Abstract
Cultivated tobacco produces secondary alkaloids involved in the formation of nitrosamines with health concerns. The recent identification of target genes in nicotine and nornicotine biosynthetic pathways now allows biotechnological approaches for their control. We demonstrate here that mutation breeding can be used as an alternative to genetically modified (GM) plants for generating nornicotine-free tobacco. Ten alleles of the NtabCYP82E4 gene (nicotine N-demethylase) were identified by screening 1,311 M2 families of tobacco ethylmethane sulphonate (EMS) mutants. Alkaloid analysis indicated that the nornicotine contents of homozygous M2 plants carrying nonsense or missense alleles of NtabCYP82E4 were very low or near-null. Backcrossing with tobacco elite varieties yielded BC1 plants phenotypically undistinguishable from parental lines. This major objective of tobacco breeders in the last few decades could be reached in a period of less than 1.5 years, including the creation of highly mutagenised tobacco mutant collections and the detection of mutated alleles using a simple and versatile detection technology (capillary electrophoresis-single strand conformation polymorphism, CE-SSCP) accessible to most breeding companies and crop species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent advances in the knowledge of metabolic pathways controlling the accumulation of secondary alkaloids in tobacco and the development of new genomic tools for detecting allelic variability now offer new opportunities to reduce the risks associated with tobacco. In the last few decades, there has been a growing concern about the possible detrimental effect on human health of specific compounds found in tobacco smoke. Lists of potentially harmful tobacco agents have been established and are now being used by governmental agencies (e.g. Health Canada) as a reference for the registration of new cigarette brands (Hoffmann et al. 1997). Among these are the secondary alkaloids derivatives, which are supposed to be implicated in the increased risks for various pathologies. As a consequence, their reduction in tobacco has become a major goal for tobacco companies. Efforts are mostly focussed on nicotine and on its conversion product, nornicotine. Nornicotine is highly undesirable because the tobacco nitrosamine N-nitrosonornicotine (NNN), which is formed from nornicotine during leaf-curing and storage (Hashimoto and Yamada 1994; Bush et al. 2001; Xu et al. 2007), has been implicated in the increased risks for certain diseases, such as oesophageal and oral tumours (Hecht 2003; Hoffmann et al. 1997). Besides, nornicotine could cause detrimental health effects by triggering aberrant protein glycation associated with metabolic diseases (Dickerson and Janda 2002). Though nornicotine levels are lower in amphidiploid-cultivated tobacco N. tabacum than in the parental species N. sylvestris and N. tomentosiformis (Gavilano et al. 2007), nicotine-to-nornicotine conversion still occurs in tobacco plants termed converter, which are mostly found in burley cultivars. Conventional tobacco breeding succeeded in creating low converter elite varieties but experience shows that an appreciable proportion of the plants (up to 20%) may reverse to converter phenotype in field conditions (Gavilano et al. 2006). The recent breakthrough demonstration that nicotine-to-nornicotine conversion in tobacco was catalysed by a Cytochrome P450 with a function of nicotine N-demethylase, referred to as NtabCYP82E4 (Gavilano et al. 2007; Siminszky et al. 2005), together with the recent availability of new genomic tools, paved the way for the successful reduction of nornicotine in cultivated tobacco by biotechnological approaches, such as RNA interference (Gavilano et al. 2006) or the mutation technology strategy described herein.
Mutation breeding has been used for decades to modify existing traits or to create new valuable traits within the cultivated varieties. The improvement of cultivated varieties largely depends on the use of new genetic variability in crop species with narrow genetic basis. Improving alleles can originate from wild germplasm and related species, as successfully experimented in various species (Tanksley and McCouch 1997; Fernie et al. 2006). Alternatively, genetic variability can be created by physical or chemical mutagens, such as X-ray, fast neutron or ethylmethane sulphonate (EMS). In the past, mutation breeding has produced a set of improved commercial varieties in a wide range of species, including tobacco (see the FAO database at http://www-infocris.iaea.org/MVD/). However, until recently, this strategy was difficult to implement by breeders because it relied on the phenotypic screening of large populations and, therefore, on lengthy breeding programs.
To become attractive in most crop species, mutation breeding must adjust to rapidly evolving markets that require the frequent releases of new varieties. Several conditions are necessary to reach this objective. First, mutant collections should display high mutation frequencies in order to reduce mutant screening effort, yet without compromising plant development, fecundity and quality. Ideally, the mutated lines entering the breeding process should show no phenotypic changes, except for the improved trait. Second, in addition to highly mutagenised plant collections, sensitive methods allowing the rapid, systematic and automated identification of mutations in targeted sequences must be available. In addition to the endonuclease-based TILLING (Targeting Induced Local Lesions IN Genomes) technology (Colbert et al. 2001; Henikoff and Comai 2003), which has been successfully used for mutation breeding in wheat (Slade et al. 2005), a number of other mutation-detection technologies are potentially amenable to mutation breeding (McCallum et al. 2000; Ellis et al. 2000; Doi et al. 2004; Bjørheim and Ekstrøm 2005; Davies et al. 2006; Yeung et al. 2005; Comai and Henikoff 2006).
To fully exploit the considerable genetic variability created by mutagenesis in crop plants for breeding, we propose a simple and highly reproducible mutation detection strategy based on the high-throughput capillary electrophoresis-single strand conformation (CE-SSCP) analysis of highly mutagenised mutant collections. Easily accessible to breeding companies and applicable to most crop species, this approach has enabled us to obtain in a very short period of time (<1.5 years) the long sought-after non-genetically modified nornicotine-free tobacco line, by targeting the tobacco nicotine N-demethylase gene.
Materials and methods
Plant material
The seeds of a burley-type tobacco accession, BB16NN (Delon et al. 1999) (Institut du Tabac de Bergerac, Accession No. 1139), were used for creating the Nicotiana tabacum mutant libraries. The main characteristics of BB16NN are its susceptibility to Potato Virus Y (PVY) and its potential to convert nicotine to nornicotine. BB16NN originates from BB16 (Institut du Tabac de Bergerac, Accession No. 450), a non-converter tobacco, among which, converter plants were found and maintained by self-pollination.
Ethylmethane sulphonate mutagenesis
Pilot experiments with ethylmethane sulphonate (EMS) concentrations ranging from 0% to 1.5% were first performed on batches of 100 seeds. EMS concentrations displaying 25% to 40% reduction in germination rate at 7 days after treatment were chosen. Two tobacco EMS mutant libraries, termed L1 and L2, were constructed by soaking tobacco seeds (6,000 seeds per library) overnight (16 h) in 0.8% EMS (L1) or 0.6% EMS (L2) solutions, followed by 12 washings of 30 min in water under shaking. In addition, L1 library seeds were pre-germinated for 2 days before EMS treatment. The mutagenised M1 seeds were grown to M1 plantlets in a greenhouse and transferred to the field to give M2 generation by self-pollination. M2 seeds were collected from each M1 plant and stored until use. Leaf material was collected from eight M2 seeds, sown in a single pot in greenhouse and pooled (two 8-mm-diameter discs for each plant, i.e. ∼100 mg fresh weight per family) to constitute the pooled M2 family. DNA was extracted using QIAGEN DNeasy 96 Plant Kit according to the manufacturer’s instructions.
Polymerase chain reaction amplification of target genes
Before screening target genes, the DNA quality of the samples was checked by amplifying the tobacco nitrate reductase gene (Genbank accession X14059) (data not shown). Primers were designed to specifically amplify the exons of tobacco N. tomentosiformis homoeologs of arginine decarboxylase ADC1 (GenBank AF127240) and of nicotine N-demethylase NtabCYP82E4 (GenBank DQ131886) genes. Forward and reverse ADC1 primers were ADC1-F (ACGCTACTGTTTCCCC) and ADC1-R (ACGACCCGAATTTGACAA). Forward and reverse NtabCYP82E4 primers were CYP82E4-F (ATTTTTGGCCAATTACGGAC) and CYP82E4-R (AAACACCGTTGCCTTAATGA). Amplicons were further sequenced to confirm the identity of the genes. Polymerase chain reaction (PCR) amplifications before CE-SSCP analysis were carried out with FAM and VIC dye-labelled primer pairs in a 20-μl volume containing 1 μl of DNA, 10× AmpliTaq buffer (Applied Biosystems, Foster City, CA), 1 μl of dNTPs (Applied Biosystems, 2.5 mM each), 50 ng of each primer and 0.05 U of AmpliTaq Polymerase (Applied Biosystems). PCR was conducted using a thermal cycler (GeneAmp® PCR System 9600, Applied Biosystems) as follows: 35 cycles of 94°C for 30 s, 58°C (ADC1) or 62°C (NtabCYP82E4) for 45 s, 72°C for 1 min, followed by 7 min at 72°C for the final extension.
Capillary electrophoresis
Fluorescent-labelled PCR products were diluted 1/20 in water before CE-SSCP analysis. Prior to loading on ABI Prism 3100Avant (Applied Biosystems), 1 μl of the diluted sample was added to 10 μl of formamide (Applied Biosystems) and 0.1 μl of Genescan-500 LIZ Size Standard (Applied Biosystems). A denaturation step of 94°C for 3 min followed by cooling on ice was used for single-strand conformation analysis.
Running conditions on the ABI Prism 3100Avant were as follows: 36-cm capillary array, run temperature of 22°C, sample injection of 1 kV for 15 s and separation of 15 kV for 40 min. The same conditions were later used for CE-SSCP analysis with 36-cm 16-capillary array on upgraded ABI3130xl (data not shown). The non-denaturing separation medium was Genescan polymer (Applied Biosystems) 5%, glycerol (Sigma-Aldrich) 10% in 1× Buffer (10×) with EDTA (Applied Biosystems). The running buffer was glycerol 10% in 1× Buffer(10×) with EDTA. The results were analysed with Genescan Analysis v1.1 software.
Cloning and sequencing
The pooled DNA from each M2 mutant family identified was used for PCR amplification with gene-specific primers (ADC1-F/ADC1-R and CYP82E4-F/CYP82E4-R). PCR products were cloned into the pGEM-T vector system (Promega, Madison, WI) and transformed into E. coli according to the manufacturer’s instructions. A total of 10 clones was sequenced for each family, using BigDye Terminator Sequencing Kit v1.1 (Applied Biosystems) and ABI Prism® 310 (Applied Biosystems).
Nornicotine analysis
A rapid test adapted from Shi et al. (2003) was used to assess the ability of a plant to convert nicotine into nornicotine. Fresh leaves from greenhouse plants were soaked in 0.8% NaHCO3 for 5 s, incubated for 4 days at 37°C and 85% relative humidity and dried at 60°C over 2 days. Nornicotine content was determined either by colourimetric test or by high-performance liquid chromatography (HPLC).
The colourimetric test was used for the rapid determination of nornicotine content of greenhouse-grown plants. Dried leaf material (250 mg) ground to a fine powder (500 μm) was extracted with 25 ml of acetic acid 5% under constant shaking over 30 min. The mixture was filtered on a paper filter (Durieux) and 2.5 ml of flow-through were transferred into a tube containing 1 ml of NaOH 10N and 0.5 ml of CHCl3. The tubes were shaken vigorously, degassed and incubated at room temperature until phase separation. Forty microlitres of CHCl3 solution were deposited on a Whatman 3MM paper, wetted in isatin solution (isatin 2 gl−1; tannic acid 1 gl−1; acetic acid 40 ml; acetone qsp 1l) (Stephens and Weybrew 1959), incubated for 5 min at room temperature and kept at 100°C for 5 min. The colour signal was quantified using the Color-Pen (Dr. Lange GmbH, Berlin, Germany) according to the L*a*b Color System and compared with references to estimate the nornicotine concentration (expressed as % of leaf dry weight).
Alkaloid analysis
HPLC analysis was used to determine the alkaloid concentrations of field-grown plants. Leaf material was air-cured until the midribs turned dry. A final step of drying was performed at 30°C for 2 days. Lamina samples were ground to a 500-μm powder. Alkaloid content (expressed as % of leaf dry weight for total alkaloids, nicotine, nornicotine, anabasine and anatabine) was quantified by HPLC as previously described (de Roton et al. 2005).
Statistical tests
F tests were performed with Statgraphics Plus 5.1 (©Statistical Graphics Corp., available at http://www.sigmaplus.fr) using one-way analysis of variance (ANOVA). The likelihood ratio chi-square test (G 2) (SAS Institute, Inc., Cary, NC) was used for the segregation ratios.
Results
Tobacco EMS mutant populations
To demonstrate the feasibility of mutation breeding for improving quality traits in tobacco, we chose the burley-type tobacco cultivar BB16NN because of its strong nicotine-to-nornicotine conversion activity. To generate populations of Nicotiana tabacum mutants with different mutation frequencies suited for both high-throughput mutation detection and mutation breeding, two batches of seeds (6,000 each) from BB16NN were mutagenised with two different concentrations of ethylmethane sulphonate (EMS) (Koornneef et al. 1982): 0.8% (L1 population) and 0.6% (L2 population). In addition, seeds used for L1 population were pre-germinated in order to increase permeability and penetration of EMS in seed tissues. Embryo lethality of EMS-treated seeds reached 36% and 20% in L1 and L2 populations, respectively. Fertility of the M1 plants was also strongly affected, since only 34.5% (L1) and 73.3% (L2) of the selfed field-grown M1 plants yielded seeds. The final ratios of harvested M1 plants to sown M1 seeds were 22% (L1) and 58.6% (L2). Seeds from each M1 plant were collected in bulk and sown (30 M2 seeds per family). The DNA was collected from eight plantlets (one pooled DNA sample per family) for CE-SSCP analysis. As already observed in the polyploid wheat (Slade et al. 2005), heavy mutagenesis did not cause strong visual phenotypic changes in the M2 mutants (data not shown). In greenhouse-grown plantlets, more phenotypes deviating from wild-type plants were, nevertheless, observed in the L1 than in the L2 population.
Targeting ADC1 and NtabCYP82E4 genes for mutation breeding in tobacco
To evaluate the potential of mutation breeding in tobacco, we targeted two genes controlling tobacco nicotine and nornicotine contents. Nicotine is synthesised either from aspartic acid or from putrescine, itself derived from arginine (Kidd et al. 2006). A key enzyme in the putrescine pathway is tobacco arginine decarboxylase, encoded by the ADC1 gene (Hashimoto and Yamada 1994; Wang et al. 2000). Nicotine-to-nornicotine conversion is catalysed by the nicotine N-demethylase NtabCYP82E4 gene, as recently shown (Gavilano et al. 2007; Siminszky et al. 2005). Tobacco (Nicotiana tabacum L.) is a natural allotetraploid species derived from the interspecific hybridisation between two ancestral species N. sylvestris and N. tomentosiformis. However, only N. tomentosiformis genes are involved in nornicotine biosynthesis in tobacco (Gavilano et al. 2007). Therefore, primers were designed to specifically amplify exons in the N. tomentosiformis homoeologs of ADC1 and NtabCYP82E4 genes (DNA fragments <600 bp). In addition, the expected effects of nucleotide changes on protein functionality in the mutants, estimated using the CODDLE program (Till et al. 2003), were taken into account to select the gene regions analysed. Primers tested in N. tabacum and in the ancestral species N. sylvestris and N. tomentosiformis failed to amplify gene fragments from N. sylvestris, as expected from the design of the primers, but succeeded in the amplification of N. tomentosiformis and the N. tabacum ADC1 and NtabCYP82E4 genes. Figure 1a shows the CE-SSCP detection of NtabCYP82E4-mutated allele in one pooled M2 family, i.e. in one pool of eight M2 plants from the same family. Additional peaks corresponding to the mutated allele labelled with two different dyes can be clearly and reproducibly identified on the chromatogram. In the L1 and L2 populations, high mutation frequencies ranging from one mutation per 30 kb to one mutation per 83 kb could be determined (Table 1). Mutation density estimates were, however, variable, depending on the gene analysed (Table 1 and data not shown) and should be considered as rough estimates given the small genome regions analysed.
Capillary electrophoresis-single strand conformation polymorphism (CE-SSCP) analysis of NtabCYP82E4 nicotine N-demethylase gene in tobacco. a CE-SSCP profiles of NtabCYP82E4 fragment in the burley tobacco BB16NN and in the L2-37 mutant (DNA from eight pooled M2 plants). Run temperature was 22°C. b CE-SSCP profiles of NtabCYP82E4 fragment in M2 progeny from L2-37 truncation mutant. W = wild type, H = heterozygous, M = homozygous NtabCYP82E4 mutant. The run temperature was 18°C. Strands labelled with VIC and FAM are shown as light and dark peaks, respectively. The positions of mutant strands with different mobility compared to wild-type strands are indicated with arrows. Size standard (LIZ500) is indicated with a star
Among the available mutant collection, we screened 1,311 M2 families, including 937 from L1 and 374 from L2 libraries. Detection of at least one loss-of-function allele for each target gene was expected, considering mutation frequencies and the predicted rate of deleterious mutations in ADC1 or NtabCYP82E4 proteins. Of 17 alleles isolated by screening 0.446 kb of ADC1 DNA, five were silent, ten were missense and two were truncation mutations. Of ten alleles isolated by screening 0.532 kb of NtabCYP82E4 DNA, one was silent, five were missense and four were truncation mutations. As a consequence of the high mutation rate, at least 0.64% to 1.49% of the screened M2 mutant families contained plants with mutated alleles of either ADC1 or NtabCYP82E4 (Table 1). Sequencing allowed the confirmation of all of the mutations detected in ADC1 and NtabCYP82E4, which were all C/G to T/A transitions (Tables 2 and 3), as expected from the large-scale TILLING analysis of Arabidopsis EMS mutants (Greene et al. 2003). Several truncation (nonsense) mutations were found in each target gene, i.e. two mutations in ADC1 and four in NtabCYP82E4. This is a clear departure from the expected truncation rate (Table 1). Moreover, two of the nonsense mutations detected in the NtabCYP82E4 gene, found in families L1-450 and L2-329, affected the same nucleotide (G to A), whereas the mutated alleles were identified in two different mutant populations created separately at two different times of the year. Some of the missense mutations found in the ADC1 and NtabCYP82E4 genes were predicted to severely affect protein function, as determined by the Sorting Intolerant From Tolerant (SIFT) program (Ng and Henikoff 2003) (Tables 2 and 3). Of the ten missense mutations found in ADC1, six were predicted to have a deleterious effect (i.e. SIFT score <0.05), while only one out of five was predicted to be deleterious for NtabCYP82E4.
Segregation analysis of ADC1 and NtabCYP82E4 mutants
Because mutation analyses were performed on pooled M2 families, and not on M2 individual plants as usually done (Henikoff and Comai 2003; Slade et al. 2005), selected M2 families were sown for the segregation analysis of the mutations (Table 4). Each of the cells from the seed embryo generating reproductive tissues (e.g. two to four “germinally effective cells” in Arabidopsis) (Henikoff and Comai 2003) can be independently mutagenised by EMS. As a consequence, several segregation patterns can be expected in M2. CE-SSCP was used to identify wild-type, heterozygous and homozygous M2 plants. An example of the CE-SSCP profiles is shown for the analysis of the NtabCYP82E4 M2 family (L2-37) carrying a nonsense mutated allele (Fig. 1b). Of 14 selected mutated families representing a wide range of neutral, missense or truncation mutations in either ADC1 or NtabCYP82E4 genes, all but one showed segregation ratios consistent with one germinally effective cell in tobacco seeds, while only six mutants displayed patterns consistent with two or three germinally effective cells.
A NtabCYP82E4 truncation tobacco mutant displays near-null nornicotine content in field conditions
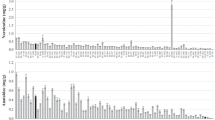
No significant effect on nicotine or nornicotine contents was observed in the heterozygous or homozygous mutant plants from the two ADC1 families carrying truncation mutations. Subsequent studies were focussed on the NtabCYP82E4 gene because of its crucial role in nicotine-to-nornicotine conversion. The nornicotine content of NtabCYP82E4 mutant M2 families was first assessed on greenhouse-grown 3-month-old plants using the colourimetric assay of nornicotine levels after the stimulation of nicotine-to-nornicotine conversion by bicarbonate (Shi et al. 2003). A set of families carrying more or less severe mutations as expected by their SIFT score were selected (Table 3). Mutations ranged from nonsense (L2-37), non-synonymous missense (P to L, L2-164), synonymous missense mutation (V to I, L2-227) to silent mutation (L1-474). The nornicotine contents of homozygous plants from L2-37 and L2-164 M2 families were clearly lower than that of wild-type plants (Fig. 2a). The nornicotine contents of L2-227 and L1-474 homozygous plants were not altered. Details of the nornicotine colourimetric test are given in Fig. 2b for the severely affected L2-37 family. A significant difference was found between homozygous plants and heterozygous or wild-type plants (P = 0.001) but not between wild-type and heterozygous plants (P = 0.765).
Alkaloids analysis of NtabCYP82E4 tobacco mutants. a Nornicotine colourimetric analysis of greenhouse-grown BB16NN and M2 progeny from L2-37, L2-164, L1-474 and L2-227 NtabCYP82E4 mutants. Tobacco plants were first genotyped by CE-SSCP and then analysed for nornicotine content using a colourimetric assay, as described in the Materials and methods section. The white, grey and black triangles represent wild-type, heterozygous and homozygous NtabCYP82E4 mutants, respectively. The y-axis represents the nornicotine content (% of leaf dry weight). b Detail of nornicotine colourimetric analysis of M2 progeny from the L2-37 mutant. Nornicotine amount in the leaf is proportional to the intensity of the blue spots. The nornicotine contents (% of leaf dry weight) estimated by quantification of the spots are indicated. W = wild type, H = heterozygous, M = homozygous. c Nornicotine HPLC analysis of field-grown BB16NN and M2 progeny from L2-37 and L1-160 NtabCYP82E4 mutants. Tobacco plants were first genotyped by CE-SSCP and then analysed for nornicotine content by HPLC. The white, grey and black bars represent wild-type, heterozygous and homozygous NtabCYP82E4 mutants, respectively. n = 3 for BB16NN, n = 12 for L2-37 (4W/3H/5M) and n = 8 for L1-160 (3W/3H/2M). The vertical bars represent the standard deviation. The y-axis represents the nornicotine content (% of leaf dry weight)
We further assessed in field conditions the individual phenotype of three mutants with altered nornicotine conversion (L2-37, L1-450 and L1-160). Alkaloid production is noticeably higher in the field conditions (Fayeulle et al. 1992), probably as a reaction to environmental stresses (Hashimoto and Yamada 1994). Alkaloids and nornicotine contents were analysed by HPLC on air-cured leaves from the medium stalk. A significant difference for nornicotine content was observed between wild-type and heterozygous plants for the truncation mutant L1-450 (data not shown). However, no homozygous plants were recovered from this mutant, which was, therefore, excluded from subsequent studies. The homozygous plants from the truncation mutant L2-37 displayed near-null nornicotine contents (Fig. 2c), while heterozygous mutants showed an intermediate phenotype. The mean nicotine-to-nornicotine conversion rate was as low as ∼3.1% in the homozygous non-converter mutant, compared to ∼59% in the heterozygous mutant and ∼77.5% in wild-type plants (Table 5). The missense mutant L1-160 (P to S) presented a similar pattern of nornicotine reduction, except that the nornicotine content of homozygous mutants remained elevated. It is noteworthy that both L2-37 and L1-160 mutants exhibited higher nornicotine and other alkaloid contents than the BB16NN control line (Fig. 2c and Table 5). As already observed in greenhouse conditions, L2-37 and L1-160 M2 plants displayed near-normal phenotype in field conditions but with slightly retarded growth, reduced height and smaller leaves than the BB16NN elite variety (Fig. 3a). Furthermore, the BC1 plants from crosses between L2-37 and elite tobacco lines presented phenotypes identical to the parental line, as exemplified in Fig. 3b.
Visual phenotype of NtabCYP82E4 tobacco mutants. a Phenotype of field-grown BB16NN and M2 progeny from L2-37 and L1-160 NtabCYP82E4 mutants. Field trial was conducted in Bergerac, France, in 2006. M2 plants were individually identified and genotyped by CE-SSCP analysis. BB16NN was more developed than M2 progeny from L2-37 and L1-160 mutants but the visual aspect of mutant plants remained very similar to BB16NN. b Phenotype of a BC1 plant carrying a NtabCYP82E4 truncation allele from L2-37 mutant and of the parental elite line used for introgression of the mutated allele. No visual phenotypic difference could be observed between the BC1 plants and the elite line
Discussion
We demonstrate here that mutation breeding can be used as an alternative to genetically modified (GM) plants (Gavilano et al. 2006) for generating nornicotine-free tobacco with reduced risks for human health. Using a non-transgenic strategy based on the generation and selection of artificially induced mutations in the nicotine N-demethylase NtabCYP82E4 target gene, we were able to identify several truncation or missense mutants displaying a very low or even near-null nicotine converter phenotype (Fig. 2a–c). The homozygous truncation mutants from the L2-37 family, for example, exhibited a nicotine-to-nornicotine conversion ratio as low as <3.4%. This remarkable reduction in nornicotine content was achieved by screening only a fraction of the available EMS tobacco mutants library (∼one third of the library, i.e. ∼1,200 M2 families).
Non-converter elite tobacco lines created by mutation breeding have several advantages over lines obtained by conventional breeding. Because the NtabCYP82E4 nicotine N-demethylase mutated alleles are stably inactivated by point mutation, introgressed elite tobacco lines display a stable non-converter phenotype, unlike the lines obtained through conventional breeding (Gavilano et al. 2006). Compared to transgenic technologies, which achieve similar a reduction in nicotine-to-nornicotine conversion rates by RNAi targeting of NtabCYP82E4 (Gavilano et al. 2006), the advantages of mutation breeding are even more pronounced. First, point mutations are stable, whereas the transgene may be silenced in some of the transgenic plants and after a number of generations (McGinnis et al. 2007). Second, low nitrosamine plants obtained through mutation technology are not considered as GM in the European Union (EU 2001/18 directive). There is, therefore, no need for the containment of plants from mutation breeding for field trials, as shown in Fig. 3a. As a consequence, the time between the targeting of a candidate gene for trait improvement and the production of the improved crop can be considerably shortened.
Our attempts to reduce nicotine level through the inactivation of the ADC1 gene were unsuccessful, despite the identification of knockout alleles. Such negative results were not surprising because nicotine is synthesised through several interconnected pathways (Kidd et al. 2006; Chintapakorn and Hamill 2003) and that arginine decarboxylase is encoded by multiple and possibly redundant genes in tobacco (Wang et al. 2000). Other likely candidate genes for nicotine content can be easily targeted using the same approach (Kidd et al. 2006). In contrast to ADC1, the NtabCYP82E4 gene was a priori an excellent target since: (i) nornicotine is synthesised through one single pathway, (ii) the NtabCYP82E4 nicotine N-demethylase function was already demonstrated both in vitro and in planta (Xu et al. 2007; Gavilano et al. 2007; Siminszky et al. 2005), (iii) the nicotine N-demethylase homoeologs of N. sylvestris origin are not active in converter tobacco plants (Gavilano et al. 2007), and (iv) the NtabCYP82E3 gene from N. tomentosiformis is inactivated by a single amino acid change, while the NtabCYP82E4 gene can be either transcriptionally active (converter phenotype) or silent (non-converter phenotype) (Gavilano et al. 2007). This favourable context is not unique to tobacco, since similar features can be found in most polyploid plant species, which constitute about two-thirds of the plant crop species. Indeed, interspecific hybridisation can result in whole-genome changes, with massive silencing and the elimination of duplicated sequences (Shaked et al. 2001; Kashkush et al. 2002; Madlung and Comai 2004; Adams and Wendel 2005; Bottley et al. 2006). Ongoing genomic programs (genome and EST sequencing, gene expression analyses) now enable the identification of transcriptionally active candidate genes in many crop species, including tobacco (http://www.estobacco.info/) and, therefore, of the most likely targets for mutation breeding.
The gene duplication due to the amphidiploid nature of cultivated tobacco may also buffer the mutation load, as demonstrated in yeast (Gu et al. 2003). This would explain the high mutation frequencies found in the polyploid species tobacco (Table 1) and wheat (Slade et al. 2005), in contrast with the diploid barley (Caldwell et al. 2004). As a consequence, small mutant libraries are sufficient to identify large allelic series comprising both severe and hypomorphic mutations in polyploids species. Moreover, the worst effects on the overall phenotype of the mutants were small changes in plant development and pollen viability. After backcrossing, BC1 plants were indistinguishable from the parental lines without negative collateral effects on visual phenotype (Fig. 3b) and alkaloid content (data not shown). It is, therefore, expected that the strategy of backcrossing until BC5 to BC6 commonly used for the introgression of valuable traits in tobacco is well adapted to the mutation breeding of commercial elite tobacco lines.
Published reports on the systematic detection of unknown mutations for mutation breeding remain scarce, with the notable exception of the waxy allele in wheat (Slade et al. 2005). Mutation detection technology should be robust, cost-effective and available in-house to the breeders. The efficiency of TILLING technology using the CEL1 endonuclease is proved for a wide range of plants (Colbert et al. 2001; Henikoff and Comai 2003; Comai and Henikoff 2006). However, the cost of commercially available enzymes (Bannwarth et al. 2006) remains prohibitive for routine use and purification of the enzyme is out of reach for non-experimental groups. Hence, the CE-SSCP analysis used here appears to be a good alternative. It requires a simple PCR on pooled mutant DNA samples followed by electrophoresis analysis (Fig. 1a), is sufficiently versatile to fulfill other routine tasks of molecular breeding (e.g. simple sequence repeat {SSR} analysis, allele sequencing), is easily handled by non-experimented technical staff and can be automated with microplates. In addition, CE-SSCP peak detection can be automated for high-throughput mutation screening, as is done for conformation-sensitive capillary electrophoresis (CSCE) analysis (Davies et al. 2006). This technology is sensitive enough to accurately detect mutations in up to four-family pools and, providing there are adjustments of the running temperature for a given target gene, in six- to eight-family pools (data not shown). The rate of mutated allele discovery is increased by the analysis of M2 families instead of M2 plants, as is usually done (Henikoff and Comai 2003). In our hands, CE-SSCP routinely enables the simultaneous detection of two differentially labelled targets (size <600 bp) in two-family pools in a single run. Screening ∼4,000 M2 mutant families with 16-capillary equipment requires ∼2 days and produces up to 35 mutated alleles per target sequence, a throughput compatible with the objective of mutation breeding of multiple quality traits in tobacco.
In summary, using as a case study the suppression of nornicotine in tobacco, the study presents evidence that crop improvement does not necessarily rely on lengthy breeding programs or on GM plant generation but can make use of a comparatively rapid and simple mutant-based strategy for modifying specific traits of interest. The likely targets are polyploid crop species (e.g. sugar beet, potato, strawberry), which are expected to sustain very high mutation rates without compromising plant survival and agricultural value of the crop.
References
Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8:135–141
Bannwarth S, Procaccio V, Paquis-Flucklinger V (2006) Rapid identification of unknown heteroplasmic mutations across the entire human mitochondrial genome with mismatch-specific Surveyor Nuclease. Nat Protoc 1:2037–2047
Bjørheim J, Ekstrøm PO (2005) Review of denaturant capillary electrophoresis in DNA variation analysis. Electrophoresis 26:2520–2530
Bottley A, Xia GM, Koebner RMD (2006) Homoeologous gene silencing in hexaploid wheat. Plant J 47:897–906
Bush LP, Cui M, Shi H et al (2001) Formation of tobacco-specific nitrosamines in air-cured tobacco. Rec Adv Tob Sci 27:23–46
Caldwell DG, McCallum N, Shaw P et al (2004) A structured mutant population for forward and reverse genetics in Barley (Hordeum vulgare L.). Plant J 40:143–150
Chintapakorn Y, Hamill JD (2003) Antisense-mediated down-regulation of putrescine N-methyltransferase activity in transgenic Nicotiana tabacum L. can lead to elevated levels of anatabine at the expense of nicotine. Plant Mol Biol 53:87–105
Colbert T, Till BJ, Tompa R et al (2001) High-throughput screening for induced point mutations. Plant Physiol 126:480–484
Comai L, Henikoff S (2006) TILLING: practical single-nucleotide mutation discovery. Plant J 45:684–694
Davies H, Dicks E, Stephens P et al (2006) High throughput DNA sequence variant detection by conformation sensitive capillary electrophoresis and automated peak comparison. Genomics 87:427–432
de Roton C, Wiernik A, Wahlberg I et al (2005) Factors influencing the formation of tobacco-specific nitrosamines in French air-cured tobaccos in trials and at the farm level. Beitr Tabakforsch Int 21:305–320
Delon R, Poisson C, Bardon JC et al (1999) Les nicotianées en collection à l’Institut du Tabac, 3rd edn. Annales du Tabac, SEITA, Paris
Dickerson TJ, Janda KD (2002) A previously undescribed chemical link between smoking and metabolic disease. Proc Natl Acad Sci USA 99:15084–15088
Doi K, Doi H, Noiri E et al (2004) High-throughput single nucleotide polymorphism typing by fluorescent single-strand conformation polymorphism analysis with capillary electrophoresis. Electrophoresis 25:833–838
Ellis LA, Taylor CF, Taylor GR (2000) A comparison of fluorescent SSCP and denaturing HPLC for high throughput mutation scanning. Hum Mutat 15:556–564
Fayeulle JP, de Salles de Hys L, Duméry B et al (1992) La nornicotine chez les variétés industrielles de tabac. I: état des connaissances, suivi du caractère et efforts visant à son élimination par sélection. Annales du Tabac, SEITA, Bergerac, France, Sect 2–24
Fernie AR, Tadmor Y, Zamir D (2006) Natural genetic variation for improving crop quality. Curr Opin Plant Biol 9:196–202
Gavilano LB, Coleman NP, Burnley LE et al (2006) Genetic engineering of Nicotiana tabacum for reduced nornicotine content. J Agric Food Chem 54:9071–9078
Gavilano LB, Coleman NP, Bowen SW et al (2007) Functional analysis of nicotine demethylase genes reveals insights into the evolution of modern tobacco. J Biol Chem 282:249–256
Greene EA, Codomo CA, Taylor NE et al (2003) Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 164:731–740
Gu Z, Steinmetz LM, Gu X et al (2003) Role of duplicate genes in genetic robustness against null mutations. Nature 421:63–66
Hashimoto T, Yamada Y (1994) Alkaloid biogenesis: molecular aspects. Annu Rev Plant Physiol Plant Mol Biol 45:257–285
Hecht SS (2003) Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer 3:733–744
Henikoff S, Comai L (2003) Single-nucleotide mutations for plant functional genomics. Annu Rev Plant Biol 54:375–401
Hoffmann D, Djordjevic MV, Hoffmann I (1997) The changing cigarette. Prev Med 26:427–434
Kashkush K, Feldman M, Levy AA (2002) Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160:1651–1659
Kidd SK, Melillo AA, Lu R-H et al (2006) The A and B loci in tobacco regulate a network of stress response genes, few of which are associated with nicotine biosynthesis. Plant Mol Biol 60:699–716
Koornneef M, Dellaert LWM, van den Veen JH (1982) EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutat Res 93:109–123
Madlung A, Comai L (2004) The effect of stress on genome regulation and structure. Ann Bot 94:481–495
McCallum CM, Comai L, Greene EA et al (2000) Targeted screening for induced mutations. Nat Biotechnol 18:455–457
McGinnis K, Murphy N, Carlson AR et al (2007) Assessing the efficiency of RNA interference for maize functional genomics. Plant Physiol 143:1441–1451
Ng PC, Henikoff S (2003) SIFT: predicting amino acid changes that affect protein function. Nucleic Acid Res 31:3812–3814
Shaked H, Kashkush K, Ozkan H et al (2001) Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13:1749–1759
Shi H, Kalengamaliro NE, Krauss MR et al (2003) Stimulation of nicotine demethylation by NaHCO3 treatment using greenhouse-grown burley tobacco. J Agric Food Chem 51:7679–7683
Siminszky B, Gavilano L, Bowen SW et al (2005) Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc Natl Acad Sci USA 102:14919–14924
Slade AJ, Fuerstenberg SI, Loeffler D et al (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23:75–81
Stephens RL, Weybrew JA (1959) Isatin: a color reagent for nornicotine. Tobacco Sci 3:48–51
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Till BJ, Reynolds SH, Greene EA et al (2003) Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res 13:524–530
Wang J, Sheehan M, Brookman H et al (2000) Characterization of cDNAs differentially expressed in roots of tobacco (Nicotiana tabacum cv Burley 21) during the early stages of alkaloid biosynthesis. Plant Sci 158:19–32
Xu D, Shen Y, Chappell J et al (2007) Biochemical and molecular characterizations of nicotine demethylase in tobacco. Physiol Plant 129:307–319
Yeung AT, Hattangadi D, Blakesley L et al (2005) Enzymatic mutation detection technologies. Biotechniques 38:749–758
Acknowledgements
The authors are grateful to Prof. Avi Levy (Weizmann Institute, IL) for the critical reading of the manuscript and helpful suggestions. We thank Béatrice Denoyes-Rothan for help with the statistical analyses. Special thanks go to members of the Altadis Research Group (Bergerac, France) for their excellent assistance with the plant culture and alkaloid analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Julio, E., Laporte, F., Reis, S. et al. Reducing the content of nornicotine in tobacco via targeted mutation breeding. Mol Breeding 21, 369–381 (2008). https://doi.org/10.1007/s11032-007-9138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-007-9138-2