, often anthocyanins, are responsible for the red color of plant flowers, leaves, and fruits and also associated with health attributes (Boyer and Liu 2004; Dixon et al. 2005). Anthocyanins are biosynthesized through a multi-step phenylpropanoid pathway, and the genetic regulation and biochemical characterization of these metabolites have been investigated in many plant species, including petunia (Petunia hybrida), maize (Zea mays), snapdragon (Antirrhinum majus), and Arabidopsis (Arabidopsis thaliana; Winkel-Shirley 2001). The coordinated regulation of genes encoding enzymes of the anthocyanin pathway suggests that this pathway is controlled by transcription factors. Among these, MYB, bHLH, and the WD40 repeat protein are implicated in the regulation of anthocyanin biosynthesis and development of red, purple, and blue colors in plant tissues (Koes et al. 2005; Allan et al. 2008).
Until recently, little was known about the genetic regulation of the anthocyanin biosynthesis pathway in fruit tissues. Based on the elucidated regulatory roles of a MYB transcription factor in other plant species, several groups have identified homologous MYB transcription factors from apple peel and cortex (Takos et al. 2006; Ban et al. 2007; Espley et al. 2007). Takos et al. (2006) clones and sequenced at least three MdMYB1 alleles in apple cultivars. One of these alleles, MdMYB1-1, was inherited by two red skin color siblings, ‘Cripps Pink’ and ‘Cripps Red’, but it was not present in their non-red skin parent ‘Golden Delicious’, leading these researchers to suggest that MdMYB1-1 (allele “1”) was inherited from the red-skinned parent ‘Lady Williams’, thereby conditioning the red skin color (known as blush or overcolor) via induction of anthocyanin synthesis and that it was dominant over non-red (non-blush) alleles “2” and “3” from ‘Golden Delicious’ (Takos et al. 2006). A derived cleaved amplified polymorphic sequence (dCAPS) DNA marker based on a single nucleotide polymorphism (SNP) at the 5’ flanking region of MdMYB1 gene was developed and associated with skin color in most of the 16 Australia-grown cultivars tested and completely in a progeny population of 136 individuals directly related to ‘Golden Delicious’ and ‘Lady Williams’ (Takos et al. 2006).
In the study reported here, the utility of this dCAPS marker across 17 diverse cultivars used as breeding parents and in two breeding progenies grown in Washington, USA, and its potential application for marker-assisted breeding were evaluated.
Materials and methods
Skin color phenotyping
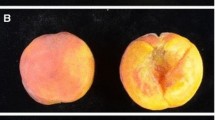
The 17 elite breeding parental cultivars and two breeding progenies included in this study were from the Tree Fruit Research and Extension Center experimental orchard, Washington State University, Wenatchee, WA, USA. The ‘Golden Delicious’ × ‘Arlet’ progeny consisted of 97 fruiting seedlings, and the ‘Honeycrisp’ × ‘Cripps Pink’ progeny included 166 fruiting seedlings, all evaluated in the 2009 season. Fruit from the outer layer of the canopy and sunny side of the trees were chosen for phenotyping fruit skin color, avoiding those inside the tree canopy or from the shady side. Fruit skin color was visually recorded on a subjective 5-point scale (0 representing non-red skin and 1–4 representing different levels of red coloration; Fig. 1) at the late ripening stage (late September to early October), with a single score given for each tree.
Skin color phenotypic categories used for two apple progenies. Fruit skin color was visually recorded on a subjective 5-point scale: 0 Non-red skin (upper panel), 1 < 50% but low-intensity coloration, 2 50–75% but low-intensity coloration, 3 75–90% and intense coloration, 4 ≥ 90% and intense coloration (lower panel)
DNA isolation and MdMYB1 marker analysis
Leaf tissue from individual breeding parent cultivars and seedlings of the two progenies were collected, frozen in liquid nitrogen, and stored at −80°C. Genomic DNA was isolated according to Cullings (1992). PCR primers for the dCAPS PCR assay were from Takos et al. (2006). PCR reactions were performed in a final mix of 25 μl containing 50 ng of template DNA, 0.25 mM of each dNTP, 0.2 μM of each primer (forward and reverse), 2.5 μl 10× PCR buffer, and 1 U of Taq polymerase (New England Biolabs, Ipswich, MA). The thermal cycling was performed in a Techne TC-512 cycler (GMI, Ramsey, MN) and consisted of one cycle of 94°C for 2 min, 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. The presence of the MdMYB1-1 allele was based on the detection of a 263-bp fragment, after BstEII (New England Biolabs) restriction of amplicons and separation on 2% agarose gels. Products that did not contain this fragment, such as those derived from the MdMYB1-2 and MdMYB1-3 alleles, were an undigested 29-bp fragment, following Takos et al. (2006).
Results
Consistency of the MdMYB1 genotype with the skin color phenotype of the breeding parents
All but one of the 17 elite breeding parent cultivars tested showed a consistent genotype–phenotype relationship, i.e., red skin color was associated with the presence of the MdMYB1-1 allele and non-red skin color was associated with the MdMYB1-2 or -3 alleles only (Table 1). Non-red-skinned ‘Golden Delicious’ and its three offspring cultivars ‘Goldrush’, ‘Orin’, and ‘Ginger Gold’ exhibited only the 291-bp fragment (MdMYB1 alleles “2” and/or “3”). Two different banding patterns for red-skinned cultivars were observed. One pattern consisted of the 263-bp DNA fragment of the MdMYB1-1 allele and the 291-bp fragment representing the other MdMYB1 alleles (presumably heterozygous for the “1” and “2”/“3” alleles of this gene) observed for ‘McIntosh’, ‘Jazz’, ‘Cripps Pink’, ‘Sunrise’, ‘Arlet’, and ‘Fortune’. The second pattern was the 263-bp DNA fragment only (presumably homozygous for the “1” allele) observed for ‘Fuji’, ‘Delicious’, ‘Honeycrisp’, ‘Sansa’, ‘Stars, and ‘Creston’ (Fig. 2). The only cultivar with an inconsistent association between genotype and phenotype was ‘Granny Smith’, which appeared to have a heterozygous genotype but a non-red skin phenotype, as also reported by Takos et al. (2006).
Banding patterns from the MdMYB1 derived cleaved amplified polymorphic sequence (dCAPS) assay of apple breeding parents. Lanes: S DNA size marker, 1 ‘Golden Delicious’, 2 ‘Goldrush’, 3 ‘Orin’, 4 ‘Ginger Gold’, 5 ‘Granny Smith’, 6 ‘Fuji’, 7 ‘Delicious’, 8 ‘McIntosh’, 9 ‘Jazz’, 10 ‘Honeycrisp’, 11 ‘Cripps Pink’ (‘Pink Lady’), 12 ‘Sansa’, 13 ‘Sunrise’, 14 ‘Arlet’, 15 ‘Stars’, 16 ‘Fortune’, 17 ‘Creston’. Arrows: top 291-bp fragment, bottom 263-bp fragment
Consistency of the MdMYB1 genotype with the skin color phenotype for a ‘Golden Delicious’ × ‘Arlet’ progeny
Red skin color was observed for 71 seedlings of this progeny, while 26 had no detectable red color (Table 2). Overall, 86% (83/97 fruiting trees) of the fruiting progeny showed the expected association between MdMYB1-1 and red skin color, with the presence of only the undigested 291-bp fragment usually (88% of seedlings) being associated with non-red skin and the presence of both bands usually (85% of seedlings) associated with red skin (Fig. 3a; Table 2). All 11 seedlings with red skin but without the MdMYB1-1 allele had fruit with a skin color score of 1, i.e., a faint blush in less than 50% of the total fruit surface (Fig. 4a). Only six seedlings with a skin color score of 1 exhibited the MdMYB1-1 allele, indicating a closer association of color score 1 with the MdMYB1-2 or -3 alleles than with the -1 allele in this progeny (Fig. 4a). All seedlings with a skin color score of ≥2 had the MdMYB1-1 allele (Fig. 4a). In this progeny, the average skin color score for seedlings with and without the MdMYB1-1 allele was 2.6 and 0.3, respectively.
Distribution of MdMYB1 genotypes and skin color phenotypes in two apple progenies. Figures immediately above each column are the number of seedlings observed in that genotype–phenotype category. Association consistency is the proportion of seedlings for each skin color score that had the expected genotype (MdMYB1-1; dash skin color of 0, plus sign skin color of 1–4). a ‘Golden Delicious’ × ‘Arlet’, b ‘Honeycrisp’ × ‘Cripps Pink’
Consistency of the MdMYB1 genotype with the skin color phenotype for a ‘Honeycrisp’ × ‘Cripps Pink’ progeny
The overall consistency between phenotype and genotype in the ‘Honeycrisp’ × ‘Cripps Pink’ progeny was 80% (Table 3). However, the specific bands associated with skin color were not the same as those identified in the ‘Golden Delicious’ × ‘Arlet’ progeny. In ‘Honeycrisp’ × ‘Cripps Pink’ progeny, the presence of both bands (i.e., inheritance of the -3 allele from ‘Cripps Pink’) was usually (79% of seedlings) associated with non-red skin, while the presence of the 263-bp band only (i.e., inheritance of the -1 allele from ‘Cripps Pink’) was usually (80% of seedlings) associated with red skin (Fig. 3b). While most of the red-skinned seedlings without the MdMYB1-1 allele from ‘Cripps Pink’ had a skin color score of 1, some had scores of 2 and 3, and thus the association of the ‘Cripps Pink’ MdMYB1-1 allele with red skin color was not complete, with the exception for the skin color score of 4 (Fig. 4b). In the ‘Honeycrisp’ × ‘Cripps Pink’ progeny, the average skin color score for seedlings with and without the MdMYB1-1 allele was 1.8 and 0.9, respectively, which was much less of a difference than that between the two scores in ‘Golden Delicious’ × ‘Arlet’ progeny. Nevertheless, because of the lower degree of blush in the former progeny, skin color scores of 1 were usually (71%) associated with the MdMYB1-1 allele (Fig. 4b).
Discussion
A robust fruit skin color marker would be useful for a marker-assisted apple breeding program. Parental combinations could be selected that provide a predictable and preferably large proportion of seedlings with target skin color types. In segregating populations, seedlings with desired skin colors could be selected at an early stage prior to resource-draining operations, such as field planting and maintenance. A previously reported allele-specific dCAPS marker was tested here for its utility in different genetic backgrounds. Our results suggest a good but less-than-perfect association between this dCAPS marker and skin color that may be dependent on genetic background. We found that the 263-bp band for this marker was not always associated with the MdMYB1-1 allele for red skin color, which is problematic for a priori predictions in some progenies.
Of the 17 apple cultivars tested in this study, all but one (‘Granny Smith’) had red skin color when the 263-bp band was present, indicating that these cultivars carried the MdMYB1-1 allele. Five of these cultivars were also tested by Takos et al. (2006), who obtained the same result and made the same interpretation. The 263-bp band of ‘Granny Smith’ appears to represent an allele associated with green skin that has the appearance—but not the function—of the MdMYB1-1 allele; we have labeled this allele as the MdMYB1-4 allele. We assume that the six cultivars with both the 291- and 263-bp bands (‘McIntosh’, ‘Jazz’, ‘Cripps Pink’, ‘Sunrise’, ‘Arlet’, and ‘Fortune’) are red-skinned because their 263-bp band is the MdMYB1-1 allele. For those six cultivars for which only the 263-bp was observed (‘Fuji’, ‘Delicious’, ‘Honeycrisp’, ‘Sansa’, ‘Stars’, and ‘Creston’), we assume that they carry at least one MdMYB1-1 allele, although some may also carry the MdMYB1-4 allele. Segregation in the two progenies examined in this study (discussed below) confirm the homozygous non-red genotype of ‘Golden Delicious’ (MdMYB1-2/3 according to Takos et al. 2006), the heterozygous genotype of ‘Arlet’ (MdMYB1-1 and either -2 or -3), and the heterozygous genotype of ‘Cripps Pink’ (MdMYB1-1/3 according to Takos et al. 2006). However, the genotype of ‘Honeycrisp’ may be MdMYB1-1/4.
Determination of the correct functional genotype of parent cultivars is important for a priori predictions of seedling outcomes. Based on our results, the MdMYB1 genotype according to the dCAPS marker appears to be accurate for non-red-skinned cultivars with only the 291-bp band (the MdMYB1-2 and/or -3 alleles) and for red-skinned cultivars with both the 291- and 263-bp bands (with the 263-bp band representing the MdMYB1-1 allele). Predictions of skin color category proportions in progenies should be accurate for crosses among such cultivars. However, the 263-bp band in non-red-skinned cultivar (Granny Smith) with both bands is probably not the MdMYB1-1 allele, and red-skinned cultivars with only the 263-bp band are not necessarily homozygous for the MdMYB1-1 allele. For these ambiguous genotypes, progeny testing involving seedling phenotyping is required before banding patterns of the dCAPS marker can be assigned to red or non-red skin color. Alternatively, a new marker that can unambiguously distinguish among functional alleles of MdMYB1 could be developed.
Genotype was a correct predictor of the presence or absence of red skin color for 79–88% of seedlings in both progenies examined, but only after accounting for genotypes of parent cultivars in the second progeny involving ‘Honeycrisp’. The presence of the MdMYB1-1 allele was usually associated with red skin color in the progeny of ‘Golden Delicious’ × ‘Arlet’, although approximately one-third of seedlings without MdMYB1-1 had a small degree of red skin (Fig. 4a). The red-skinned parent in this cross, ‘Arlet’, has no known pedigree link with ‘Lady Williams’, the red-skinned cultivar with MdMYB1-1, from which the dCAPS marker was originally developed. Therefore, there appears to be a general association of MdMYB1-1 with the red skin phenotype in apple cultivars. While the banding pattern for the MdMYB1 dCAPS marker suggest that ‘Honeycrisp’ was homozygous for MdMYB1-1 (Fig. 2), observed genotypes and phenotypes in the ‘Honeycrisp’ × ‘Cripps Pink’ progeny indicate that only the MdMYB1-1 allele of ‘Cripps Pink’ was clearly segregating in association with red skin color. Most seedlings with both bands had non-red skinned fruit and because these had inherited MdMYB1-3 from ‘Cripps Pink’ (the 291-bp band), the 263-bp band in such seedlings was deduced to represent an allele other than MdMYB1-1 (perhaps MdMYB1-4). As seedlings with only the 263-bp band usually bore red-skinned fruit, MdMYB1-1 inherited from ‘Cripps Pink’ was identified as the primary contributor to their red skin phenotype. Therefore, at least one of the 263-bp bands of ‘Honeycrisp’ is not MdMYB1-1. While it is tempting to consider that neither 263-bp allele of ‘Honeycrisp’ represents MdMYB1-1 given that the segregation of ‘Cripps Pink’ alleles in the cross predicts the phenotype of most seedlings, ‘Honeycrisp’ is a red-skinned cultivar (given a phenotypic score of 3). If this cultivar has the MdMYB1-1/4 genotype, half the seedlings with both bands from the cross with ‘Cripps Pink’ would be expected to be MdMYB1-1/3 and thus red-skinned. However, only 23 of 70 seedlings with both bands were red-skinned (Fig. 4b), and if about a third of seedlings without MdMYB1-1 could have a skin color score of 1, as observed for the ‘Golden Delicious’ × ‘Arlet’ progeny, then only the six seedlings with a score of 2 and two seedlings with a score of 3 (Fig. 4), or 11% (8/70), appear to have inherited an allele associated with red skin from ‘Honeycrisp’. If ‘Honeycrisp’ does not carry MdMYB1-1, another locus may account for its red-skinned phenotype and that of the eight aforementioned red-skinned seedlings.
Takos et al. (2006) reported a perfect (100%) association between the MdMYB1-1 allele and red skin in a progeny from an unnamed red skin sibling of ‘Cripps Pink’ crossed with ‘Golden Delicious’, which was therefore germplasm immediately related to that in which the dCAPS marker was developed. We widened the genetic backgrounds under investigation by using ‘Arlet’ in the first progeny and ‘Honeycrisp’ in the second, both of which are unrelated to ‘Lady Williams’. The incomplete genotype–phenotype association observed in our study may be due to this wider genetic background. The phenotyping method may also contribute to discrepancies between our results and those of Takos et al. (2006). As the fruit color intensity or the degree of surface pigmentation varies quantitatively within a progeny, in practice it is difficult to clearly separate high and low coloration. The color intensity of apple fruit skin is also influenced by many other factors, such as amount of light reaching fruit (affected by pruning and other horticultural practices, and genetic aspects of tree architecture), nutritional status, and fruit maturity. Lower night-time temperatures were also more likely to have occurred in the Wenatchee orchard than in the location in the Australian study, a factor known to induce red skin color formation (Ban et al. 2007). For purposes of calculating genotype–phenotype association in this study, even faint a blush was included as “red color skin”. Takos et al. (2006) grouped individuals with very poor pigmentation together with those that had none; such differences in stringency of phenotyping most likely contributed to the less-than-perfect association between marker and phenotype in our data. In our observations, a large proportion of those seedlings demonstrating an inconsistent genotype–phenotype association had a very low level of coloration. Effort is underway to establish standardized phenotyping methods to improve the determination of allele functionality.
The intensity of red skin coloration varied widely among seedlings with the MdMYB1-1 allele from the same parentage (0–4 in both progenies examined), indicating the existence of other genetic factors in addition to MdMYB1 that co-regulate the biosynthesis of anthocyanin, as suggested by Allan et al. (2008). Two families of transcription factors, the bHLH and MYB proteins, are conserved in the regulation of the anthocyanin and condensed tannins (CTs) biosynthesis pathways in all species analyzed to date (Koes et al. 2005). The bHLH (also called MYC) proteins may have overlapping regulatory targets (Hartmann et al. 2005; Zimmermann et al. 2004). In fact, a recent study has shown that an apple gene encoding a bHLH transcription factor enhanced the transcriptional activity of the MdMYB10 gene (Espley et al. 2009). Alternatively, an inhibitor protein could mediate the induction of MYB gene expression by sunlight (Hernandez et al. 2004). More studies are needed to gain a detailed understanding of the regulation of the anthocyanin pathway and interacting partners of MYB transcription factors and to investigate genetic variation among cultivars for the underlying genes, for a more accurate prediction of apple fruit skin color in any genetic background. Such data will then enable a more accurate prediction of fruit skin color in any genetic background.
For those seedlings that did not have the expected genotype–phenotype association, several scenarios could exist. As discussed above, it is likely that there are other genetic factors that co-regulate anthocyanin biosynthesis and coloration. Alternatively, MdMYB1-1 may be responsible only for intense and large areas of color, while other MdMYB1 alleles may produce low-intensity and limited areas of blush, such as those seedlings with low-intensity color and no MdMYB1-1 allele. However, seedlings with no detectable red color of the fruit skin which were confirmed to carry MdMYB1-1, including three seedlings in the ‘Golden Delicious’ × ‘Arlet’ progeny and 11 in ‘Cripps Pink’ × ‘Honeycrisp’ (Table 2; Fig. 4), are more difficult to explain. It is possible that an inhibitor protein was the cause of failure of these seedlings to develop red color even while carrying MdMYB1-1. The dCAPS marker should also amplify the gene MdMYB10 (Espley et al. 2007) that controls red versus white flesh color. The ‘Royal Gala’ MdMYB10 sequence representing cultivated apple for this gene is expected to have the same dCAPS allele as MdMYB1-1, and the M. niedzwetzkyana sequence is expected to generate banding patterns equivalent to the “2” and “3” alleles of MdMYB1. These two genes may actually be allelic, and further alleles at this locus which are not distinguishable by the dCAPS marker may underlie the current imperfect association between the marker and skin color phenotype.
Our results suggest that more work is needed to perfect the genotypic prediction of red skin color. However, the dCAPS marker of MdMYB1 appears to have immediate utility for many progenies in typical apple breeding programs carried out around the world. High-throughput application will require conversion of the dCAPS polymorphism to a simple PCR test or SNP assay without the need for restriction digestion. Genotypic monitoring of MdMYB1-1 inheritance in a background of MdMYB1-2 and -3 alleles provides a predictability of about 80%, which may be an acceptable level of reliability in many breeding programs, particularly those aimed at culling seedlings with a predicted very low or no skin blush.
References
Allan AC, Hellens R, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13:99–102
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Boyer J, Liu R (2004) Apple phytochemicals and their health benefits. Nutr J 3:5
Cullings KW (1992) Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Mol Ecol 1:233–240
Dixon RA, Xie D, Sharma SB (2005) Proanthocyanidins—a final frontier in flavonoid research? New Phytol 165:9–28
Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC (2007) Red colouration in apple fruit is due to the activity of MYB transcription factor, MdMYB10. Plant J 49:414–427
Espley RV, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, Allana AC (2009) Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21:168–183
Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B (2005) Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light responsive and tissue specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol 57:155–171
Hernandez JM, Heine GF, Irani NG, Feller A, Kim M-G, Matulnik T, Chandler VL, Grotewold E (2004) Different mechanisms participate in the R-dependent activity of the R2R3 MYB transcription factor C1. J Biol Chem 279:48205–48213
King MC, Cliff MA (2002) Development of a model for prediction of consumer liking from visual attributes of new and established apple cultivars. J Am Pomol Soc 56:223–229
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker Amanda R (2006) Light-induced expression of a myb gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142:1216–1232
Winkel-Shirley B (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40:22–34
Acknowledgments
The authors are grateful to Fred Bliss, James McFerson, and Tianbao Yang for their helpful comments. We wish to thank Mallela Magana and Bonnie Konishi for their contribution in tissue collection, DNA extraction, and PCR amplification. This study was supported by Washington Tree Fruit Research Commission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Evans, K. & Peace, C. Utility testing of an apple skin color MdMYB1 marker in two progenies. Mol Breeding 27, 525–532 (2011). https://doi.org/10.1007/s11032-010-9449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-010-9449-6