Abstract
Erect panicle (EP) is one of the more important traits of the proposed ideotype of high-yielding rice. Several rice cultivars with the EP phenotype, which has been reported to be controlled by a dominant gene, have been successfully developed and released for commercial production in North China. To analyze the inheritance of the EP trait, we generated segregating F2 and BC1F1 populations by crossing an EP-type variety, Liaojing 5, and a curved-panicle-type variety, Fengjin. Our results confirmed that a dominant gene controls the EP trait. Simple-sequence repeat (SSR) and bulked segregant analyses of the F2 population revealed that the EP gene is located on chromosome 9, between two newly developed SSR markers, RM5833-11 and RM5686-23, at a genetic distance of 1.5 and 0.9 cM, respectively. Markers closer to the EP gene were developed by amplified fragment length polymorphism (AFLP) analysis with 128 AFLP primer combinations. Three AFLP markers were found to be linked to the EP gene, and the nearest marker, E-TA/M-CTC200, was mapped to the same location as SSR marker RM5686-23, 1.5 cM from the EP gene. A local map around the EP gene comprising nine SSR and one AFLP marker was constructed. These markers will be useful for marker-assisted selection (MAS) for the EP trait in rice breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first breakthrough in increasing rice yield was the development of the semidwarf cultivar. The breeding of super-high-yielding rice lines has been the focus of rice breeders since the end of last century. One of the more important aspects for the breeding of super-rice lines has been the improvement of the plant type, denoted as ideotype breeding. Breeding programs aimed at developing dwarf or ideotype lines are mostly concerned with improving parts of the plant, such as the stem, leaf and tiller. More recently, however, panicle traits, such as spike number, panicle length and spike density, have received much attention, but to date little information on the erect panicle (EP) type has been reported.
In the 1960s, the EP phenotype in a wheat line was found to reduce the amount of shading and confer a high photosynthetic capacity, while curved panicles (CP) appeared to reduce the efficiency of the line with respect to the utilization of light energy (Yin 1961; Donald 1968). Twenty years later, an EP-type rice variety Liaojing 5 with a high yield was developed and grown commercially (Xiong and Wang 1992). Subsequent studies have provided some evidence that rice EP-type plants can utilize solar energy effectively, improve ecological conditions, such as canopy illumination, temperature, humidity and CO2, and increase population growth rate, which would ultimately increase yield (Gao et al. 1999), while CP-type rice plants have been reported to lodge more easily than their EP counterparts (Du and Fang 2004). This led to the model of EP and super-high yield being the aim in Japonica breeding; validation of this strategy has been the successful development of another high-yielding EP-type variety, Shennong 265, which is successfully cultivated throughout the northern part of China.
Although the EP trait has been extensively propagated, there has been little advancement in the study of the EP gene. In the northern part of China, the EP gene mostly originates from Balilla, an old Italian variety that is representative of the EP type (Zhang et al. 2002). Field statistical results obtained from crossing EP-type Liaojing 5 with CP-type Fengjin and Shennong 129 indicated that a dominant gene controlled the EP trait (Xu et al. 1995). However, information on linkage markers and the chromosome location of the EP gene is currently lacking. Consequently, the objectives of this study were to tag and map the EP gene using molecular markers and to develop a linkage map of the area encompassing the EP gene. This information will provide valuable tools for marker-assisted selection (MAS) in rice EP breeding and further molecular cloning of the EP gene.
Material and methods
Plant materials
EP parent Liaojing 5 was crossed with CP parent Fengjin. The progeny of this cross were used to develop an F2 population, and a BC1F1 population with CP parent Fengjin. Rice plants were cultivated in the experimental field of Shenyang Agricultural University under natural growing conditions.
Scanning electron microscopy
Samples were prepared as described previously (Mou et al. 2000; Zhong et al. 1997; Zhong and Ye 1999), with some modifications. Briefly, stem tissues of rice panicles were excised with a razor and immediately placed in 2.5% glutaraldehyde, dehydrated in ethanol and then dried in a Samdri point dryer (Hitachi, Tokyo, Japan). The samples were coated with gold using E-1010 ion sputter (Hitachi) and observed with a scanning electron microscope (S-3000N; Hitachi).
Statistical and genetic analyses
The numbers of EP plants and CP plants in the F2 and BC1F1 populations were counted and calculated. χ2 tests were performed to determine whether the goodness-of-fit was a ratio of 3:1 in the F2 populations and a ratio of 1:1 in BC1F1 populations.
DNA isolation
Leaves from parent, BC1F1 and F2 plants were harvested and DNA extracted using the standard sodium dodecyl sulfate (SDS) method (McCouch et al. 1988). The DNA concentration was adjusted to 100 ng μl-1 with TE buffer (pH 8.0) for subsequent analyses.
Simple sequence repeats (SSR) analysis
Bulked segregant analysis (BSA; Michelmore et al. 1991) was performed in conjunction with the SSR analysis. The SSR analysis was carried out in a 1× volume of reaction buffer containing 0.1 mM of each dNTP, 1.0 U Taq polymerase, 0.2 μM primer and 20 ng template DNA; the final volume was adjusted to 20 μl with ultra-pure water and one drop of mineral oil was added. The amplification reaction consisted of one cycle at 94°C for 4 min, followed by 35 cycles at 94°C for 45 s, 55°C for 45 s, 72°C for 1 min, with a final extension step at 72°C for 5 min. The amplification products were then separated on 6% polyacrylamide-gel electrophoresis (PAGE) sequencing gels at 85 W for 1.5 h after an initial pre-electrophoresis for 30 min. The gel was then removed from the apparatus and visualized by silver staining (Xu et al. 2002).
Amplified fragment length polymorphism (AFLP) analysis
AFLP (Vos et al. 1995) was performed in conjunction with BSA analysis following the manufacturer’s instructions for the Gibco-BRL AFLP Analysis System kit (Life Technologies, Gaithersburg, Md.), with minor modifications (Chen et al. 2004). A total of 128 primer combinations were screened. The amplification products were separated on a 6% PAGE sequencing gel at 100 W for 2 h after an initial pre-electrophoresis for 30 min. The gels were then silver-stained, followed by SSR analysis.
Mapping of the EP gene
Primary mapping was performed by SSR analysis combined with BSA analysis, in which the mapped SSR markers were used as anchor loci to map the EP gene. Following the identification of potential linkage markers, co-segregation analysis was carried out with the F2 population to verify the linkage of the markers to the EP gene. This method enabled the EP gene to be mapped primarily to a specific chromosome. In order to develop more markers closely linked to the EP gene, we performed AFLP analysis together with BSA analysis in the F2 population. Using the SSR and AFLP data together with the phenotypic data, we developed a local linkage map with MAPMAKER ver. 3.0 (Lander et al. 1987). Recombination fractions were converted into genetic map distance (centiMorgans, cM) using the Kosambi mapping function (Kosambi 1944).
Results
Observation of the EP-trait phenotype
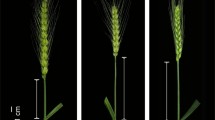
The EP and CP types differed significantly with respect to phenotype. The panicles of both types remained equally erect from heading to flowering (Fig. 1a); however, the panicles of the CP-type cultivar started to droop towards the end of the flowering period. The maximum amount of curving in the CP-type cultivars occurred about 25 days after the start of flowering, while EP-type cultivars remained erect at this time (Fig. 1b, c). The leaves of the EP-type variety were greener and wider than those of the CP type. In addition, the culm nodule of the EP type was thicker than that of CP-type cultivars, resembling that of the culm dwarf. The EP type also had more primary branches and more spikelets per primary branch than the CP type (data was not shown).
The phenotype of the erect panicle (EP)-type parent Liaojing 5 and the curved panicle (CP)-type parent Fengjin at different growth stages. a The phenotype of EP and CP plants at the flowering stage, b the phenotype of an EP plant at the mature stage, c the phenotype of a CP type plant at the mature stage
Anatomical characterization of the two phenotypes
The panicle stem was used as experimental material to compare the anatomical structure difference between two phenotypes. Cell-wall morphology was examined by scanning electron microscopy. The EP type had more vascular bundles (Fig. 2a, b) and thicker sclerenchyma cell walls at the mature stage (Fig. 2c, d) than its CP counterpart; however, no difference in cell width and length were found between the two types.
Genetic analysis of the EP trait
Field tests performed once a year for two consecutive years showed that all of the F1 individuals were of the EP type. Table 1 shows the genetic analysis of the progeny of the cross Liaojing 5 × Fengjin. A total of 6342 plants from the F2 population were examined over the 2 years of the study. Of these, 4846 were EP and 1496 were CP, which fits a 3:1 ratio; this result was confirmed by χ2 tests (Table 1), thereby indicating that a dominant gene controls the EP trait.
Similar observations on the BC1F1 population generated from the same cross over the two consecutive years of the study confirmed the conclusion drawn from the F2 data. Of a total of 227 BC1F1 plants, 110 were EP and 117 were CP. This result (P > 0.05; Table 1) fits the expected 1:1 ratio. It is therefore safe to conclude that the EP trait is controlled by one dominant gene. This conclusion is in agreement with that reported earlier by Xu et al. (1995) based on field statistical data.
Screening of SSR and AFLP markers linked to the EP gene and primary mapping of the EP gene
One hundred plants of the F2 population derived from the cross Liaojing 5 × Fengjin were used to screen for molecular markers putatively linked to the EP gene. In total, 120 SSR primer-pairs that were evenly distributed throughout the 12 chromosomes were selected and screened. The screened markers were further checked with 100 recessive individuals of the F2 population to verify their linkage with the EP gene. Of these, one marker, RM242 on chromosome 9, showed linkage with the EP gene. Subsequently, all of the SSR markers flanking RM242 on the updated linkage map were analyzed, and five markers (RM1189, RM257, RM242, RM3787 and RM1013) were identified as being linked to the EP gene. The amplification pattern of RM257 is shown in Fig. 3.
The amplification pattern of the SSR marker, RM257, in 30 F2 plants of the CP type generated from the cross Liaojing 5 × Fengjin. The samples in each lane are: P E , EP parent Liaojing 5, P C , CP parent Fengjin, B E , EP bulk, B C , CP bulk, 1–30 F2 CP individuals. A 3.5% agarose gel was used in the electrophoresis, and the gels were subsequently stained with EtBr. Asterisks indicate the recombinant
In order to find new markers that are more closely linked to the EP gene, we synthesized SSR primers flanking the EP gene, based on the GRAMENE public database (http://www.gramene.org), and subsequently analyzed their linkage relationship to the EP gene. The results indicated that four new SSR markers (RM5707-18, RM5093-5, RM5686-23 and RM5833-11; Table 2) were linked to the EP gene. The EP gene was located between the RM5686-23 and RM5833-11 markers, with genetic distances of 1.5 and 0.9 cM, respectively. According to the physical mapping GRAMENE data, the two markers were located on bacterial artificial chromosome (BAC) accessions AP005686 and AP005833, respectively, and the physical distance between RM5686-23 and RM5833-11 was about 400 kb.
In the AFLP-BSA analysis, about 50–80 bands appeared in each lane of the PAGE gel when amplified with a 2 + 3 primer-combination and visualized using silver staining (Xu et al. 2002). In total, 128 primer-combinations were screened, of which three combinations (E-TA/M-CTC, E-AG/M-CGA, E-TA/M-CGG) generated three markers that were linked to the EP gene. The most closely linked marker, E-TA/M-CTC200, was mapped to the exact same location as the SSR marker, RM5686-23.
Construction of a local linkage map of the region encompassing the EP gene on chromosome 9
Based on the results of the linkage analysis, we determined that ten markers were linked with the EP gene, nine of which are SSR markers and only one is an AFLP marker. The phenotypic, SSR and AFLP data were combined for linkage analysis using MAPMAKER ver. 3.0. This analysis produced a local linkage map of the EP gene-encompassing region on chromosome 9 (Fig. 4). The most closely linked markers flanking the EP gene are RM5686-23 and RM5833-11, with genetic distances of 1.5 and 0.9 cM, respectively.
Discussion
The EP-and CP-type rice plants differed distinctly from each other in both phenotype and anatomical structure. These differences were only seen after the flowering period was over – not during the heading period – which indicates that expression of the EP gene may occur during the stages of spikelet development. The two notable anatomical differences between the EP-type and CP-type plants in this study were that the former have more vascular bundles and thicker sclerenchyma cell walls than the latter. These two typical EP-type plant characteristics can provide mechanical strength by enhancing the mechanical strength of the stem towards the panicle, thereby allowing the panicle to stay erect more easily. These traits also may be beneficial to the whole population of rice plants by allowing the plants a better exposure to solar energy and enhancing photosynthesis in the reproductive growth period, thus increasing the ultimate rice yield (Jin et al. 2003). To date, the EP trait is only found in the Japonica super-high-yielding cultivar, with the CP type being the ideotype of the Indica high-yielding cultivar in South China. When the EP-type cultivar was grown in South China, the seed setting rate was reduced. It would therefore appear that the choice of EP or CP depends on the different growth conditions and growth areas.
The EP-type cultivar, with a high spikelet density, usually tends to suffer from panicle disease, such as false smut. However, Chen (2003) mentioned that the panicle curvature was negatively associated with spikelet density and incidence of false smut. The correlation coefficient with incidence of false smut was too low to discern the difference level, which indicated that no direct relation existed between erect panicle trait and incidence of false smut. Subsequent studies confirmed that the incidence of false smut was correlated with high spike density, which is the primary causal factor of severe false smut in the EP-type cultivar. Therefore, in rice breeding of the EP-type cultivar, reducing the spikelet density properly could completely decrease the incidence of false smut.
The EP gene, which originated from Balilla, was always thought to be a single dominant gene or an additive major gene plus a minor gene (Wang et al. 1997; Xu et al. 1995). In this investigation, Liaojing 5, the donor of the EP gene, also originated from Balilla; it was crossed with Fengjin, and thought to have a single dominant gene. Genetic analysis of the F2 and BC1F1 progeny confirmed this. The results from chromosome mapping of the EP trait obtained here will be useful for further molecular cloning of the EP gene through the map-based cloning approach. A large number of studies have shown that the panicle curvature was negatively related with the number of primary branch and primary branch grains and positively related with panicle length, yet the correlation coefficient did not reach the difference level. Whether the relation between EP trait and other traits is due to the pleiotrpic effects of the EP gene or to linkage to the EP gene is still to be investigated.
This study is the first to report the chromosome mapping of the EP gene. Ten markers were linked to EP gene, which was mapped to the long arm of chromosome 9. These two closely linked markers, SSR markers RM5686-23 and RM5833-11, will be useful for MAS of the EP trait in breeding programs.
References
Chen CX, Yang DE, Jin DM,Wang B (2004) Molecular tagging and genetic mapping of the disease resistance gene RppQ to southern corn rust. Theor Appl Genet 108:945–950
Chen WF (2003) The rice erect panicle trait and super-high yield breeding. In: Chen WF, Xu ZJ, Zhang LB (eds) Physiological basis of rice breeding for super high yield. Liaoning Science and Technology Publisher, Hangzhou, China, pp 199–225
Donald CM (1968) The breeding of crop ideotypes. Euphytica 17:385–403
Du XL, Fang XQ (2004) Theoretical analysis and simulated determination of the relationship between rice erect panicle type and lodging resistance. J Jilin Agric Sci 29:3–4
Gao SJ, Chen WF, Zhang BL (1999) Studies of erect-panicle in rice. J Jilin Agric Sci 24:12–15
Jin XH, Wang JY, Xu ZJ, Zhang BL (2003) Genetic multiple-effects of erect panicle type rice. J Shenyang Agric Univ 34:332–335
Kosambi DD (1944) The estimation of map from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Etoh T (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
McCouch SR, Kochert G, Yu ZH, Wang ZY, Khush GS, Coffman WR, Tanksley SD (1988) Molecular mapping of rice chromosomes. Theor Appl Genet 76:815–829
Michelmore R, Paran I, Kesseli RV (1991) Identification of marker linked to disease resistance gene by bulk segregant analysis: a rapid method to detect markers in specific genomic regions using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Mou Z, He Y, Dai Y, Liu X, Li JY (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alteration in plant morphology. Plant Cell 12:405–417
Wang BL, Dong YH, Wang S (1997) Studies on genetic activities of semidwarfism and erect-panicle in rice. J Shenyang Agric Univ 28:83–87
Vos P, Hongers R, Bleeker M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Xiong ZM, Wang JL (1992) The theory and practice of breeding high yielding varieties of super-rice in China. Abstr Rice 11:1–8
Xu SB, Tao YF, Yang SQ, Zhe JY (2002) A simple and rapid DNA silver staining and gel preservation. Yi Chuan 24:335–336
Xu ZJ, Chen WF, Zhang BL, Zhang CL (1995) The heredity of the erect panicle character and relations with other characters in rice. J Shenyang Agric Univ 26:1–7
Yin HZ (1961) The research about rice and wheat population. Shanghai Scientific and Technical Publisher, China, pp 40–50
Zhang WZ, Xu ZJ, Zhang BL, Chen WF (2002) The value and character of erect-panicle in rice. Liaoning Agric Sci 5:24–27
Zhong RQ, Ye ZH (1999) IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell 11:2139–2152
Zhong RQ, Taylor J, Ye ZH (1997) Disruption of interfasciculr fiber differentiation in an Arabidopsis mutant. Plant Cell 9:2159–2170
Acknowledgements
This work was partially supported by the State Key Basic Research and Development Plan of China (Grant No. 2001CB108802) and the National Five Year Research Program of Science and Technology of China (Grant No. 2004BA525B04)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kong, F.N., Wang, J.Y., Zou, J.C. et al. Molecular tagging and mapping of the erect panicle gene in rice. Mol Breeding 19, 297–304 (2007). https://doi.org/10.1007/s11032-006-9062-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-006-9062-x