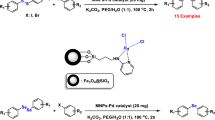
Abstract
A new eco-friendly catalytic system is devised for C–C bond formation through Suzuki coupling, using an impressive nanocatalyst (Fe3O4/L-(+)-tartaric acid/Pd-NPs). It contains immobilized palladium (0) onto magnetite nanoparticles, stabilized by tartaric acid, and is characterized by FT-IR, XRD, EDS, SEM, TEM, TGA, and VSM. The catalyst is used in an efficient synthesis of biaryls in EtOH/H2O (1:1), in the presence of K2CO3. Our Fe3O4/tartaric acid/Pd-NPs exhibit magnetic recoverability and reusability for five cycles without measurable loss of its activity.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Palladium-catalyzed cross-coupling reactions have long been considered as efficient methods for C–C, C–N, C–O, and C–S bond formations [1, 2]. In 2010, Suzuki, Heck, and Negishi won the Nobel Prize for palladium-catalyzed cross-coupling synthesis [3,4,5]. Coupling reactions play strategic roles in synthesizing natural products and pharmacological compounds [6,7,8]. Developing and designing new catalytic systems for these reactions is an attractive research field in organic synthesis.
Cross-coupling reactions have recently focused on magnetic nanocarrier-supported palladium catalysts [9,10,11]. These catalysts, as one of the efficient metal–organic catalytic systems, have benefits such as high surface‐to‐volume ratio, eco-friendliness, high dispersibility, high coercivity, convenient recoverability by an external magnet, and reusability [12,13,14]. The Fe3O4 nanoparticle is used as the magnetic core of these systems and is usually covered with some coating materials such as silica [15, 16]. This coat protects the magnetic core against agglomeration and oxidation and helps maintain the magnetic properties [17,18,19]. The silica shell has other advantages, such as easy functionalization and readying the surface of the core for more modifications [20]. Suzuki coupling reaction of organoboron compounds with aryl halides can be carried out in the presence of versatile functional groups [21, 22]. This reaction is considered an efficient and flexible method for synthesizing biaryls, which are found in the structure of conducting polymers, natural products, and herbicides [23, 24]. Modifications to Suzuki coupling include the design of novel catalysts, solid-phase synthesis, and the use of biocompatible solvents.[25,26,27,28] Given the facts mentioned above and in line with our continued work on nanocatalyst [29,30,31,32,33]. Here, we have immobilized palladium (II) onto magnetic nanoparticles stabilized by tartaric acid and applied it as a nanocatalyst in the Suzuki cross-coupling reaction (Scheme 1).
Results and discussion
Magnetic nanoparticles (Fe3O4 MNPs) were obtained from the Fe2+/Fe3+ salt solution in a basic medium, then functionalized with tartaric acid and anchored by palladium (Scheme 2).
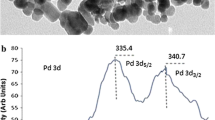
The Fe3O4/tart/Pd-NPs nanocatalyst and its components were characterized using FT-IR, XRD, EDS, SEM, and VSM spectroscopic analysis. After characterization of the nanocatalyst, its catalytic activity is examined in the Suzuki coupling reaction. Phenylboronic acid and iodobenzene are selected as model substrates to optimize the reaction conditions (Table 1). Initially, several solvents such as toluene, DMF, EtOH, water, and EtOH–H2O (1:1) were tested. The best solvent turns out to be H2O–EtOH (1:1) (Table 1, entry 1–6). To optimize the loading amount of the catalyst, different quantities (5–20 mg) of the catalyst were employed (Table 1, entries 6–9). The best result is obtained with 0.01 g of the catalyst (Table 1, entry 7).
The catalyst's efficiency is investigated by using a range of substituted aryl halides subjected to the optimized reaction conditions (Table 1, entry 7 Table). Aryl iodide shows better reactivity and product yields compared to aryl bromide (Table 2, entries 1, 7). This is due to the strength of the halogen-carbon bond, which weakens from bromine to iodine. In addition, the electronic properties of substitute rings influence the reaction yield. Efficiency is higher in the presence of electron-withdrawing groups than electron‐donating ones (Table 2, entries 1–5, 7–13).
Ortho‐substituted derivatives show a lower yield compared with the para‐substituted ones, indicating the sensitivity of the reaction to steric effects (Table 2, entries 5 and 6). Based on the catalytic cycle for the Suzuki coupling reaction and the aforementioned results, we suggest a plausible mechanism for the formation of biaryls in the presence of Fe3O4/tartaric acid/Pd(0) NPs (Scheme 3).
The FT-IR spectrum of Fe3O4 nanoparticles, tartaric acid, Fe3O4/tartaric acid nanoparticles, and Fe3O4/tartaric acid/Pd(0) nanoparticles are obtained (Fig. 1). The FT-IR spectrum of Fe3O4 NPs shows peaks at 567, 1619, and 3427 cm−1, indicating the basic nature of the NPs obtained. The FT-IR spectrum of tartaric acid shows a strong peak at 1739 cm−1, along with a broad peak at 2600–3600 cm−1, corresponding to the characteristics of a carboxylic acid group. Absorption at 1066–1132 cm−1 shows stretching for the C–O single bonds. In the IR spectrum of Fe3O4/tartaric acid NPs, peaks of C=O stretchings appear at 1654 and 1630 cm−1. Also, this spectrum shows peaks at 1364 and 1365 cm−1 related to the C–O stretchings of carboxylate groups. These observations revealed the chemisorption of tartaric acid on Fe3O4 NPs as a carboxylate. In the Fe3O4/tartaric acid/Pd(0) NPs spectrum, a shift in peaks of C=O and C–O to lower wavenumber, a shift to higher wavenumber for the OH of carboxyl groups, and also an increase in the intensity of C–O peak are observed. This evidence proves the interaction between the carboxyl group of tartaric acid and palladium (Fig. 1).
FT-IR spectrum of Fe3O4 nanoparticles, tartaric acid, Fe3O4/tartaric. The structural composition is examined using EDS, which displays the catalyst containing Pd, Si, Fe, O, and C (Fig. 2)
The size and morphology of the catalyst nanoparticles were evaluated via SEM (Fig. 3) and TEM analyses (Fig. 4). Spherical particles with an average size of 25 nm are observed with weak agglomeration. TEM image shows magnetic nanoparticles covered with organic groups.
The magnetization vs. applied magnetic field curves at the room temperature range from + 10,000 to − 10,000 Oersted is illustrated for the Fe3O4 NPs and the catalyst (Fig. 5). The magnetic saturation (Ms) value is varied from 63 emug−1 for Fe3O4 NPs to 41 emug−1 for the catalyst. The decrease in magnetic saturation for Fe3O4/tart/Pd(0)-NPs can be attributed to the modification on the surface of the Fe3O4 with tartaric acid.
The properties of crystallinity and phase purity of the as‐synthesized Fe3O4/tart/Pd(0)-NPs were investigated by wide angle XRD. (Fig. 6). The decrease in the intensity of peaks is related to the functionalization and modification of the Fe3O4 surface. Both the catalyst and recycled catalyst exhibit the six characteristics.
The organic content and the thermal stability on the surface of the magnetic NPs are determined by TGA whose thermogram shows the initial weight loss is below 200 °C, which is attributed to the removal of physically adsorbed solvent and water. Also, the mass loss at the temperature range 200–400 °C is due to the decomposition of l-(+)-tartaric acid, grafted on the surface of the magnet (Fig. 7).
To check leaching of the Pd NPs, after completion of reaction, the catalyst is separated from the solution by an external magnet, analysis of the aqueous phases of reaction mixture using inductively coupled plasma analysis show that the leaching of Palladium is negligible and the catalyst is mainly heterogeneous in nature.
Given commercial and industrial applications and the principles of green chemistry and environmentally friendly processes, the recyclability and reusability of catalysts are very important. The reusability of our catalyst is probed through the model reaction, where the catalyst is simply removed from the reaction medium using an external magnet. Before each run, the catalyst is washed thoroughly with ethanol and acetone and dried in an oven. The results showed that the catalyst could be reused at least five times with a slight decrease in its catalytic activity (Fig. 8).
Experimental
Chemical reagents in high purity were purchased from Merck and Aldrich and used without further purification. Melting points were determined in open capillaries using an Electrothermal 9100 apparatus and are uncorrected. Fourier transform infrared (FT‐IR) spectra were recorded using KBr pellets in the range of 400–4000 cm−1 on a Nicolet IR‐100 infrared spectrometer. NMR spectra were recorded in DMSO‐d6 on Brucker DRX 500‐Avance spectrometer. The particle morphology is examined by SEM (KYKY EM3200–25 kV). X‐ray diffraction (XRD) is performed on Philips XPert 1710 diffractometer. The latter appears with Co Kα (α = 1.79285 Å) with 40 kV voltage.
Preparation of Fe3O4 magnetic nanoparticles (MNPs)
FeCl3⋅6H2O (5.4 g, 20 mmol) and FeCl2⋅4H2O (1.98 g, 10 mmol) were dissolved in 100 ml deionized water at 80 °C under Ar atmosphere by vigorous mechanical stirring. Then, 10 ml of 28% aqueous ammonia solution was quickly injected into the reaction mixture in one portion. The addition of the base to the Fe2+/Fe3+ salt solution resulted in the immediate formation of a black precipitate of MNPs. The stirring continued for another 2 h, and the mixture was cooled to room temperature. Subsequently, the produced ultra-fine magnetic particles were separated by a magnet and washed several times with deionized water.[49] The resulting nanoparticle weighed 2.1 g.
Fe3O4/tart-NPs preparation
A round-bottom flask was charged with Fe3O4 (500 mg) in EtOH (50 ml), and the flask was sonicated for 30 min. Then, the sonication was stopped, and l-(+)-tartaric acid (1 mmol) was added. The mixture was stirred for 12 h at 40 °C. Finally, Fe3O4/tart NPs were separated by an external magnet washed with ethanol and dried in an oven. The resulting nanoparticle weighed 700 mg.
Fe3O4/tart/Pd(0)-NPs preparation
A round-bottom flask was charged with Fe3O4/tart NPs (500 mg) and sonicated in ethanol for half an hour. Then, the flask was removed, followed by the addition of palladium acetate (50 mg), and the mixture was stirred at 50 °C for 4 h. NaBH4 (100 mg) was added to the mixture, and the stirring continued for 2 h at 40 °C. The resulting magnetic nanoparticles were washed with ethanol and dried in an oven at 50 °C for 4 h. The resulting nanoparticle weighed 540 mg.
General procedure for the Suzuki cross-coupling reaction
A round-bottom flask was charged with MNP supported palladium (0) (Fe3O4/tart/Pd(0)-NPs) (10 mg), aryl halide (1.0 mmol), phenylboronic acid (1.2 mmol), K2CO3 (1.5 mmol) and EtOH:H2O (1:1, 3 ml). Then, the reaction mixture was stirred at 60 °C for 20 min. The reaction progress was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature, and the catalyst was separated by an external magnet. The product was purified by thin-layer chromatography using n-hexane and ethyl acetate (5:2) as eluents. Here we have immobilized palladium (II) onto magnetic nanoparticles stabilized by tartaric acid and applied it as a nanocatalyst in Suzuki cross-coupling (Scheme 1).
4-methoxy-1,1′-biphenyl (Table 2, Entry 3): 1H NMR (300 MHz, CDCl3) δ 7.60–7.54 (m, 4H), 7.467.41 (m, 2H), 7.32–7.26 (m, 1H), 7.02–6.99 (m, 2H), 3.87 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3) δ 159.15, 140.82, 133.78, 128.70, 128.14, 126.72, 126.64, 114.20, 55.35 ppm.
1,1′-biphenyl (Table 2, Entry 1): 1H NMR (300 MHz, CDCl3) δ 7.66–7.63 (dd, 4H), 7.49–7.42 (t, 4H), 7.42–7.26 (t, 2H) ppm; 13C NMR (75 MHz, CDCl3) δ 141.26, 128.77, 127.26, 127.18 ppm.
Conclusions
Here, we present a recoverable heterogeneous catalyst by stabilizing palladium on the surface of tartaric acid-activated magnetic iron. The catalytic activity of the synthesized magnetic nanoparticles in the Suzuki reaction, i.e., the interaction of aryl halides with phenylboronic acid, was investigated. The advantages of this new heterogeneous nanocatalyst include easy separation by magnets, compatibility with aquatic environments, easy access, thermal stability, high efficiency, recyclability, green conditions, and short reaction time.
References
Kumar S (2019) Recent advances in the schiff bases and N-heterocyclic carbenes as ligands in the cross-coupling reactions: a comprehensive review. J Heterocycl Chem 56:1168–1230. https://doi.org/10.1002/jhet.3504
Li P, Wang L, Zhang L, Wang G (2012) Magnetic nanoparticles-supported palladium: a highly efficient and reusable catalyst for the Suzuki, Sonogashira, and Heck reactions. Adv Synth Catal 354:1307–1318. https://doi.org/10.1002/adsc.201100725
Nuri A, Mansoori Y, Bezaatpour A (2019) N-heterocyclic carbene–palladium (II) complex supported on magnetic mesoporous silica for Heck cross-coupling reaction. Appl Organomet Chem 33:e4904. https://doi.org/10.1002/aoc.4904
Hajipour AR, Kalantari Tarrari M, Jajarmi S (2018) Synthesis and characterization of 4-AMTT-Pd (II) complex over Fe3O4@SiO2 as supported nanocatalyst for Suzuki-Miyaura and Mizoroki-heck cross-coupling reactions in water. Appl Organomet Chem 32:e4171. https://doi.org/10.1002/aoc.4171
Yousaf M, Zahoor AF, Akhtar R et al (2020) Development of green methodologies for Heck, Chan-Lam, Stille and Suzuki cross-coupling reactions. Mol Divers 24:821–839. https://doi.org/10.1007/s11030-019-09988-7
Byun S, Chung J, Kwon J, Moon Kim B (2015) Mechanistic studies of magnetically recyclable Pd-Fe3O4 heterodimeric nanocrystal-catalyzed organic reactions. Chem Asian J 10:982–988. https://doi.org/10.1002/asia.201403201
Begum T, Mondal M, Gogoi PK, Bora U (2015) Palladium-Schiff-base-silica framework as a robust and recyclable catalyst for Suzuki-Miyaura cross-coupling in aqueous media. RSC Adv 5:38085–38092. https://doi.org/10.1039/C5RA01574J
Akbarzadeh P, Koukabi N, Kolvari E (2020) Polythiophene-functionalized magnetic carbon nanotube-supported copper (I) complex: a novel and retrievable heterogeneous catalyst for the “Phosphine-and Palladium-Free” Suzuki-Miyaura cross-coupling reaction. Mol Divers 24:1125–1137. https://doi.org/10.1007/s11030-019-10016-x
Sheykhan M, Yahyazadeh A, Rahemizadeh Z (2016) Cu–EDTA-modified APTMS-Fe3O4@SiO2 core–shell nanocatalyst: a novel magnetic recoverable catalyst for the Biginelli reaction. RSC Adv 6:34553–34563. https://doi.org/10.1039/C6RA02415G
Duan X, Liu J, Hao J et al (2018) Magnetically recyclable nanocatalyst with synergetic catalytic effect and its application for 4-nitrophenol reduction and Suzuki coupling reactions. Carbon N Y 130:806–813. https://doi.org/10.1016/j.carbon.2018.01.038
Zolfigol MA, Khazaei A, Alaie S et al (2016) Experimental and theoretical approving of anomeric based oxidation in the preparation of 2-sbstituted benz-(imida, oxa and othia)-zoles using [2,6-DMPy-NO2]C(NO2)3 as a novel nano molten salt catalyst. RSC Adv 6:58667–58679. https://doi.org/10.1039/C6RA13231F
Hajipour AR, Tavangar-Rizi Z (2017) Methionine-functionalized chitosan–Pd(0) complex: a novel magnetically separable catalyst for Heck reaction of aryl iodides and aryl bromides at room temperature in water as only solvent. Appl Organomet Chem 31:e3638. https://doi.org/10.1002/aoc.3638
Hadian-Dehkordi L, Hosseini-Monfared H (2016) Enantioselective aerobic oxidation of olefins by magnetite nanoparticles at room temperature: a chiral carboxylic acid strategy. Green Chem 18:497–550. https://doi.org/10.1039/C5GC01774B
Rohani S, Mohammadi Ziarani G, Badiei A (2019) Pd embedded N, S co-doped graphene wrapped core-shell magnetic nanospheres: engineered stable nanocatalyst for Suzuki couplings. Appl Organomet Chem 33:e5142. https://doi.org/10.1002/aoc.5142
Keypour H, Saremi SG, Noroozi M, Veisi H (2017) Synthesis of magnetically recyclable Fe3O4@[(EtO)3Si–L1H]/Pd (II) nanocatalyst and application in Suzuki and Heck coupling reactions. Appl Organomet Chem 31:e3558. https://doi.org/10.1002/aoc.3558
Azad S, Mirjalili BBF (2019) One-pot solvent-free synthesis of 2,3-dihydro-2-substituted-1H-naphtho [1,2-e][1,3] oxazine derivatives using Fe3O4@nano-cellulose/TiCl as a bio-based and recyclable magnetic nano-catalyst. Mol Divers 23:413–420. https://doi.org/10.1007/s11030-018-9884-6
Ghorbani-Choghamarani A, Tahmasbi B, Moradi Z (2017) S-Benzylisothiourea complex of palladium on magnetic nanoparticles: a highly efficient and reusable nanocatalyst for synthesis of polyhydroquinolines and Suzuki reaction. Appl Organomet Chem 31:e3665. https://doi.org/10.1002/aoc.3665
Dehkordi S, Jafari AA, Albadi J, Samimi HA (2022) Mesoporous epoxidized soybean oil-supported copper-based magnetic nanocatalyst and amberlite-supported azide as a green and efficient catalytic system for 1, 2, 3-triazole synthesis. Mol Divers 26:1–16. https://doi.org/10.1007/s11030-022-10408-6
Ferdousian R, Behbahani FK, Mohtat B (2022) Synthesis and characterization of Fe3O4@Sal@Cu as a novel, efficient and heterogeneous catalyst and its application in the synthesis of 2-amino-4H-chromenes. Mol Divers 26:1–13
Zhang L, Li P, Liu C et al (2014) A highly efficient and recyclable Fe3O4 magnetic nanoparticle immobilized palladium catalyst for the direct C-2 arylation of indoles with arylboronic acids. Catal Sci Technol 4:1979–1988. https://doi.org/10.1039/C4CY00040D
Kandathil V, Fahlman BD, Sasidhar BS et al (2017) A convenient, efficient and reusable N-heterocyclic carbene-palladium (ii) based catalyst supported on magnetite for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions. New J Chem 41:9531–9545. https://doi.org/10.1039/C7NJ01876B
Han F, Xu Y, Zhu R et al (2018) Highly active NHC–Pd (ii) complexes for cross coupling of aryl chlorides and arylboronic acids: an investigation of the effect of remote bulky groups. New J Chem 42:7422–7427. https://doi.org/10.1039/C8NJ01047A
Sharma KN, Satrawala N, Srivastava AK et al (2019) Palladium (ii) ligated with a selenated (Se, C NHC, N−)-type pincer ligand: an efficient catalyst for Mizoroki-Heck and Suzuki-Miyaura coupling in water. Org Biomol Chem 17:8969–8976. https://doi.org/10.1039/C9OB01674K
Nayan Sharma K, Satrawala N, Kumar Joshi R (2018) Thioether–NHC-ligated PdII complex for crafting a filtration-free magnetically retrievable catalyst for Suzuki-Miyaura coupling in water. Eur J Inorg Chem 2018:1743–1751. https://doi.org/10.1002/ejic.201800209
Zhan J, Wu K, Yu X et al (2019) α-Fe2O3 nanoparticles decorated C@MoS2 nanosheet arrays with expanded spacing of (002) plane for ultrafast and high Li/Na-ion storage. Small 15:1901083. https://doi.org/10.1002/smll.201901083
Veerakumar P, Thanasekaran P, Lu K-L et al (2017) Functionalized silica matrices and palladium: a versatile heterogeneous catalyst for Suzuki, Heck, and Sonogashira reactions. ACS Sustain Chem Eng 5:6357–6376. https://doi.org/10.1021/acssuschemeng.7b00921
Karami K, Abedanzadeh S, Vahidnia O et al (2017) Orthopalladated complexes of phosphorus ylide: Poly (N-vinyl-2-pyrrolidone)-stabilized palladium nanoparticles as reusable heterogeneous catalyst for Suzuki and Heck cross-coupling reactions. Appl Organomet Chem 31:e3768. https://doi.org/10.1002/aoc.3768
Sahu D, Silva AR, Das P (2016) Facile synthesis of palladium nanoparticles supported on silica: an efficient phosphine-free heterogeneous catalyst for Suzuki coupling in aqueous media. Catal Commun 86:32–35. https://doi.org/10.1016/j.catcom.2016.08.005
Bai L, Wang J (2008) Reusable, polymer-supported, palladium-catalyzed, atom-efficient coupling reaction of aryl halides with sodium tetraphenylborate in water by focused microwave irradiation. Adv Synth Catal 350:315–320. https://doi.org/10.1002/adsc.200700361
Eidi E, Kassaee MZ, Nasresfahani Z (2015) Nanocrystalline TiO2, via green combustion synthesis, as an efficient and reusable catalyst for the preparation of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines. Appl Organomet Chem 29:793–797. https://doi.org/10.1002/aoc.3370
Eidi E, Kassaee MZ, Nasresfahani Z (2016) Synthesis of 2,4,5-trisubstituted imidazoles over reusable CoFe2O4 nanoparticles: an efficient and green sonochemical process. Appl Organomet Chem 30:561–565. https://doi.org/10.1002/aoc.3470
Nasresfahani Z, Kassaee MZ, Eidi E (2016) Homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H) as an efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols. New J Chem 40:4720–4726. https://doi.org/10.1039/C5NJ02974K
Nasresfahani Z, Kassaee MZ (2018) Cu (II) immobilized on mesoporous organosilica as an efficient and reusable nanocatalyst for one-pot Biginelli reaction under solvent-free conditions. Appl Organomet Chem 32:e4106. https://doi.org/10.1002/aoc.4106
Fakhri A, Naghipour A (2018) Organometallic polymer-functionalized Fe 3 O 4 nanoparticles as a highly efficient and eco-friendly nanocatalyst for C-C bond formation. Transit Met Chem 43:463–472. https://doi.org/10.1007/s11243-018-0233-5
Ghorbani-Choghamarani A, Rabiei H (2016) Synthesis, characterization, and application of palladium-dithizone immobilized on magnetic nanoparticles as an efficient and recoverable catalyst for Suzuki type coupling reactions. Tetrahedron Lett 57:159–162. https://doi.org/10.1016/j.tetlet.2015.11.096
Cho S-D, Kim H-K, Yim H et al (2007) Suzuki-Miyaura coupling reaction of aryl chlorides using di (2, 6-dimethylmorpholino) phenylphosphine as ligand. Tetrahedron 63:1345–1352. https://doi.org/10.1016/j.tet.2006.12.001
Jarret RM, Keil N, Allen S et al (1989) Friedel Crafts acylation and alkylation with acid chlorides. J Chem Educ 66:1056. https://doi.org/10.1021/ed066p1056
Ghorbani-Choghamarani A, Tahmasbi B, Moradi P (2016) Synthesis of a new Pd (0)-complex supported on boehmite nanoparticles and study of its catalytic activity for Suzuki and Heck reactions in H 2 O or PEG. RSC Adv 6:43205–43216. https://doi.org/10.1039/C6RA02967A
Alacid E, Najera C (2008) First cross-coupling reaction of potassium aryltrifluoroborates with organic chlorides in aqueous media catalyzed by an oxime-derived palladacycle. Org Lett 10:5011–5014. https://doi.org/10.1021/ol802024j
Beygzadeh M, Alizadeh A, Khodaei MM, Kordestani D (2013) Biguanide/Pd (OAc)2 immobilized on magnetic nanoparticle as a recyclable catalyst for the heterogeneous Suzuki reaction in aqueous media. Catal Commun 32:86–91. https://doi.org/10.1016/j.catcom.2012.11.028
Lei L (2019) Pd–Schiff base complex supported on Fe3O4 magnetic nanoparticles: A new and highly efficient reusable catalyst for C-C bond formation in water. Appl Organomet Chem 33:e5158. https://doi.org/10.1002/aoc.5158
Nikoorazm M, Ghorbani F, Ghorbani-Choghamarani A, Erfani Z (2018) Pd (0)-S-propyl-2-aminobenzothioate immobilized onto functionalized magnetic nanoporous MCM-41 as efficient and recyclable nanocatalyst for the Suzuki, Stille and Heck cross coupling reactions. Appl Organomet Chem 32:e4282. https://doi.org/10.1002/aoc.4282
Eslahi H, Sardarian AR, Esmaeilpour M (2021) Green and sustainable palladium nanomagnetic catalyst stabilized by glucosamine-functionalized Fe3O4@SiO2 nanoparticles for Suzuki and Heck reactions. Appl Organomet Chem 35:e6260. https://doi.org/10.1002/aoc.6260
Nikoorazm M, Ghorbani-Choghamarani A, Jabbari A (2016) A facile preparation of palladium Schiff base complex supported into MCM-41 mesoporous and its catalytic application in Suzuki and Heck reactions. J Porous Mater 23:967–975. https://doi.org/10.1007/s10934-016-0154-7
Nasrollahzadeh M, Sajadi SM, Rostami-Vartooni A, Khalaj M (2015) Green synthesis of Pd/Fe3O4 nanoparticles using Euphorbia condylocarpa M. Bieb root extract and their catalytic applications as magnetically recoverable and stable recyclable catalysts for the phosphine-free Sonogashira and Suzuki coupling reactions. J Mol Catal A Chem 396:31–39. https://doi.org/10.1016/j.molcata.2014.09.029
Elhampour A, Mirhosseyni MS, Nemati F (2018) Palladium nanoparticles supported on modified hollow Fe3O4@TiO2: Preparation, characterization, and catalytic activity in Suzuki cross-coupling reactions. J Chin Chem Soc 65:875–882. https://doi.org/10.1002/jccs.201700341
Nouri K, Ghassemzadeh M, Mohsenzadeh F, Afsharpour M (2020) Pd (0) complex of fuberidazole modified magnetic nanoparticles: a novel magnetically retrievable high-performance catalyst for Suzuki and Stille C-C coupling reactions. Appl Organomet Chem 34:e5771. https://doi.org/10.1002/aoc.5771
Chen T, Gao J, Shi M (2006) A novel tridentate NHC–Pd (II) complex and its application in the Suzuki and Heck-type cross-coupling reactions. Tetrahedron 62:6289–6294. https://doi.org/10.1016/j.tet.2006.04.034
Khazaei A, Sarmasti N, Seyf JY (2018) Anchoring high density sulfonic acid based ionic liquid on the magnetic nano-magnetite (Fe3O4), application to the synthesis of hexahydroquinoline derivatives. J Mol Liq 262:484–494. https://doi.org/10.1016/j.molliq.2018.04.125
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Souri, S.M., Eidi, E. & Kassaee, M.Z. Efficient Suzuki coupling over novel magnetic nanoparticle: Fe3O4/L-(+)-tartaric acid/Pd(0). Mol Divers 27, 1469–1479 (2023). https://doi.org/10.1007/s11030-022-10507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10507-4