Abstract
A series of 1-substituted-1H-tetrazole-5-thiol building blocks were synthesized and introduced to the N-4 piperazinyl group at C-7 position of the quinolone core, and these novel compounds (5a–g and 8a–g) were screened for their antibacterial and antiproliferative activities. Bioactive assay studies manifested that most of new compounds exhibited significant antibacterial activity against the tested strains, including multi-drug-resistant MRSA in comparison with reference drugs ciprofloxacin, streptomycin B and pipemidic acid. Among the synthesized compounds, only ciprofloxacin (5a–g) derivatives displayed significant activity (\(\mathrm{MIC}=15.6~\upmu \hbox {g}/\hbox {mL}\)) compared to reference drugs. In addition, these compounds were evaluated for their in vitro inhibition of human cancer cell lines viz human cervical carcinoma cell line (SiHA), breast adenocarcinoma (MDA-MB-235) and human pancreas carcinoma (PANC-1) cell lines by using the SRB assay method. Most of the target compounds showed broad potent growth inhibition activity (\(\hbox {GI}_{50}\le ~0.1~\upmu \hbox {M}\)) against all the tested cancer cell lines compared with reference drug. The most promising active compounds in this series were 5c, 5d, 8c, 8d and 8f endowed with excellent antiproliferative activity.
Graphical abstract
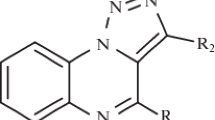
A new class of compounds was designed rationally by introducing tetrazole building block on N-4 piperazinyl group at C-7 position of quinolones core. The titled compounds were evaluated for their preliminary antibacterial and antiproliferative activities.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) has been increasing danger to public health attention due to the occurrence of various drug-resistant microbial infections by an inappropriate and irrational use of the currently marketed antimicrobial drugs [1, 2]. In 2014, WHO stated that the resistance to common bacteria has gaining alarming levels in several parts of the world [3]. In view of the growing threat from these drug-resistant Gram-positive and Gram-negative bacterial strains, there is an essential need to discover new drug candidates.
Quinolones are one of the most essential synthetic drug classes used for the treatment of community or hospital acquired infectious diseases in view of their excellent antibacterial activity with minimum side effects (Fig. 1). Quinolones successfully restrain the synthesis of DNA and functionally exert their effect by inhibition of two type bacterial topoisomerases II, namely DNA gyrase and topoisomerase IV [4]. Among the quinolones, fluoroquinolones (FQs) are powerful antibiotics with a broad spectrum of antibacterial activity and these are commonly used to treat a range of related bacterial infections like urinary tract, respiratory, gastrointestinal, chronic osteomyelitis and sexually transmitted diseases [5]. Moreover, some congeners of fluoroquinolone (FQs) family exhibited antiproliferative activity. For instance, ciprofloxacin (CP) showed antiproliferative and apoptosis-inducing activities on prostate and bladder cancer cells [6,7,8,9]. In addition, fleroxacin, ofloxacin and levofloxacin also showed to inhibit the growth of transitional cell bladder carcinoma cell lines [10,11,12].
Structure–activity relationship (SAR) studies of quinolones revealed that modification at the C-7 position with an additional functional moiety was reported to be greatly influence their antibacterial potency, spectrum and safety [13, 14]. Thus, several hybrid quinolone analogues derived by modification at C-7 position showed stronger antitumor [15] antibacterial or antitubercular [16, 17] activity as well as increased lipophilicity compared with the parent quinolones. Above SAR studies concluded that modification of basic group of the C-7 position leads to enhancement of antibacterial potency as well as antitumor potency.
Similarly, tetrazoles are privileged heterocyclic scaffolds and appear in many drugs such as, valsartan or losartan, that are nonpeptidic angiotensin-II-receptor blockers and cilostazol, which is a phosphodiesterase inhibitor. Their structures are showed in Fig. 2 [18]. Medicinal chemistry has led to the use of tetrazoles in pharmaceuticals as lipophilic spacers and carboxylic acid surrogates [19]. Bioisosteric replacement of a functional moiety (i.e., tetrazole replacing a carboxylic acid group) is a widely used known strategy in medicinal chemistry for the discovery of more selective and potent drug candidates related to a parent drug [20]. In addition, tetrazole derivatives have displayed pharmacological and biological properties such as antiviral, antibacterial, antifungal, antiallergic, antiulcer, anticonvulsant, anti-inflammatory and antitubercular activities [21,22,23].
In view of the previous rationale and in continuation of our research program to develop new potential biological active compounds, a hybrid pharmacophoric approach [24,25,26] was adopted in which modification at C-7 position on the quinolone core and substituted tetrazoles were combined into a one hybrid structure possessing improved biological activity. The present work describes the synthesis of a new series of ciprofloxacin derivatives (5a–g) and pipemidic (8a–g) derivatives.
Results and discussion
Chemistry
Target compounds 5a–g and 8a–g were obtained in a two-step synthesis as depicted in Schemes 1 and 2. The synthesis of 1-substituted 5-mercaptotetrazoles 2a–g derivatives were obtained by the reaction of \(\hbox {NaN}_{3}\) with isothiocyanates 1a–g (Scheme 1) in water at \(80\,{^{\circ }}\hbox {C}\). On the other hand, ciprofloxacin 3 and pipemidic acid 6 were treated with 3-chloropropionyl chloride and triethylamine in dichloromethane yielded compounds 7-(4-(3-chloropropanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4 and 2-(4-(3-chloropropanoyl)piperazin-1-yl)-8-ethyl-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid 7 in 75%.
Target compounds ciprofloxacin derivatives 5a–g and pipemidic acid derivatives 8a–g were prepared by S-alkylation of 1-substituted 5-mercaptotetrazoles with compounds 4 and 7 using triethylamine in ethanol under reflux conditions to afford products in 82 to 92% yield (Table 1, Scheme 2). The target compounds were confirmed by \(^{1}\hbox {H-NMR}\), \(^{13}\)C NMR and mass spectrometry. The NMR data for compounds 5a–g and 8a–g showed a characteristic pattern for all products: a characteristic peak S-CH \(_{2}\) appeared as a triplet range at \(\delta \) = 3.2–3.6 ppm, whereas \(^{13}\)CNMR data showed a characteristic S-CH\(_{2}\) signal at \(\delta \) = 34.2–36.5 ppm.
Biology
Antibacterial activity
A total of fourteen novel analogues (5a–g and 8a–g) were synthesized and tested for antibacterial activity against six microorganisms, out of which three are Gram-positive (Bacillus subtilis, Bacillus megaterium, Micrococcus luteus) and the other three are Gram-negative (Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa) bacterial strains and were compared against ciprofloxacin, streptomycin B and pipemidic acid. The zone of inhibition (summarized in Table 2) of all tested compounds 5a–g and 8a–g showed similar activity compared to the reference drugs. The antimicrobial activity profile of the synthesized compounds revealed that they could be divided into active and moderately active based on antibacterial data against Gram-positive and Gram-negative bacterial strains. The compounds of ciprofloxacin derivatives (5a–g) showed better zone of inhibition over the pipemidic acid derivatives (8a–g); this might be ascribed to its enhanced stability and increased clogP values than the pipemidic acid (8a–g) derivatives as shown in Table 1. It can be noted from the data that the lower lipophilicity (clogP = 0.66–2.45) of the analogues 8a–g of pipemidic acid (clog\(P=-\,2.47\)) seems to be related to a lower antimicrobial activity, whereas the higher lipophilicity of the analogues 5a–g (clogP = 2.45–4.21) of ciprofloxacin (clogP = 1.63) appears to be positively correlated to antimicrobial activity.
Though the activity profile of active compounds 5a–g was effective on almost all tested strains, one of the bacterial strains, P. aeruginosa, was resistant to compounds 5a–c and 8a–g; however, compounds 5d–g exhibited moderate zone of inhibition against bacterial strain P. aeruginosa. Among all examined compounds the best activity profile was found for compounds 5e and 5f. Compound 8g did not display zone of inhibition activity against all the tested the bacterial strains. It is evident from Table 2 that compounds having an electron-withdrawing group (e.g., trifluoromethyl, fluoro, bromine) on the tetrazole ring of ciprofloxacin derivatives exhibited good zone inhibition against all the bacterial strains compared to the standard ciprofloxacin. The remaining compounds were exhibited by a smaller zone of inhibition against all the bacterial strains. In view of this preliminary zone of growth inhibition data, only ciprofloxacin derivatives 5a–g were further analyzed for their minimal inhibitory concentrations (MIC \(\upmu \)g/mL) at the selected microbial strains.
Generally, MIC is the minimum concentration of a drug that is required to arrest the growth of bacterium. In the present study, the MIC values of compounds, 5a–g, were determined by using the tube dilution method and the results are presented in Table 3. In general, most of the target compounds 5a–g exhibited considerable antibacterial activity against all the tested Gram-positive and Gram-negative bacterial strains except for compounds 5a, 5b and 5c that did not exhibit antibacterial activity against Gram-negative strain P. aeruginosa. All the compounds 5a–g showed similar promising activity (\(\mathrm{MIC}=15.6~\upmu \hbox {g}/\hbox {mL}\)) compared to ciprofloxacin (\(\mathrm{MIC}=7.8~\upmu \hbox {g}/\hbox {mL}\)), streptomycin B (\(\mathrm{MIC}=15.6~\upmu \hbox {g}/\hbox {mL}\)) and pipemidic acid (\(\mathrm{MIC}=31.2~\upmu \hbox {g}/\hbox {mL}\)).
Antiproliferative activity
In addition to antibacterial activity, the target hybrid compounds 5a–g and 8a–g were screened for their in vitro antiproliferative activity against a panel of three different human cancer cell lines, namely cervix (SiHa), breast (MDA-MB-231) and pancreatic carcinoma cell lines by using the sulforhodamine B (SRB) assay method [25, 27]. Tamoxifen and DMSO were used as positive and negative controls, respectively. The \(\hbox {GI}_{50}\) values (\(\hbox {GI}_{50}\) = molar concentration of a test compound that inhibits 50% net cell growth) are listed in Table 4. The antiproliferative activity (\(\hbox {GI}_{50}\) values expressed in \(\upmu \hbox {M}\) concentration) results was exhibited for the synthesized ciprofloxacin analogues (5a–g) and pipemidic acid analogues (8a–g) after 48 h of incubation time. Among the 14 compounds synthesized, 5 against SiHa cancer cell line, 11 against MDA-MB-231 and 1 against PANC-1 cancer cell lines exhibited greater inhibition growth than tamoxifen. Compounds 5c, 5d, 8c, 8d and 8f showed twofold greater activity than tamoxifen (\(\hbox {GI}_{50}=0.12~\upmu \hbox {M}\)), as evidenced by \(\hbox {GI}_{50}\) values of 0.06–0.08 \(\upmu \)M against the SiHa cancer cell line. On the other hand, compounds 5a, 5c–5 g, 8a, 8b, 8d–8f showed three to tenfold greater activity (\(\hbox {GI}_{50}\) = 0.08–0.02 \(\upmu \hbox {M}\)) than tamoxifen (\(\hbox {GI}_{50}=0.24~\upmu \hbox {M}\)) against MDA-MB-231 cancer cell line, whereas the only compound 8d showed twofold greater activity (\(\hbox {GI}_{50}=0.07~\upmu \hbox {M}\)) than tamoxifen (\(\hbox {GI}_{50}=0.15~\upmu \hbox {M}\)) against PANC-1 cancer cell line. Overall, 8d showed the most potent activity against the tested all cancer cell lines.
From structure–activity relationship (SAR) point of view, different substituents were employed on the tetrazole moiety attached to ciprofloxacin (5a–g) and pipemidic acid (8a–g) derivatives to investigate antiproliferative activity. Tetrazole has different substituents at position 1, namely ethyl, benzyl, phenyl and substituted phenyl ring such as 4-bromo, 4-trifluoro methyl, 2,-3,-4-trifluoro and 3,-4,-5-trimethoxy phenyl groups. The resulting compounds were tested for their growth inhibition effect. Further, the target compounds are divided into two series: one group (a–c) with aliphatic, aromatic and benzylic substituents on the tetrazole where a \(\pi \)-electron cloud is absent, conjugated or not conjugated with tetrazole; and another group with aromatic substitution, namely derivatives (d–g) to be compared with the corresponding b (phenyl) as reference.
Compounds 5b and 8b bearing an un-substituted phenyl moiety on the tetrazole ring showed growth inhibition (\(\hbox {GI}_{50}\) values) at concentrations ranges from 0.085 to \(1.15~\upmu \hbox {M}\) against various tested cancer cell lines, whereas compounds 5d–g and 8d–g, bearing different substituents on the phenyl linked to the tetrazole ring, showed enhanced growth inhibition (\(\hbox {GI}_{50}=0.066\)–\(1.3~\upmu \hbox {M}\)) against various tested cancer cell lines. Benzyltetrazole linked to ciprofloxacin analogue 5c showed a potent activity against SiHa and MDA-MB-231 cell lines, while benzyltetrazole linked to pipemidic acid analogue 8c showed a potent activity against only SiHa cell line. Compounds 5d and 8d bearing bromine group (electron-withdrawing group) on phenyl ring attached to tetrazole showed enhancement of growth inhibition activity against all the tested cancer cell lines. Electron donating groups were detrimental for the pipemidic acid system (8g), but not for the ciprofloxacin derivatives where 5g is even more active than un-substituted phenyl derivative 5b.
Conclusion
In conclusion, a series of novel ciprofloxacin (5a–g) and pipemidic acid (8a–g) analogues were synthesized and evaluated for their in vitro antibacterial and antiproliferative activities. These preliminary investigations showed that a modification on the N-4 piperazinyl group at C-7 position of ciprofloxacin and pipemidic acid can influence biological activity. Most of the target compounds showed moderate to significant antimicrobial activity. Among them, compounds of all ciprofloxacin derivatives 5a–g showed significant improved activity compared to the corresponding reference drugs. In addition, all the target compounds evaluated for their in vitro antiproliferative studies. The preliminary antiproliferative studies revealed that compounds 5c, 5d, 8c and 8f displayed significant potent antiproliferative activity with GI\(_{50}\) \(\le \) 0.1 \(\upmu \)M against all the SiHa and MDA-MD-231 cancer cell lines compared to Tamoxifen. Most significantly, compounds 8d showed the broad spectrum of antiproliferative activity against all the tested cancer cell lines.
Experimental
Chemistry
General
All the reagents and starting materials were obtained from commercial sources and were used without further purification. The progress of reactions was determined by analytical thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated plates, and a UV lamp and \(\hbox {I}_{2}\) stain for visualization of the TLC plates. Column chromatography was done using Merck 60–120 sized mesh silica gel using chloroform and methanol as eluents. \(^{1}\)HNMR spectra (300 and 500 MHz) and \(^{13}\hbox {C}\) NMR spectra (75 and 126 MHz) were recorded on a Bruker Avance spectrometer using CDCl\(_{3}\) or DMSO-\(\hbox {d}_{6 }\) as solvents and TMS as internal standard. Chemical shifts were reported in parts per million (ppm, \(\delta )\) downfield from tetramethylsilane. The following abbreviations are used for NMR signals: s = singlet, br s = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets. ESI (HRMS) spectra were recorded on “High Resolution QSTAR XL hybrid MS/ MS system using methanol as a solvent. Melting points were recorded on a Buchi R-535 apparatus and are uncorrected.
Synthetic procedures and spectroscopic data
Procedure for the synthesis of 7-(4-(3-chloropropanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4- oxo-1,4-dihydroquinoline-3-carboxylic acid ( 4 )
Ciprofloxacin 3 (2.5 g, 7.54 mmol) and triethylamine (1.3 mL, 9 mmol) were added in 30 mL of dry \(\hbox {CH}_{2}\hbox {Cl}_{2}\) at 0 \({^{\circ }}\mathrm{C}\), after stirring for 10 min 3-chloropropionyl chloride (0.87 mL, 9.05 mmol) was added drop-wise under stirring, and the reaction mixture was stirred for about 1.2 h at room temperature. After the completion of reaction (confirmed by TLC), the reaction mixture was extracted with \(\hbox {CH}_{2}\hbox {Cl}_{2 }\) (\(40~\hbox {mL}~\times \) 3 times). The organic layers were collected, washed with saturated brine solution, dried over anhydrous \(\hbox {MgSO}_{4}\) and concentrated in vacuo. The remaining residue was washed with excess diethyl ether and purified by silica gel chromatography (\(\hbox {CHCl}_{3}\)/MeOH 40:1) to yield desired product.
Procedure for the synthesis of 2-(4-(3-chloropropanoyl)piperazin-1-yl)-8-ethyl-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 7 )
Pipemidic acid 7 (3.0 g, 9.90 mmol) and triethylamine (2.06 mL, 14.8 mmol) were added in 35 mL of dry \(\hbox {CH}_{2}\hbox {Cl}_{2}\) at \(0\,~{^{\circ }}\hbox {C}\), after stirring for 10 min 3-chloropropionyl chloride (1.14 mL, 11.88 mmol) was added drop-wise under stirring, and the reaction mixture was stirred for about 1.2 h at room temperature. After the completion of reaction (confirmed by TLC), the reaction mixture was extracted with \(\hbox {CH}_{2}\hbox {Cl}_{2 }\) (\(40~\hbox {mL}~\times ~3\) times). The organic layers were collected, washed with saturated brine solution, dried over anhydrous \(\hbox {MgSO}_{4}\) and concentrated in vacuo. The remaining residue was washed with excess diethyl ether and purified by silica gel chromatography \((\hbox {CHCl}_{3}\)/MeOH 35:1) to yield the desired product.
Procedure for the synthesis of 1-cyclopropyl-6-fluoro-4-oxo-7-(4-(3-((1-phenyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid ( 5b )
To a solution of substituted 1-phenyl-1H-tetrazole-5-thiol 2b (1.123 mmol) in ethanol (4 mL) and triethylamine (0.23 mL, 1.685 mmol) were added and stirred for 10 min at rt under N\(_{2}\) atmosphere. To the resultant mixture, compound 4 (1.123 mmol) was added and stirred for 3 h at 80 \({^{\circ }}\hbox {C}\). After the reaction was complete, as indicated by TLC, ethanol was evaporated in vacuo. The reaction mixture was extracted with \(\hbox {CH}_{2}\hbox {Cl}_{2 }\) (\(20~\hbox {mL}~\times ~3\) times). The organic layers were collected, washed with saturated brine solution, dried over anhydrous \(\hbox {MgSO}_{4}\) and concentrated in vacuo. The resulting crude material was purified by column chromatography (\(\hbox {CHCl}_{3}\)/MeOH 33:1) to yield desired product. All other remaining target compounds were prepared as similar manner.
Procedure for the synthesis of 8-Ethyl-5-oxo-2-(4-(3-((1-phenyl-1H-tetrazol-5-yl)thio)propanoyl) piperazin-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8b )
To a solution of substituted 1-phenyl-1H-tetrazole-5-thiol 2b (1.123 mmol) in ethanol (4 mL) and triethylamine (0.156 mL, 1.345 mmol) were added and stirred for 10 min at rt under N\(_{2}\) atmosphere. To the resultant mixture, compound 4 (1.123 mmol) was added and stirred for 3 h at \(80\,{^{\circ }}\hbox {C}\). After the reaction was complete, as indicated by TLC, ethanol was evaporated in vacuo. The reaction mixture was extracted with \(\hbox {CH}_{2}\hbox {Cl}_{2 }\) (\(20~\hbox {mL}~\times \) 3 times). The organic layers were collected, washed with saturated brine solution, dried over anhydrous \(\hbox {MgSO}_{4}\) and concentrated in vacuo. The resulting crude material was purified by column chromatography (\(\hbox {CHCl}_{3}\)/MeOH 30:1) to get the title compound. All other remaining target compounds were prepared as similar manner.
1-Cyclopropyl-7-(4-(3-((1-ethyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ( 5a )
Yield 88%, White crystalline solid, m.p. 156–157 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.88 (br s, 1H), 8.77 (s, 1H), 8.05 (s, 1H), 7.35 (s, 1H), 4.27 (q, J = 7.5 Hz, 2H), 4.01–3.70 (m, 4H), 3.63 (t, J = 6.5 Hz, 2H), 3.58–3.51 (m, 1H), 3.42–3.26 (m, 4H), 3.07 (t, J = 6.5 Hz, 2H), 1.52 (t, J = 7.5 Hz, 3H), 1.31–1.14 (m, 4H); \(^{ 13}\)C NMR (126 MHz, CDCl\(_{3}) \quad \delta \) 177.0, 169.0, 166.8, 147.6, 139.0, 128.8, 126.9, 120.3, 112.6, 108.2, 105.2, 49.9, 45.0, 42.6, 35.3, 33.2, 28.4, 14.2, 8.3; HRMS (ESI) calcd for \(\hbox {C}_{23}\hbox {H}_{27}\hbox {O}_{4}\hbox {N}_{7}\)FS, 516.18238 \([\mathrm{M}+\mathrm{H}]^{+}\); found, 516.18077.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(3-((1-phenyl-1H-tetrazol-5-yl)thio) propanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid ( 5b )
Yield 90%, White solid, m.p. 234–235 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.91 (br s, 1H), 8.72 (s, 1H), 7.98 (d, J = 3.2 Hz, 1H), 7.61–7.51 (m, 5H), 7.37 (s, 1H), 4.00–3.79 (m, 4H), 3.70 (t, J = 6.5 Hz, 2H), 3.59 (s, 1H), 3.42–3.29 (m, 4H), 3.09 (t, J = 6.5 Hz, 2H), 1.44–1.37 (m, 2H), 1.28–1.16 (m, 2H); \(^{13}\)C NMR (75 MHz, CDCl\(_{3}\) + DMSO-d\(_{6}) \quad \delta \) 173.8, 171.6, 159.6, 152.4, 150.0, 143.7, 138.1, 134.3, 128.7, 125.0, 117.0, 112.6, 110.3, 54.2, 49.7, 40.4, 37.8, 33.5, 12.9; HRMS (ESI) calcd for \(\hbox {C}_{27}\hbox {H}_{27}\hbox {O}_{4}\hbox {N}_{7}\)FS, 564.18238 [M + H]\(^{+}\); found, 516.18103.
7-(4-(3-((1-Benzyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl) -1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ( 5c )
Yield 92%, Light brown solid, m.p. 181–182 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.91 (br s, 1H), 8.75 (s, 1H), 8.02 (d, 1H), 7.45–7.18 (m, 6H), 5.41 (s, 2H), 3.99–3.64 (m, 5H), 3.58 (t, J = 5.7 Hz, 2H), 3.42–3.32 (m, 4H), 3.03 (t, J = 5.7 Hz, 2H), 1.45–1.35 (m, 2H), 1.15–1.25 (m, 2H);\(^{ 13}\)C NMR (75 MHz, CDCl\(_{3}\) + DMSO-\(\hbox {d}_{6 }\)) \(\delta \) 177.0, 169.0, 166.8, 154.2, 151.9, 147.5, 145.2, 139.0, 132.7, 129.1, 128.8, 128.2, 126.9, 112.7, 108.2, 105.1, 51.0, 49.7, 45.0, 41.47, 35.3, 33.1, 28.8, 8.2; HRMS (ESI) calcd for \(\hbox {C}_{28}\hbox {H}_{29}\hbox {O}_{4}\hbox {N}_{7}\)FS, 578.19803 [M + H]\(^{+}\); found, 578.19683.
7-(4-(3-((1-(4-Bromophenyl)-1H-tetrazol-5-yl)thio)propanoyl) piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ( 5d )
Yield 88%, White solid, m.p. 209–210 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.89 (br s, 1H), 8.78 (s, 1H), 8.06 (s, 1H), 7.71 (d, J = 8.6 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 7.37 (s, 1H), 4.04–3.72 (m, 4H), 3.69 (t, J = 6.2 Hz, 2H), 3.56 (s, 1H), 3.43–3.24 (m, 4H), 3.10 (t, J = 6.2 Hz, 2H), 1.45–1.34 (m, 2H), 1.24–1.14 (m, 2H); \(^{13}\)C NMR (126 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 175.8, 167.9, 165.6, 153.6, 146.6, 138.0, 131.9, 126.3, 124.3, 122.9, 118.7, 110.9, 106.6, 104.7, 48.6, 43.7, 40.2, 34.5, 31.8, 28.4, 27.6, 7.0; HRMS (ESI) calcd for \(\hbox {C}_{27}\hbox {H}_{26}\hbox {O}_{4}\hbox {N}_{7}\)BrFS, 642.09289 [M + H]\(^{+}\); found, 642.09208.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(3-((1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid ( 5e )
Yield 84%, White solid, m.p. 195–196 \({^{\circ }}\mathrm{C}\); \(^{1}\hbox {H}\) NMR (300 MHz, \(\hbox {DMSO-d}_{6}) \quad \delta \) 14.86 (br s, 1H), 8.63 (s, 1H), 7.88 (s, 1H), 7.83–7.69 (m,2H), 7.49–7.39 (m, 3H) 4.04–3.66 (m, 7H), 3.48 (br s, 1H), 3.46–3.24 (m, 4H), 3.09 (t, J = 5.8 Hz, 2H), 1.48–1.29 (m, 2H), 1.27–1.08 (m, 2H); \(^{ 13}\)C NMR (75 MHz, DMSO-\(\hbox {d}_{6}) \quad \delta \) 175.1, 164.9, 163.4, 153.4, 146.1, 143.6, 137.6, 134.8, 126.4, 126.0, 125.6, 122.9, 110.0, 109.8, 105.8, 104.6, 48.09, 47.7, 43.1, 39.6, 34.2, 31.2, 27.3, 6.4; HRMS (ESI) calcd for \(\hbox {C}_{28}\hbox {H}_{26}\hbox {O}_{4}\hbox {N}_{7}\hbox {F}_{4}\)S, 632.16976 [M + H]\(^{+}\); found, 632.16920.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(3-((1-(2,3,4-trifluorophenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid ( 5f )
Yield 86%, Light brown solid, m.p. 172–173 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3}\) + DMSO-\(\hbox {d}_{6 }\)) \(\delta \) 15.00 (br s, 1H), 8.77 (s, 1H), 8.02 (s, 1H), 7.44–7.33 (m, 1H), 7.30–7.14 (m, 2H), 4.01–3.71 (m, 4H), 3.68 (t, J = 6.0 Hz, 2H), 3.67–3.57 (m, 1H), 3.43–3.32 (m, 4H), 3.07 (t, J = 6.0 Hz, 2H) 1.49–1.35 (m, 2H), 1.27–1.15 (m, 2H); \(^{13}\)C NMR (101 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 177.7, 166.0, 165.6, 161.2, 160.9, 157.7, 155.4, 149.1, 148.7, 137.7, 128.9, 126.9, 111.1, 109.6, 46.5, 45.0, 43.8, 33.2, 28.7, 14.7, 8.6; HRMS (ESI) calcd for \(\hbox {C}_{27}\hbox {H}_{26}\hbox {O}_{4}\hbox {N}_{7}\hbox {F}_{4}\hbox {S}\), 618.15403 [M + H]\(^{+}\); found, 618.15322.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-(3-((1-(3,4,5-trimethoxyphenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid ( 5g )
Yield 90%, White solid, m.p. 215–216 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.90 (br s, 1H), 8.73 (s, 1H), 8.00 (dd, J = 12.8, 5.4 Hz, 1H), 7.37 (s, 1H), 6.78 (s, 2H), 3.89 (s, 9H), 3.87–3.72 (m, 4H), 3.70 (t, J = 6.5 Hz, 2H), 3.57 (s, 1H), 3.42–3.28 (m, 4H), 3.09 (t, J = 6.5 Hz, 2H), 1.47–1.34 (m, 2H), 1.30–1.18 (m, 2H); \(^{13}\hbox {C}\) NMR (126 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 176.9, 168.9, 166.7, 154.5, 153.9, 147.5, 139.2, 138.9, 128.8, 126.9, 120.0, 112.4, 108.0), 105.2, 101.5, 61.0, 56.5, 45.0, 41.4, 35.4, 33.1, 28.6, 8.2; HRMS (ESI) calcd for \(\hbox {C}_{30}\hbox {H}_{33}\hbox {O}_{7}\hbox {N}_{7}\hbox {FS}\), 654.21407 [M + H]\(^{+}\); found, 654.21395.
8-Ethyl-2-(4-(3-((1-ethyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8a )
Yield 90%, White solid, m.p. 191–192 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.48 (br s, 1H), 9.31 (d, J = 3.5 Hz, 1H), 8.67 (d, J = 1.9 Hz, 1H), 4.35 (q, J = 7.2 Hz, 2H), 4.27 (q, J = 7.3 Hz, 2H), 4.06 (q, J = 7.6 Hz, 6H), 3.91–3.77 (m, 8H), 3.72 (q, J = 7.2 Hz, 1H), 3.64 (t, J = 6.3 Hz, 2H), 3.08 (t, J = 6.3 Hz, 2H), 1.51 (t, J = 7.6 Hz, 2H), 1.48 (t, J = 7.2 Hz, 2H); \(^{13}\)C NMR (126 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 177.6, 169.2, 166.0, 161.2, 160.8, 155.4, 153.6, 149.1, 128.9, 126.9, 111.0, 109.6, 46.5, 43.9, 42.6, 33.4, 28.4, 14.7, 14.2; HRMS (ESI) calcd for \(\hbox {C}_{20}\hbox {H}_{26}\hbox {O}_{4}\hbox {N}_{9}\)S, 488.18230 [M + H]\(^{+}\); found, 488.18203.
8-Ethyl-5-oxo-2-(4-(3-((1-phenyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8b )
Yield 90%, White solid, m.p. 213–214 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.48 (br s, 1H), 9.32 (s, 1H), 8.67 (s, 1H), 7.63–7.45 (m, 5H), 4.35 (q, J = 7.2 Hz, 2H), 4.18–3.88 (m, 4H), 3.82–3.75 (m, 2H), 3.68 (t, J = 6.5 Hz, 2H), 3.64–3.58 (m, 2H), 3.10 (t, J = 6.5 Hz 2H), 1.50 (t, J = 7.2 Hz, 3H); \(^{13}\)C NMR (75 MHz, CDCl\(_{3}\) + DMSO-\(\hbox {D}_{6 }\)) \(\delta \) 175.7, 167.4, 163.9, 159.2, 158.7, 153.6, 152.9, 148.6, 131.6, 128.4, 122.3, 108.5, 107.4, 44.5, 42.8, 42.1, 31.2, 26.9, 12.9; HRMS (ESI) calcd for \(\hbox {C}_{24}\hbox {H}_{26}\hbox {O}_{4}\hbox {N}_{9}\)S, 536.18230 [M + H]\(^{+}\); found, 536.18082.
2-(4-(3-((1-Benzyl-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-8-ethyl-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8c )
Yield 89%, White solid, m.p. 220–221 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (500 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.49 (br s, 1H), 9.27 (s, 1H), 8.67 (s, 1H), 7.44–7.24 (m, 5H), 5.43 (s, 2H), 4.36 (q, J = 5.4 Hz, 2H), 4.27–3.68 (m, 8H), 3.59 (t, J = 6.0 Hz, 2H), 3.02 (t, J = 6.0 Hz, 2H), 1.50 (t, J = 5.4 Hz, 3H); \(^{13}\)C NMR (75 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 177.6, 169.2, 166.0, 161.0, 155.4, 154.2, 149.1, 132.7, 129.1, 128.2, 127.0, 111.0, 109.5, 51.0, 46.5, 43.9, 33.2, 28.7, 14.7; HRMS (ESI) calcd for \(\hbox {C}_{25}\hbox {H}_{28}\hbox {O}_{4}\hbox {N}_{9}\hbox {S}\), 550.19795 [M + H]\(^{+}\); found, 550.19630.
2-(4-(3-((1-(4-Bromophenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-8-ethyl-5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8d )
Yield 88%, White solid, m.p. 195–196 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (500 MHz, DMSO) \(\delta \) 14.73 (br s, 1H), 9.21 (s, 1H), 8.91 (s, 1H), 7.94–7.79 (m, 2H), 7.67–7.59 (m, 2H), 4.39 (q, J = 6.4 Hz, 2H), 4.07–3.69 (m, 8H), 3.43 (t, J = 6.0 Hz, 2H), 2.97 (t, J = 6.0 Hz, 2H), 1.35 (t, J = 6.4 Hz, 3H); \(^{13}\)C NMR (75 MHz, CDCl\(_{3}\) + DMSO-\(\hbox {d}_{6 }\)) \(\delta \) 175.2, 174.3, 167.1, 163.6, 158.9, 158.3, 153.3, 152.9, 148.7, 131.1, 124.5, 121.8, 107.9, 107.6, 44.1, 41.9, 41.6, 39.1, 36.1, 30.7, 26.9, 12.5; HRMS (ESI) calcd for \(\hbox {C}_{24}\hbox {H}_{25}\hbox {O}_{4}\hbox {N}_{9}\hbox {BrS}\), 614.09281 [M + H]\(^{+}\); found, 614.09235.
8-Ethyl-5-oxo-2-(4-(3-((1-(4-(trifluoromethyl)phenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8e )
Yield 82%, White solid, m.p. 185–186 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.48 (br s, 1H), 9.29 (s,1 H), 8.68 (s, 1H), 7.90–7.73 (m, 4H), 4.35 (q, J = 7.1 Hz, 2H), 4.24–3.88 (m, 4H), 3.90–3.56 (m, 6H), 3.10 (t, J = 6.1 Hz, 2H), 1.50 (t, J = 7.1 Hz, 3H); \(^{13}\)C NMR (75 MHz, \(\hbox {CDCl}_{3}\,+\,\)DMSO-d\(_{6}\)) \(\delta \) 177.0, 168.5, 165.4, 160.5, 160.1, 154.8, 154.2, 148.8, 135.8, 126.5, 123.4, 110.2, 108.9, 45.9, 44.3, 43.3, 32.7, 28.2, 14.1; HRMS (ESI) calcd for \(\hbox {C}_{25}\hbox {H}_{25}\hbox {O}_{4}\hbox {N}_{9}\hbox {F}_{3}\)S, 604.16968 [M + H]\(^{+}\); found, 604.16888.
8-Ethyl-5-oxo-2-(4-(3-((1-(2,3,4-trifluorophenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8f )
Yield 86%, Light brown solid, m.p. 177–178 \({^{\circ }}\hbox {C}\); \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.54 (br s, 1H), 9.28 (s, 1H), 8.66 (s, 1H), 8.07–7.02 (m, 2H), 4.35 (q, J = 7.5 Hz, 2H) 4.21–3.92 (m, 4H), 4.23–3.60 (m, 6H), 3.26 (t, J = 6.7 Hz, 2H), 0.98 (t, J = 7.5 Hz, 3H); \(^{13}\)C NMR (75 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 177.0, 168.7, 166.8, 155.2, 151.9, 147.5, 145.3, 128.8, 126.9, 112.6, 112.3, 108.1, 105.1, 49.6, 45.0, 41.5, 35.3, 27.0, 10.1; HRMS (ESI) calcd for \(\hbox {C}_{24}\hbox {H}_{23}\hbox {O}_{4}\hbox {N}_{9}\hbox {F}_{3}\)S, 590.15392 [M + H]\(^{+}\); found, 590.15307.
8-Ethyl-5-oxo-2-(4-(3-((1-(3,4,5-trimethoxyphenyl)-1H-tetrazol-5-yl)thio)propanoyl)piperazin-1-yl)-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid ( 8g )
Yield 92%, White solid, m.p. 204–205 \({^{\circ }}\mathrm{C}\); \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3}) \quad \delta \) 14.48 (br s, 1H), 9.29 (s, 1H), 8.68 (s, 1H), 6.78 (s, 2H), 4.34 (t, J = 6.8 Hz, 2H), 4.17–3.96 (m, 6H), 3.90 (s, 9H), 3.83–3.71 (m, 2H), 3.69 (t, J = 6.0 Hz, 2H), 3.10 (t, J = 6.0 Hz, 2H), 1.50 (t, J = 6.8 Hz, 3H); \(^{13}\)C NMR (75 MHz, CDCl\(_{3}\) + CD\(_3\)OD) \(\delta \) 179.1, 171.3, 168.1, 162.5, 162.3, 155.1, 151.3, 140.3, 130.2, 111.4, 110.7, 103.2, 62.2, 57.8, 48.1, 45.4, 34.5, 29.9, 15.8; HRMS (ESI) calcd for \(\hbox {C}_{27}\hbox {H}_{32}\hbox {O}_{7}\hbox {N}_{9}\)S, 626.21399 \([\hbox {M}~+~\hbox {H}]^{+}\); found, 626.21341.
Experimental biology
Antiproliferative activity
The antiproliferative activity of the prepared tetrazole containing ciprofloxacin and pipemidic acid hybrids was evaluated on the basis of measurement of in vitro growth of tumor cell lines in 96 well plates by SRB cell proliferation assay [25, 27] using tamoxifen as a standard control. Cancer cell lines utilized for antiproliferative assay, namely SiHa, MDA-MB-231 and PANC-1 were procured from the American Type Culture Collection. The required cell lines were grown in the specific growth medium (Dulbecco’s modified Eagle’s medium) containing 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% \(\hbox {CO}_{2}\) at \(37\,{^{\circ }}\hbox {C}\), and these subconfluent cells were trypsinized from T25 flasks/60-mm dishes and seeded in 96-well plates. A protocol of 48-h continuous drug exposure was used and evaluated by SRB cell proliferation assay was used to estimate cell viability or growth. The \(\hbox {GI}_{50 }\) values (50% inhibitory concentration) were calculated from the plotted absorbance data for the dose response curves. \(\hbox {GI}_{50}\) values (in \(\upmu \)M) are expressed as the average of two independent experiments.
Antimicrobial activity
The antimicrobial activity [25, 28] of the synthesized compounds was evaluated by two methods (1) zone of growth inhibition by agar well diffusion method (2) tube dilution method to determine minimum inhibitory concentration (MIC). The antimicrobial activity was performed against six bacterial strains. Among the six bacterial strains tested, three are Gram-positive (Bacillus subtilis, Micrococcus luteus and Bacillus megatherium) and the other three are Gram-negative (Escherichia coli, Salmonella typhi andPseudomonas aeruginosa). Also, in the present study, three standards (ciprofloxacin, pipemidic acid and streptomycin B) were used as positive controls for comparative studies. All the compounds (synthesized and positive controls) were solubilized in DMSO at a concentration of 1 mg/mL. In order to assess any DMSO effect, a well was loaded with DMSO serving as negative control.
Zone of growth inhibition test
Initially, the media required for bacterial growth, and the Petri dishes used for performing activity were autoclaved at \(121~{^{\circ }}\hbox {C}\) for 15 min. Later, the sterilized media was poured in to Petri plates and kept aside for 30 min to solidify. After solidification, the plates were spread with 60 \(\upmu \)L of test inoculums using sterile cotton swabs. Wells were made with sterile cork borer, and in each well exactly 100 \(\upmu \)L of sample was loaded along with control and standard in separate wells. Then, the plates were incubated at \(4~{^{\circ }}\hbox {C}\) for 20–30 min to allow the compounds to diffuse into the agar and then subsequently incubated at \(37~{^{\circ }}\hbox {C}\). After 24 h of incubation, the diameters of the zones of inhibition were measured in millimeters using a calibrated scale
Tube dilution method
Compounds exhibiting significant activity in the agar well diffusion method were selected for determining MIC (minimum inhibitory concentration). The concentrations of synthesized and positive controls were serially diluted concentration range from 1000 to \(3.90~\upmu \hbox {g}/\hbox {mL}\) were added to sterile test tubes, and one tube without drug serves as control. Then, the tubes were inoculated with 1.0 mL of tested culture having their growth absorbance of 0.2 optical densities at 540 nm. Later, the tubes were incubated at \(37~{^{\circ }}\hbox {C}\) for growth. After 12–16 h incubation time, the turbidity of each tube is visually observed with respect to control tube and the MIC values were noted where no visual observation of growth appeared.
Supporting information
Full experimental details, spectral data of the products, and \(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR spectra of all the new compounds can be found via the Supplementary content section of this article’s Web page.
References
Janin YL (2007) Antituberculosis drugs: ten years of research. Bioorg Med Chem 15:2479–2513. https://doi.org/10.1016/j.bmc.2007.01.030
Balsalobre LC, Dropa M (2014) An overview of antimicrobial resistance and its public health significance. Braz J Microbiol 45:1–5. https://doi.org/10.1590/S1517-83822014005000033
Morgan LSK, Becnel BL, Steffen D, Zechiedrich L (2009) Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob Agents Chemother 53:235–241. https://doi.org/10.1128/AAC.00665-08
Peraman R, Varma RV, Reddy YP (2015) Re-engineering nalidixic acid’s chemical scaffold: a step towards the development of novel anti-tubercular and anti-bacterial leads for resistant pathogens. Bioorg Med Chem Lett 25:4314–4319. https://doi.org/10.1016/j.bmcl.2015.07.071
Bartlett JG, Dowell SF, Mandell LA, File TM Jr, Musher DM, Fine MJ (2000) Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 31:347–382. https://doi.org/10.1086/313954
Kamat AM, DeHaven JI, Lamm DL (1999) Quinolone antibiotics: a potential adjunct to intravesical chemotherapy for bladder cancer. Urology 54:56–61. https://doi.org/10.1016/S0090-4295(99)00064-3
Aranha O, Grignon R, Fernandes N, McDonnell TJ, Wood DP, Sarkar FH (2003) Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol 22:787–794. https://doi.org/10.3892/ijo.22.4.787
Landuyt HW, Van K, Magerman GB (1990) The importance of the quinolones in antibacterial therapy. J Antimicrob Chemother 26:1–6. https://doi.org/10.1093/jac/26.suppl_D.1
El-Rayes BF, Grignon R, Aslam N, Aranha O, Sarkar FH (2002) Ciprofloxacin inhibits cell growth and synergises the effect of etoposide in hormone resistant prostate cancer cells. Int J Oncol 21:207–211. https://doi.org/10.3892/ijo.21.1.207
Seay TM, Peretsman SJ, Dixon PSJ (1996) Inhibition of human transitional cell carcinoma in vitro proliferation by fluoroquinolone antibiotics. Urol 155:757–762. https://doi.org/10.1016/S0022-5347(01)66516-9
Ebisuno S, Inagaki T, Kohjimoto Y, Ohkawa T (1997) The cytotoxic effect of fleroxacin and ciprofloxacin on transitional cell carcinoma in vitro. Cancer 80:2263–2267. https://doi.org/10.1002/(SICI)1097-0142(19971215)80
Yamakuchi M, Nakata M, Kawahara K, Kitajima I, Maruyama T (1997) New quinolones, ofloxacin and levofloxacin, inhibit telomerase activity in transitional cell carcinoma cell lines. Cancer Lett 119:213–219. https://doi.org/10.1016/S0304-3835(97)00269-3
Dang Z, Yang YS, Ji RY, Zhang SH (2007) New quinolones, ofloxacin and levofloxacin, inhibit telomerase activity in transitional cell carcinoma cell lines. Med Chem Lett 17:4523–4526. https://doi.org/10.1016/S0304-3835(97)00269-3
Shen LL, Mitscher LA, Sharma PN, Odonnell TJ, Chu DWT, Cooper CS, Rosen T, Pernet AG (1989) Mechanism of inhibition of DNA gyrase by quinolone antibacterials: a cooperative drug–DNA binding model. Biochemistry 28:3886–3894. https://doi.org/10.1021/bi00435a039
Sharma PC, Jain A, Jain S, Pahwa R, Yar MS (2010) Ciprofloxacin: review on developments in synthetic, analytical, and medicinal aspects. J Enzyme Inhib Med Chem 25:557–589. https://doi.org/10.3109/14756360903373350
Feng LL, Liu ML, Zhang S, Chai Y, Wang B, Zhang YB, Lv K, Guan Y, Guo HY, Xiao CL (2011) Synthesis and in vitro antimycobacterial activity of 8-OCH(3) ciprofloxacin methylene and ethylene isatin derivatives. Eur J Med Chem 46:341–348. https://doi.org/10.1016/j.ejmech.2010.11.023
Foroumadi A, Emami S, Mehni M, Moshafi MH, Shafiee A (2005) Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2–5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg Med Chem Lett 15:4536–4539. https://doi.org/10.1016/j.bmcl.2005.07.005
Butler RN, Katritzky AR, Rees CW, Scriven EFV (1996) Comprehensive heterocyclic chemistry II. Pergamon Press, Oxford, p 621
Herr RJ (2002) 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem 10:3379–3393. https://doi.org/10.1016/S0968-0896(02)00239-0
Matta CF, Arabi AA, Weaver DF (2010) The bioisosteric similarity of the tetrazole and carboxylate anions: clues from the topologies of the electrostatic potential and of the electron density. Eur J Med Chem 45:1868–1872. https://doi.org/10.1016/j.ejmech.2010.01.025
Shemyakina OA, Mal’kina AG, Albanov AI, Trofimov BA (2011) Regio- and stereodirection of addition of tetrazole to \(\alpha \),\(\beta \)-acetylenic \(\gamma \)-hydroxy nitrile: synthesis of 1- and 2-(Z)-(2-cyanoethenyl-1-hydroxyalkyl)tetrazoles. Chem Heterocycl Compd 47:464–469. https://doi.org/10.1007/s10593-011-0782-4
Kumar CNSSP, Parida DK, Santhoshi A, Kota AK, Sridhar B, Rao VJ (2011) Synthesis and biological evaluation of tetrazole containing compounds as possible anticancer agents. Med Chem Commun 2:486–492. https://doi.org/10.1039/C0MD00263A
Leyla Y, Zafer AK, Halide ET, Gülşen AÇ (2016) Novel tetrazole derivatives: synthesis, anticholinesterase activity and cytotoxicity evaluation. Turk J Biochem. https://doi.org/10.1515/tjb-2016-0207
Murty MSR, Raju P, Polepalli S, Jain N (2016) Synthesis and biological evaluation of novel resveratrol-oxadiazole hybrid heterocycles as potential antiproliferative agents. Med Chem Res 25:627–643. https://doi.org/10.1007/s00044-016-1514-1
Murty MSR, Katiki MR, Babu NJ, Garimella S, Polepalli S, Jain N, Buddana SK, Prakasham RS (2016) Synthesis and biological evaluation of novel tamoxifen-1,2,4-triazole conjugates. Mol Divers 20:687–703. https://doi.org/10.1007/s11030-016-9677-8
Murty MSR, Rao BR, Katiki MR, Nath LR, Anto RJ (2013) Synthesis of piperazinyl benzothiazole/benzoxazole derivatives coupled with 1,3,4-oxadiazole-2-thiol: novel hybrid heterocycles as anticancer agents. Med Chem Res 22:4980–4991. https://doi.org/10.1007/s00044-013-0510-y
Reddy MA, Jain N, Yada D, Kishore C, Vangala JR, Surendra PR, Addlagatta A, Kalivendi SV, Sreedhar B (2011) Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J Med Chem 54:6751–6760. https://doi.org/10.1021/jm200639r
Amsterdam D (1996) Susceptibility testing of antimicrobials in liquid medium. In: Lorian V, Baltimore MD (eds) Antibiotics in laboratory medicine, 4th edn. Williams & Wilikins, Baltimore, pp 52–111
Acknowledgements
The authors are thankful to the Director, Indian Institute of Chemical Technology, Hyderabad, for the encouragement, KD thankful to CSIR, New Delhi, India, for the award of research fellowships. We thank CSIR for financial support under the 12th Five Year plan projects “Affordable Cancer Therapeutics (ACT)” (CSC 0301) and “Small Molecules in Lead Exploration (SMiLE)” (CSC0111).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2017_9795_MOESM1_ESM.docx
SUPPORTING INFORMATION Full experimental details, spectral data of the products, and \(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR spectra of all the new compounds can be found via the Supplementary content section of this article’s Web page.
(DOCX 4,538 KB)
Rights and permissions
About this article
Cite this article
Dileep, K., Polepalli, S., Jain, N. et al. Synthesis of novel tetrazole containing hybrid ciprofloxacin and pipemidic acid analogues and preliminary biological evaluation of their antibacterial and antiproliferative activity. Mol Divers 22, 83–93 (2018). https://doi.org/10.1007/s11030-017-9795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9795-y