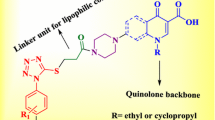
Abstract
Background
Quinolones are well known antibacterial chemotherapeutics. Furthermore, they were reported for other activities such as anticancer and urease inhibitory potential. Modification at C7 of quinolones can direct these compounds preferentially toward target molecules.
Methods
Different derivatives of ciprofloxacin by functionalization at the piperazinyl N-4 position with arylidenehydrazinecarbonyl and saturated heterocyclic-carbonyl moieties have been synthesized and characterized using different spectral and analytical techniques. The synthesized compounds were evaluated for anticancer, antibacterial, and urease inhibitory activities.
Results
Among the synthesized compounds derivatives 3f and 3g experienced a potent antiproliferative activity against the breast cancer BT-549 cell line, recording growth percentages of 28.68% and 6.18%, respectively. Additionally, compound 3g revealed a remarkable antitumor potential toward the colon cancer HCT-116 cells (growth percentage 14.76%). Activity of compounds 3f and 3g against BT-549 cells was comparable to doxorubicin (IC50 = 1.84, 9.83, and 1.29 µM, respectively). Test compounds were less active than their parent drug, ciprofloxacin toward Klebsiella pneumoniae and Proteus mirabilis. However, derivative 4a showed activity better than chloramphenicol against Klebsiella pneumoniae (MIC = 100.64 and 217.08 µM, respectively). Meanwhile, many of the synthesized compounds revealed a urease inhibitory activity greater than their parent. Compound 3i was the most potent urease inhibitor with IC50 of 58.92 µM, greater than ciprofloxacin and standard inhibitor, thiourea (IC50 = 94.32 and 78.89 µM, respectively).
Conclusion
This study provided promising derivatives as lead compounds for development of anticancer agents against breast and colon cancers, and others for optimization of urease inhibitors.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among bioactive molecules, fluoroquinolones represent an important drug class that made a revolution in the field of antibacterial chemotherapy. Thanks to their broad spectrum of activity and favorable pharmacokinetic profile, a wide range of contagious diseases could be managed using fluoroquinolone drugs including Gram-positive, Gram-negative, anaerobic, atypical, and mycobacterial infections [1, 2]. In addition to clinically used drugs that constitute different generations, several new fluoroquinolone derivatives with up-and-coming activity are now under clinical investigation [3, 4]. Other derivatives with potent antibacterial activity were also described in different researches, for example ciprofloxacin hydrazones such as compound I experienced anti-mycobacterial activity comparable to or more than isoniazid [5].
Quinolones were additionally reported for anticancer activity with the first member in clinical use, voreloxin, in addition to quarfloxin, which failed in phase III of clinical trials due to some pharmacokinetic problems [6,7,8]. Numerous other candidates with promising antitumor potential were reported in many literature studies [9]. Structural features for anticancer fluoroquinolones have been well determined, with the most important sites for modification at C3 carboxylic acid functionality and at position 7 of the ring core. Substitution at C7 exhibited a major influence on fluoroquinolone activity including preferential binding to bacterial type II topoisomerases, gyrase or topoisomerase IV, as well as physicochemical properties and hence, distribution and access to target molecules [10, 11]. Introducing aromatic or heteroaromatic moieties at position 7 can also increase the affinity of quinolone molecules toward mammalian enzymes and potentiate the antiproliferative tendency [12, 13]. Among the prepared compounds in this direction, hydrazone derivatives of ciprofloxacin and norfloxacin such as compounds II (QNT11), III and IV afforded potent antitumor activity [14,15,16].
On the other hand, urease had emerged as an attractive molecular target for different health benefits, with the advantage that it is not expressed by human host cells [17, 18]. This enzyme is produced by numerous microorganisms and represents a crucial factor for their growth and survival [19]. Urease (or urea amidohydrolase EC 3.5.1.5) is a nickel containing metalloenzyme that acts by converting urea into ammonia and carbamates, which in turn hydrolyzed to ammonia and carbonic acid with a net result of elevating pH of the medium [20,21,22]. Such catalytic activity plays a vital role for pathogenesis, growth, and maintenance of different microorganisms like Helicobacter pylori and Proteus mirabilis [23]. Helicobacter pylori urgently need urease to alkalinize surrounding medium in the gastric juice to achieve colonization. Moreover, urease was evidenced for prognosis and long lasting of H. pylori infection as well as its consequent peptic ulcer and even gastric carcinoma [24, 25]. Urease also rises pH of urine to a favorable level for growth of some pathogens and represents a leading cause for renal injury and urinary calculi during P. mirabilis and other urease positive bacterial infections [26]. Furthermore, urease was found to be a virulence factor in hepatic coma, encephalopathy in addition to different chronic diseases such as atherosclerosis and rheumatoid arthritis [27, 28]. Hence, hindrance of urease activity can be useful for cure of different infectious diseases and other health conditions. Targeting both bacteria and urease in the same time was proposed to be a good strategy for treatment of some GIT diseases [29]. Some fluoroquinolone drugs exhibited anti-urease activity such as norfloxacin [30], ciprofloxacin, sparafloxacin [31], metal complexes of sparafloxacin [32], ciprofloxacin capped nanoparticles [33] in addition to different ciprofloxacin analogs like compounds V and VI. Compound V experienced anti-urease along with anti P. mirabilis activities more than acetohydroxamic acid (AHA) and N-acetylciprofloxacin, respectively [34]. Meanwhile, compound VI showed urease inhibitory activity more than the parent drug, ciprofloxacin and thiourea as a standard inhibitor [35].
Based on the above findings, the present study aimed at the synthesis, characterization, and investigation of different C7 modified ciprofloxacin analogs, bearing urea like scaffold (Series I and II) that may be able to bind to urease and hence, can target both urease enzyme and urease producing bacteria. Also, compounds of Series I contain aryl moieties that may enhance anticancer activity. The synthesized compounds were tested for both anticancer and antibacterial potential. Moreover, urease inhibitory activity and molecular docking onto urease protein (PDB: 1E9Y) [36] have been carried out.
Materials and methods
Chemical synthesis and characterization
All chemicals used for preparation of the target compounds were of the commercially available analytical grade quality. Reaction progress was monitored using TLC (Kieselgel 60 G F254 precoated plates, E. Merck, Dermastadt, Germany). Spots were detected by exposure to UV lamp (Spectroline CM-10, Seattle, USA) at λ 254 and 365 nm. Melting points were determined on Stuart SMP1 electrothermal melting point apparatus (Stuart Scientific, Staffordshire, UK) and were uncorrected. IR Spectra were recorded with Bruker Alpha Platinum-ATR FTIR spectrometer (Bruker Corporation, Germany), applying attenuated total reflection technique (ATR) and were expressed as cm−1. 1H-NMR And 13C-NMR spectra were recorded on BRUKER Avance III400 MHz spectrophotometer (Bruker AG, Switzerland) at 400 MHz for 1H and 100 MHz fo 13C. TMS was used as an internal standard and CDCl3 or DMSO-d6 as a solvent. Chemical shift (δ) values are expressed in parts per million (ppm) and coupling constants (J) in Hertz (Hz). Signals are designated as follows: s singlet, d doublet, t triplet, q quartet, m multiplet, brs broad singlet. Mass spectroscopy was performed using DI-50 unit of Shimadzu GC/MS-QP 5050A apparatus. Elemental analyses were carried out at the regional center for mycology and biotechnology, Al-Azhar University, Cairo, Egypt.
Synthesis of intermediate 1 (1-cyclopropyl-6-fluoro-7-{4-[(4-nitrophenoxy)carbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid)
A mixture of ciprofloxacin (0.994 g, 0.003 mol), 4-nitrophenyl chloroformate (0.605 g, 0.003 mol) and pyridine (0.356 g, 0.0045 mol) in acetonitrile (10 mL) was heated at reflux for 5 h, then cooled to room temperature and poured to 0.1 M HCl. The formed precipitate was collected, washed successively with water and dried [41].
Yield 0.968 g (65%); canary yellow powder, mp: 290–92 °C; 1H-NMR (400 MHz, CDCl3) δ 14.95 (s, 1H, COOH), 8.82 (s, 1H, C2–H), 8.32 (d, J = 8.8 Hz, 2H, Ar–H), 8.10 (d, J = 12.0 Hz, 1H, C5–H), 7.47–7.35 (m, 3H, C8-H & 2Ar–H), 3.99–3.86 (m, 4H, piperazine-4H), 3.64–3.52 (m, 1H, cyclopropyl-H), 3.50–3.38 (m, 4H, piperazine-4H), 1.34–1.14 (m, 4H, cyclopropyl-H); MS m/z calcd for C24H21FN4O7 [M+]: 496.14, found: 496.57; Anal. calcd for C24H21FN4O7: C, 58.06; H, 4.26; N, 11.29; found: C, 58.34; H, 4.35; N, 11.43.
Synthesis of intermediate 2 (1-cyclopropyl-6-fluoro-7-[4-(hydrazinecarbonyl)piperazin-1-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid)
A mixture of ciprofloxacin carbamate intermediate 1 (0.992 g, 0.002 mol) and hydrazine hydrate (0.200 g, 0.004 mol) in ethanol (10 mL) was heated at reflux for 5 h. After the reaction was completed, the mixture was cooled to room temperature and the formed precipitate was filtered off, washed with ethanol and dried [42].
Yield 0.623 g (80%); off white powder, mp: 248–50 °C; 1H-NMR (400 MHz, DMSO-d6) δ 15.25 (brs, 1H, COOH), 8.68 (s, 1H, C8–H), 8.09 (s, 1H, NH), 7.93 (d, J = 12.8 Hz, 1H, C5–H), 7.58 (d, J = 7.6 Hz, 1H, C8–H), 3.87–3.79 (m, 1H, cyclopropyl-H), 3.58–3.46 (m, 4H, piperazine-4H), 3.36–3.24 (m, 4H, piperazine-4H), 1.37–1.29 (m, 2H, cyclopropyl-H), 1.24 (s, 2H, NH2), 1.23–1.14 (m, 2H, cyclopropyl-H); MS m/z calcd for C18H20FN5O4 [M+]: 389.15, found: 389.07; Anal. calcd for C18H20FN5O4: C, 55.52; H, 5.18; N, 17.99; found: C, 55.68; H, 5.29; N, 18.27.
General procedure for synthesis of target compounds 3a–i
A mixture of hydrazide intermediate 2 (0.001 mol) and the appropriate aldehyde (0.003 mol) in methanol (15 mL) containing few drops of glacial acetic acid was heated at reflux for 2 h then cooled to room temperature. The found precipitate was filtered off, washed with methanol and dried [43].
(E/Z)-7-[4-(2-Benzylidenehydrazinecarbonyl)piperazin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3a
Yield 0.435 g (90%); off white powder, mp: 242–44 °C; IR (KBr) 3332 (NH str), 3097 (aromatic C–H str), 2957 (aliphatic C–H str), 1727 (carboxylic C=O str), 1668 (amidic C=O str) 1626 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.03 (brs, 1H, COOH), 10.42 (s, 1H, NH), 8.67 (s, 1H, C2–H), 8.17 (s, 1H, =CH), 7.93 (d, J = 13.2 Hz, 1H, C5–H), 7.68–7.57 (m, 3H, C8–H & 2Ar–H), 7.47–7.34 (m, 3H, Ar–H), 3.90–3.79 (m, 1H, cyclopropyl-H), 3.76–3.67 (m, 4H, piperazinyl-H), 3.45–3.35 (m, 4H, piperazinyl-H), 1.38–1.30 (m, 2H, cyclopropyl-H), 1.25–1.17 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.86, 166.29, 154.98, 153.47 (d, JC–F = 249.0 Hz), 148.43, 145.49 (d, JC–F = 10.1 Hz), 143.39, 139.66, 137.92, 135.46, 129.61, 129.15, 126.89, 119.34, 111.49 (d, JC–F = 23.0 Hz), 107.21, 49.82, 44.21, 36.33, 8.06; MS m/z calcd for C25H24FN5O4 [MH+]: 478.19, found: 478.38; Anal. calcd for C25H24FN5O4: C, 62.88; H, 5.07; N, 14.67; found: C, 62.74; H, 5.11; N, 14.79.
(E/Z)-7-{4-[2-(4-Chlorobenzylidene)hydrazinecarbonyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3b
Yield 0.471 g (92%); pale yellow powder, mp: 234–36 °C; IR (KBr) 3333 (NH str), 3062 (aromatic C–H str), 2955 (aliphatic C–H str), 1716 (carboxylic C=O str), 1665 (amidic C=O str) 1627 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.01 (brs, 1H, COOH), 10.50 (s, 1H, NH), 8.68 (s, 1H, C2–H), 8.16 (s, 1H, =CH), 7.93 (d, J = 13.1 Hz, 1H, C5–H), 7.70–7.57 (m, 3H, C8–H & 2Ar–H), 7.48 (d, J = 7.9 Hz, 2H, Ar–H), 3.89–3.79 (m, 1H, cyclopropyl-H), 3.76–3.66 (m, 4H, piperazinyl-H), 3.46–3.33 (m, 4H, piperazinyl-H), 1.40–1.29 (m, 2H, cyclopropyl-H), 1.25–1.16 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.89, 166.31, 154.84, 153.47 (d, JC–F = 248.8 Hz), 148.47, 145.49 (d, JC–F = 11.8 Hz), 142.06, 139.68, 134.42, 134.05, 129.25, 128.50, 126.90, 119.34, 111.50 (d, JC–F = 23.0 Hz), 107.23, 49.81, 44.19, 36.33, 8.06; MS m/z calcd for C25H23ClFN5O4 [M+]: 511.14, found: 511.86; Anal. calcd for C25H23ClFN5O4: C, 58.65; H, 4.53; N, 13.68; found: C, 58.51; H, 4.47; N, 13.54.
(E/Z)-1-Cyclopropyl-6-fluoro-7-{4-[2-(4-methylbenzylidene)hydrazinecarbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3c
Yield 0.319 g (65%); pale beige powder, mp: 240–42 °C; IR (KBr) 3326 (NH str), 3043 (aromatic C–H str), 2919 (aliphatic C–H str), 1727 (carboxylic C=O str), 1670 (amidic C=O str) 1626 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.05 (brs, 1H, COOH), 10.34 (s, 1H, NH), 8.67 (s, 1H, C2–H), 8.13 (s, 1H, =CH), 7.92 (d, J = 13.2 Hz, 1H, C5–H), 7.60 (d, J = 6.1 Hz, 1H, C8–H), 7.53 (d, J = 7.6 Hz, 2H, Ar–H), 7.23 (d, J = 7.6 Hz, 2H, Ar–H), 3.90–3.78 (m, 1H, cyclopropyl-H), 3.75–3.65 (m, 4H, piperazinyl-H), 3.44–3.35 (m, 4H, piperazinyl-H), 2.34 (s, 3H, Ph–CH3), 1.39–1.29 (m, 2H, cyclopropyl-H), 1.24–1.17 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.86, 166.30, 155.03, 153.46 (d, JC–F = 249.0 Hz), 148.42, 145.49 (d, JC–F = 11.5 Hz), 143.52, 139.66, 139.29, 132.75, 129.75, 126.87, 119.27, 111.48 (d, JC–F = 23.2 Hz), 107.40, 107.01, 49.82, 44.21, 36.33, 21.38, 8.06; MS m/z calcd for C26H26FN5O4 [M+]: 491.20, found: 491.56; Anal. calcd for C26H26FN5O4: C, 63.53; H, 5.33; N, 14.25; found: C, 63.46; H, 5.29; N, 14.37.
(E/Z)-1-Cyclopropyl-6-fluoro-7-{4-[2-(4-methoxybenzylidene)hydrazinecarbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3d
Yield 0.320 g (63%); pale beige powder, mp: 148–50 °C; IR (KBr) 3255 (NH str), 3075 (aromatic C–H str), 2972 (aliphatic C–H str), 1716 (carboxylic C=O str), 1654 (amidic C=O str) 1622 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.10 (brs, 1H, COOH), 10.26 (s, 1H, NH), 8.62 (s, 1H, C2–H), 8.11 (s, 1H, =CH), 7.93 (d, J = 13.0 Hz, 1H, C5–H), 7.81 (d, J = 6.6 Hz, 1H, C8–H), 7.67–7.53 (m, 2H, Ar–H), 7.06 (d, J = 6.8 Hz, 2H, Ar–H), 4.06–3.94 (m, 1H, cyclopropyl-H), 3.84 (s, 3H, Ph–OCH3), 3.76–3.64 (m, 4H, piperazinyl-H), 3.46–3.34 (m, 4H, piperazinyl-H), 1.41–1.29 (m, 2H, cyclopropyl-H), 1.27–1.16 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.88, 166.31, 162.20, 160.72, 155.14, 148.46, 145.55 (d, JC–F = 13.1 Hz), 143.42, 139.68, 130.41, 128.39, 127.14, 114.90, 111.50 (d, JC–F = 23.1 Hz), 107.42, 107.04, 55.88, 49.84, 44.22, 36.33, 8.06; MS m/z calcd for C26H26FN5O5 [M + 2H+]: 509.21, found: 509.60; Anal. calcd for C26H26FN5O5: C, 61.53; H, 5.16; N, 13.80; found: C, 61.37; H, 5.13; N, 13.91.
(E/Z)-1-Cyclopropyl-6-fluoro-7-{4-[2-(2-hydroxybenzylidene)hydrazinecarbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3e
Yield 0.469 g (95%); off white powder, mp: 234–36 °C; IR (KBr) 3318 (NH str), 3034 (aromatic C–H str), 2950 (aliphatic C–H str), 1728 (carboxylic C=O str), 1672 (amidic C=O str) 1625 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.08 (brs, 1H, COOH), 11.42 (s, 1H, OH), 10.72 (s, 1H, NH), 8.67 (s, 1H, C2–H), 8.36 (s, 1H, =CH), 7.92 (d, J = 12.6 Hz, 1H, C5–H), 7.61 (d, J = 6.0 Hz, 1H, C8–H), 7.41 (d, J = 6.4 Hz, 1H, Ar–H), 7.30–7.19 (m, 1H, Ar–H), 6.99–6.83 (m, 2H, Ar–H), 3.91–3.79 (m, 1H, cyclopropyl-H), 3.77–3.65 (m, 4H, piperazinyl-H), 3.50–3.35 (m, 4H, piperazinyl-H), 1.34 (s, 2H, cyclopropyl-H), 1.27–1.14 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.87, 166.29, 157.63, 154.22, 153.96 (d, JC–F = 250.5 Hz), 149.33, 148.45, 145.45, 145.06, 139.66, 130.81, 129.78, 119.55, 119.39, 116.76, 111.50 (d, JC–F = 22.7 Hz), 107.40, 107.07, 49.73, 43.87, 36.33, 8.07; MS m/z calcd for C25H24FN5O5 [M + H+]: 494.18, found: 494.17; Anal. calcd for C25H24FN5O5: C, 60.85; H, 4.90; N, 14.19; found: C, 60.68; H, 4.93; N, 14.16.
(E/Z)-7-{4-[2-(4-Bromobenzylidene)hydrazinecarbonyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3f
Yield 0.490 g (88%); pale beige powder, mp: 236–38 °C; IR (KBr) 3363 (NH str), 3078 (aromatic C–H str), 2867 (aliphatic C–H str), 1715 (carboxylic C=O str), 1652 (amidic C=O str) 1627 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 14.86 (s, 1H, COOH), 10.51 (s, 1H, NH), 8.67 (s, 1H, C2-H), 8.14 (s, 1H, =CH), 7.92 (d, J = 13.2 Hz, 1H, C5–H), 7.67–7.51 (m, 5H, C8–H & 4Ar–H), 3.89–3.78 (m, 1H, cyclopropyl-H), 3.77–3.66 (m, 4H, piperazinyl-H), 3.45–3.34 (m, 4H, piperazinyl-H), 1.40–1.29 (m, 2H, cyclopropyl-H), 1.25–1.16 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.88, 166.30, 154.83, 153.46 (d, JC–F = 248.4 Hz), 148.45, 145.48 (d, JC–F = 11.9 Hz), 142.12, 139.67, 134.78, 132.16, 128.75, 122.68, 119.29, 111.50 (d, JC–F = 23.0 Hz), 107.40, 107.03, 49.79, 44.18, 36.33, 8.06; MS m/z calcd for C25H23BrFN5O4 [M-H+]: 554.08, found: 554.59; Anal. calcd for C25H23BrFN5O4: C, 53.97; H, 4.17; N, 12.59; found: C, 53.87; H, 4.19; N, 12.52.
(E/Z)-1-Cyclopropyl-6-fluoro-7-{4-[2-(naphthalen-1-ylmethylene)hydrazinecarbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3g
Yield 0.401 g (76%); white powder, mp: 262–64 °C; IR (KBr) 3253 (NH str), 3034 (aromatic C–H str), 2856 (aliphatic C–H str), 1688 (carboxylic C=O str), 1669 (amidic C=O str) 1625 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.06 (brs, 1H, COOH), 10.53 (s, 1H, NH), 8.86–8.76 (m, 2H, Ar–H), 8.68 (s, 1H, C2–H), 8.03–7.89 (m, 3H, C5-H, =CH & Ar–H), 7.84 (d, J = 7.1 Hz, 1H, Ar–H), 7.67–7.53 (m, 4H, C8–H & 3Ar–H), 3.89–3.81 (m, 1H, cyclopropyl-H), 3.80–3.71 (m, 4H, piperazinyl-H), 3.48–3.38 (m, 4H, piperazinyl-H), 1.39–1.29 (m, 2H, cyclopropyl-H), 1.25–1.17 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.87, 166.30, 154.95, 153.48 (d, JC–F = 249.6 Hz), 148.44, 145.49 (d, JC–F = 11.1 Hz), 143.22, 139.66, 136.93, 135.65, 134.06, 130.57, 130.04, 129.15, 127.43, 127.11, 126.56, 125.99, 124.70, 119.29, 111.50 (d, JC–F = 23.1 Hz), 107.22, 49.83, 44.20, 36.33, 8.06; MS m/z calcd for C29H26FN5O4 [M+]: 527.20, found: 526.99; Anal. calcd for C29H26FN5O4: C, 66.02; H, 4.97; N, 13.28; found: C, 65.91; H, 4.94; N, 13.36.
(E/Z)-1-Cyclopropyl-6-fluoro-7-{4-[2-(furan-2-ylmethylene)hydrazinecarbonyl]piperazin-1-yl}-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 3h
Yield 0.313 g (67%); beige powder, mp: 206–08 °C; IR (KBr) 3333 (NH str), 3091 (aromatic C–H str), 2846 (aliphatic C–H str), 1711 (carboxylic C=O str), 1672 (amidic C=O str) 1624 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.03 (brs, 1H, COOH), 10.38 (s, 1H, NH), 8.67 (s, 1H, C2–H), 8.08 (s, 1H, =CH), 7.91 (d, J = 13.1 Hz, 1H, C5–H), 7.75 (s, 1H, furan C5–H), 7.60 (d, J = 6.8 Hz, 1H, C8–H), 6.76 (s, 1H, furan C3–H), 6.59 (s, 1H, furan C4–H), 3.89–3.79 (m, 1H, cyclopropyl-H), 3.74–3.64 (m, 4H, piperazine-4H), 3.44–3.34 (m, 4H, piperazine-4H), 1.38–1.30 (m, 2H, cyclopropyl-H), 1.24–1.17 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.85, 166.29, 153.48 (d, JC–F = 256 Hz), 150.55, 148.42, 145.46 (d, JC–F = 12.3 Hz), 144.59, 139.66, 133.81, 131.31, 120.46, 119.26, 112.15, 111.47 (d, JC–F = 21.2 Hz), 107.38, 107.00, 49.77, 44.11, 36.32, 8.05; MS m/z calcd for C23H22FN5O5 [M+]: 467.16, found: 467.59; Anal. calcd for C23H22FN5O5: C, 59.10; H, 4.74; N, 14.98; found: C, 59.28; H, 4.87; N, 14.83.
(E/Z)-1-Cyclopropyl-6-fluoro-4-oxo-7-{4-[2-(thiophen-2-ylmethylene)hydrazinecarbonyl]piperazin-1-yl}-1,4-dihydroquinoline-3-carboxylic acid 3i
Yield 0.406 g (84%); beige powder, mp: 234–36 °C; IR (KBr) 3337 (NH str), 3083 (aromatic C–H str), 2876 (aliphatic C–H str), 1710 (carboxylic C=O str), 1671 (amidic C=O str) 1625 (quinolone C=O str); 1H-NMR (400 MHz, DMSO-d6) δ 15.06 (brs, 1H, COOH), 10.37 (s, 1H, NH), 8.67 (s, 1H, C2–H), 8.39 (s, 1H, =CH), 7.92 (d, J = 13.2 Hz, 1H, C5–H), 7.60 (d, J = 7.0 Hz, 1H, C8–H), 7.55 (d, J = 4.2 Hz, 1H, thiophene C5–H), 7.32 (s, 1H, thiophene C3–H), 7.10 (s, 1H, thiophene C4–H), 3.89–3.79 (m, 1H, cyclopropyl-H), 3.74–3.63 (m, 4H, piperazinyl-H), 3.44–3.34 (m, 4H, piperazinyl-H), 1.39–1.29 (m, 2H, cyclopropyl-H), 1.25–1.16 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, DMSO-d6) δ 176.86, 166.29, 154.81, 153.45 (d, JC–F = 248 Hz), 148.43, 145.47 (d, JC–F = 10.4 Hz), 140.37, 139.66, 138.84, 132.95, 129.33, 128.05, 127.90, 119.34, 111.48 (d, JC–F = 23.0 Hz), 107.20, 49.79, 44.16, 36.32, 8.06; MS m/z calcd for C23H22FN5O4S [M+]: 483.14, found: 383.13; Anal. calcd for C23H22FN5O4S: C, 57.13; H, 4.59; N, 14.48; found: C, 57.32; H, 4.66; N, 14.61.
General procedure for synthesis of target compounds 4a–c
A mixture of ciprofloxacin carbamate intermediate 1 (0.496 g, 0.001 mol) and the corresponding amine [either as 0.003 mol in 10 mL dioxane, for compounds 4a and 4b or neat using 2 mL of morpholine (0.023 mol), for compound 4c] was heated at reflux for 3 h, then cooled to room temperature and poured to 0.1 M HCl (20 mL). The obtained precipitate was collected, washed successively with water and dried.
1-Cyclopropyl-6-fluoro-4-oxo-7-[4-(pyrrolidine-1-carbonyl)piperazin-1-yl]-1,4-dihydroquinoline-3-carboxylic acid 4a
Yield 0.274 g (64%); beige powder, mp: 278–80 °C; 1H-NMR (400 MHz, CDCl3) δ 14.81 (brs, 1H, COOH), 8.63 (s, 1H, C2–H), 7.87 (d, J = 13.0 Hz, 1H, C5–H), 7.28 (d, J = 6.4 Hz, 1H, C8–H), 3.53–3.42 (m, 5H, cyclopropyl-H & piperazinyl-H), 3.41–3.30 (m, 4H, piperazinyl-H), 3.33–3.22 (m, 4H, pyrrolidinyl-H), 1.85–1.75 (m, 4H, pyrrolidinyl-H), 1.38–1.27 (m, 2H, cyclopropyl-H), 1.23–1.12 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, CDCl3) δ 177.00, 166.83, 162.39, 153.63 (d, JC–F = 251.5 Hz), 147.38, 145.81 (d, JC–F = 9.8 Hz), 139.07, 119.85, 112.35 (d, JC–F = 23.4 Hz), 108.10, 104.93, 49.65, 48.36, 45.88, 35.31, 25.52, 8.21; MS m/z calcd for C22H25FN4O4 [M+]: 428.19, found: 428.16; Anal. calcd for C22H25FN4O4: C, 61.67; H, 5.88; N, 13.08; found: C, 61.90; H, 6.04; N, 12.79.
1-Cyclopropyl-6-fluoro-4-oxo-7-[4-(piperidine-1-carbonyl)piperazin-1-yl]-1,4-dihydroquinoline-3-carboxylic acid 4b
Yield 0.354 g (80%); pale yellow powder, mp: 286–88 °C; 1H-NMR (400 MHz, CDCl3) δ 14.83 (brs, 1H, COOH), 8.67 (s, 1H, C2–H), 7.90 (d, J = 12 Hz, 1H, C5–H), 7.33 (d, J = 8.0 Hz, 1H, C8–H), 3.58–3.35 (m, 5H, cyclopropyl-H & piperazinyl-H), 3.32–3.23 (m, 4H, piperazinyl-H), 3.22–3.14 (m, 4H, piperidinyl-H), 1.61–1.47 (m, 6H, piperidinyl-H), 1.40–1.28 (m, 2H, cyclopropyl-H), 1.22–1.10 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, CDCl3) δ 177.09, 166.84, 163.91, 153.68 (d, JC–F = 251.8 Hz), 147.44, 145.82 (d, JC–F = 9.1 Hz), 139.07, 120.05, 112.49 (d, JC–F = 23.4 Hz), 108.24, 104.97, 49.64, 47.79, 46.70, 35.29, 25.76, 24.64, 8.24; MS m/z calcd for C23H27FN4O4 [M+]: 442.20, found: 442.51; Anal. calcd for C23H27FN4O4: C, 62.43; H, 6.15; N, 12.66; found: C, 62.67; H, 6.34; N, 12.90.
1-Cyclopropyl-6-fluoro-7-[4-(morpholine-4-carbonyl)piperazin-1-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4c
Yield 0.338 g (76%); pale beige powder, mp: 268–72 °C; 1H-NMR (400 MHz, CDCl3) δ 14.81 (brs, 1H, COOH), 8.64 (s, 1H, C2–H), 7.90 (d, J = 12 Hz, 1H, C5–H), 7.30 (d, J = 6.0 Hz, 1H, C8–H), 3.70–3.58 (m, 4H, morpholinyl-H), 3.52–3.40 (m, 5H, cyclopropyl-H & piperazinyl-H), 3.33–3.22 (m, 8H, piperazinyl-H & morpholinyl-H), 1.38–1.28 (m, 2H, cyclopropyl-H), 1.18–1.08 (m, 2H, cyclopropyl-H); 13C-NMR (100 MHz, CDCl3) δ 176.97, 166.78, 163.52, 153.63 (d, JC–F = 251.7 Hz), 147.41, 145.68 (d, JC–F = 9.9 Hz), 139.03, 119.99, 112.38 (d, JC–F = 23.6 Hz), 108.11, 105.04, 66.60, 49.56, 47.27, 46.52, 35.32, 8.22; MS m/z calcd for C22H25FN4O5 [M+]: 444.18, found: 444.06; Anal. calcd for C22H25FN4O5: C, 59.45; H, 5.67; N, 12.61; found: C, 59.31; H, 5.80; N, 12.67.
Biological investigation
Evaluation of anticancer activity
NCI anticancer screening
According to the protocol of Drug Evaluation Branch of the National Cancer Institute (NCI), Bethesda, USA (described in details at http://www.dtp.nci.nih.gov) [44,45,46], the process of in vitro screening utilized about 60 different human tumor cell lines (NCI-60 cell lines panel) representing leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney. Briefly, compounds were added to the cancer cell lines at a concentration of 10 μM and incubated for 48 h, then cellular growth was terminated by adding the protein binding dye, sulforhodamine B (SRB). Anticancer activity for each compound was recorded as the growth percent of cells treated with the test compound as compared with control untreated ones. Growth percentages were measured spectrophotometrically.
In vitro MTT cytotoxicity assay
Cell line cells were obtained from American Type Culture Collection. Cells were cultured using DMEM (Invitrogen/Life Technologies) supplemented with 10% FBS (Hyclone), 10 μg/mL of insulin (Sigma), and 1% penicillin–streptomycin. All of the other chemicals and reagents were from Sigma. Cells (cells density 1.2–1.8 × 10,000 cells/well) were plated in a volume of 100 µL complete growth medium + 100 µL of the tested compound per well in a 96-well plate for 24 h before the MTT assay.
The MTT method for monitoring in vitro cytotoxicity [47] is well suited for use with multi-well plates. For best results, cells in the log phase of growth were employed and final cell number did not exceed 106 cells/cm2. Each test included a blank containing complete medium without cells. Cultures were removed from incubator into a sterile work area. Each vial of MTT [M-5655] was reconstituted to be used with 3 mL of medium without phenol red and serum. Reconstituted MTT were added to an amount equal to 10% of the culture medium volume. Cultures were returned to incubator for 2–4 h. After the incubation period, cultures were removed from incubator and the resulting formazan crystals were dissolved by adding an amount of MTT solubilization solution [M-8910] equal to the original culture medium volume. Dissolution was enhanced by gentle mixing in a gyratory shaker or occasionally by pipetting up and down (trituration) to completely dissolve the MTT formazan crystals. Absorbance was measured spectrophotometrically at a wavelength of 570 nm. The background absorbance of multiwell plates was measured at 690 nm and subtracted from the 450 nm measurement. Tests performed in multi-well plates were read after transferring contents to appropriate size cuvettes for spectrophotometric measurement.
Screening of antibacterial activity
Antibacterial activity of test compounds were determined according to the agar cup diffusion method [48] using Klebsiella pneumoniae and Proteus mirabilis as representatives for Gram-negative urease producing bacteria. These strains represent common contaminants of the environment and involved in human and animal diseases. Bacterial strains were kindly provided by the Assiut University Mycological Centre (AUMC). To prepare inocula for bioassay, bacterial strains were individually cultured for 48 h in a universal tube 20 mL nutrient broth medium. Test was done in 10 cm sterile plastic Petri plates in which microbial suspension (1 mL/plate) and 15 mL of nutrient agar medium were poured. After solidification of the media, 5 mm diameter cavities were cut in the solidified agar (three cavities/plate) using sterile cork borer. Test compounds dissolved in dimethyl sulfuxide (DMSO) at a concentration of 2000 μM were pipetted in the cavities (50 µL/cavity). Cultures were then incubated at 28 °C for 48 h. Results were read as the diameter (in mm) of inhibition zone around cavities. For determination of minimum inhibitory concentrations (MICs), chemical compounds giving positive results were diluted with DMSO to prepare series of two fold descending concentrations (2000–31.25 μM) and similarly assayed as mentioned before till the concentration that gave no activity.
Evaluation of urease inhibitory activity
This study was performed using indophenol method, which depends on color production relative to ammonia liberated from the enzyme catalytic activity on urea [38]. An assay mixture consists of 1 mL buffer (phosphate pH 6.7, 50 mM; EDTA, 2 mM; sodium salicylate, 400 mM and sodium nitroprossid, 10 mM), 30 μL of enzyme (30,000 U/L), 10 μL of test compounds at different concentrations and 10 μL urea (50 mg/dL) was incubated at 37 °C for 10 min. End point was achieved by adding 200 μL of colouring reagent (sodium hypochlorite 140 mM and dodium hydroxide 150 mM), making a final volume of 1.25 mL and the mixture was reincubated for additional 10 min at 37 °C. Experiments were carried out in triplicate and thiourea was used as a standard inhibitor. A mixture prepared and treated as before but inhibitors were omitted was used as a control. Absorbance was measured at 578 nm and percentages of inhibition were calculated using the formula: 100-(optical densitytest/optical densitycontrol) × 100 [40, 49].
Molecular docking study
Docking simulation study was performed using Molecular Operating Environment (MOE®) version 2008.10 (Chemical Computing Group Inc., Montreal, Canada) [50]. Test compound 3i was docked onto the binding pocket of active site of urease obtained from protein data bank (PDB: 1E9Y) [36]. Preparation of the compound for docking was achieved via building its 3D structure by MOE and database formation. Test compound was subjected to a conformational search, and all conformers were subjected to energy minimization, that was performed with MOE until a RMSD gradient 0.01 kcal mol−1 A˚−1 with MMFF94X force-field.3D. Preparation of the protein was performed by 3D protonation, removal of unwanted water and finally surfaces and maps were taken before docking of test compounds on the enzyme active site. Flexible ligand–rigid receptor docking of the most stable conformers was done with MOE-DOCK using triangle matcher as the placement method, London dG as the scoring function, and refinement of the results was achieved using force field energy. Docking results had appeared in a DBV window (dock.mdb). The S field, that the docking poses are ranked by the MM/GBVI binding free energy calculation is identical to the E_refine score. Database browser was used for comparing docking poses of the ligand in the co-crystallized structure. Thirty of the most stable docking models for the ligand were retained with the best scored conformation.
Results and discussion
Chemistry
Protocols for synthesis of the target compounds are illustrated in Schemes 1. Ciprofloxacin was converted to the corresponding carbamate 1 by heating at reflux with 4-nitrophenyl chloroformate in acetonitrile containing pyridine as a base. Conventional utilization of ethyl chloroformate gave ethyl carbamate of ciprofloxacin with a poor reactivity toward nucleophilic attack by amines. This was consistent with the previously reported data regarding tertiary carbamates [37]. However, 4-nitrophenol is known for its better nucleofugality than ethanol and consequently, 4-nitrophenyl carbamate of ciprofloxacine 1 could be effectively reacted with different nucleophiles. Compound 1 was heated at reflux with hydrazine hydrate in ethanol to afford the hydrazide intermediate 2. Condensation of compound 2 with different aromatic aldehydes in methanol gave the target compounds 3a–i. Alternatively, ciprofloxacin carbamate intermediate 1 was reacted with the appropriate cyclic amines to afford the titled derivatives 4a–c. Different spectroscopic and analytical tools were applied for identification of the synthesized compounds including IR, 1H-NMR, 13C-NMR, Mass spectroscopy, and elemental analyses. Compounds 3a–i were characterized by two singlet signals (1H each) at δ ~ 10.40 and 8.16 ppm assigned as amidic NH and N=CH protons, respectively. 13C-NMR Spectra of compounds 3a–i revealed three carbonyl signals appeared at δ ~ 176.87, 166.30, and 155.14–154.22 ppm, assigned to C=O of the quinolone nucleus, carboxylic group and that of carbamoyl bridge, respectively. Similarly, derivatives 4a–c showed the same signals at δ ~ 177.00, 166.80, and 163.91–162.39 ppm, respectively. Elemental analysis and MS furtherly confirmed the assigned structures of the synthesized compounds.
Biological evaluation
Evaluation of anticancer activity
NCI anticancer screening
All of the target compounds 3a–i and 4a–c were selected for in vitro anticancer screening according to the applied rules for compounds selection by drug evaluation branch of the National Cancer Institute, Bethesda, USA. Selected compounds were tested at a single concentration of 10 μM, using sixty different tumor cell lines known as NCI-60 cell line panel, representing nine types of human cancers including both solid and liquid tumors. Compounds were applied at determined concentration and incubated for 28 h then growth was terminated by adding a protein binding dye, sulforhodamine B (SRB). Antitumor activity was recorded as the growth percent of cells treated with test compound in comparison with control untreated ones.
Compound 3a revealed a moderate antitumor activity against only leukemia SR cell line with a growth percentage of 54.41%. No other considerable cell growth suppression was observed except with non-small cell lung cancer NCI-H522 and renal cancer UO-31 cell lines, where growth percentages of 78.04 and 74.00%, respectively, were recorded (Supplementary data). On the other hand, derivative 3b showed a weak anticancer activity, where the most pronounced cell growth inhibition noted was against leukemia MOLT-4, non-small cell lung cancer NCI-H522, renal cancer UO-31, and breast cancer BT-549 cell lines with growth percentages 82.92, 82.03, 81.15, and 85.64%, respectively (Supplementary data). Similarly, a weak cell growth inhibition was indicated with compounds 3c (Supplementary data). Activity of interest exerted by compound 3c was against leukemia MOLT-4, CNS cancer SF-268, SNB-75, melanoma LOX IMVI, renal cancer CAKI-1, and UO-31 cells (growth percentages 82.45, 84.21, 83.64, 85.16, 83.45, and 72.23%, respectively). Compounds 3d and 3e revealed also a weak activity, where the most intense cell growth inhibition was observed toward the renal cancer UO-31 cell line with growth percentages 84.34 and 84.11%, respectively (Supplementary data).
It is worth to note that compound 3f experienced a remarkable cell growth inhibition against the breast cancer BT-549 cell line, recording a growth percentage of 28.68%. A moderate activity was also observed by compound 3f against Leukemia CCRF-CEM, melanoma LOX IMVI, UACC-62, renal cancer UO-31, and breast cancer T-47D cells (growth percentages 62.87, 58.54, 49.35, 64.83, and 67.67%, respectively). Inversely, the activity against other cell lines was weak, Table 1. Compound 3g revealed also a potent antitumor activity against colon cancer HCT-116 and breast cancer BT-549 cell lines (growth percentages 14.76 and 6.18%, respectively). The compound exhibited also a moderate activity against melanoma LOX IMVI cells with a growth percentage of 67.78%. However, it showed a weak cell growth inhibition with some of the other cell lines, Table 1. Compounds 3h and 3i showed a weak antitumor activity against some of the NCI panel cell lines (Supplementary data).
On the other hand, compound 4a experienced a weak anticancer activity (Supplementary data), where the most pronounced cell growth inhibition recorded was against non-small cell lung cancer NCI-H522, melanoma UACC-62, and renal cancer UO-31 cell lines (growth percentages 76.36, 74.90, and 79.73%, respectively). However, compounds 4b and 4c revealed no significant antitumor potential, where the highest activity was obtained by compound 4b against CNS cancer SNB-75 and renal cancer UO-31 cells with growth percentages of 87.92 and 86.42%, respectively (Supplementary data).
From the above results, it is obvious that most of the tested compounds experienced moderate to weak activity against the tested cell lines. Relatively, compounds 4a–c having no aromatic or heteroaromatic moiety at position 7 showed a very weak activity, this finding is consistent with that reported in literatures [9]. The most promising antitumor results were obtained with compound 3f toward breast cancer BT-549, derivative 3g against breast cancer BT-549 and colon cancer HCT-116 as well as analog 3a on leukemia SR cell lines with growth percentages of 28.68, 6.18, 14.76, and 54.41%, respectively (Fig. 1).
In vitro MTT cytotoxicity assay
Cytotoxic activity of compounds 3f and 3g was evaluated against two specific cell lines, namely BT-549 and MCF10a, using doxorubicin (DOX) as a reference drug. These two cell lines were selected based on the sensitivity of the tested cells shown by the previously mentioned NCI results. The growth inhibition is expressed as the median growth inhibitory concentration (IC50), which corresponds to the concentration required for 50% inhibition of cell viability. Compounds 3f and 3g revealed IC50 comparable to the standard drug, doxorubicin toward the breast cancer BT-549 cell line (1.84, 9.83, and 1.29 µM, respectively), where derivative 3f was nearly equipotent to standard. On the other hand, the test compounds were less toxic than doxorubicin toward the non-tumorigenic MCF10a breast cell line (Fig. 2).
Evaluation of antibacterial activity
Standard agar cup diffusion method was applied for antibacterial screening of the target compounds 3a–i and 4a–c along with intermediates 1 and 2, using two Gram-negative urease producing bacterial strains, Proteus mirabilis and Klebsiella pneumonia. Chloramphenicol and the parent drug, ciprofloxacin were used as positive controls. Compound 4a revealed activity against Klebsiella pneumonia more than that of the reference drug, chloramphenicol (MIC = 100.64 and 217.08 µM, respectively). However, the antibacterial activity of this derivative was less than the parent drug, ciprofloxacin (MIC = 40.16 µM). Compound 4c showed anti-Klebsiella pneumonia activity only at the highest concentration used, 2 mM, while other derivatives revealed no activity at 2 mM. On the other hand, only compound 3e exhibited activity against Proteus mirabilis at the initial concentration used, 2 mM. Meanwhile, other derivatives experienced no activity up to the highest concentration applied. Antibacterial screening results are shown in Table 2.
Evaluation of urease inhibitory activity
Indophenol test for detection of ammonia (Weatherburn method) [38] was applied for evaluation of urease inhibitory activity of the synthesized compounds along with ciprofloxacin, using thiourea as a reference enzyme inhibitor [39]. This enzyme assay depends on the colored indophenol produced by the reaction of phenolic compounds and chlorine with ammonia liberated as a result of the enzyme catalytic activity on urea. Reduction in color intensity of samples treated with the test compounds and standard inhibitor at different concentrations was measured in comparison with untreated control one. Percentages of enzyme inhibition were calculated using the formula:
100-(optical densitytest/optical densitycontrol) × 100 [40].
Assay results revealed that most of the tested compounds have a urease inhibitory activity better than the parent drug, ciprofloxacin (CIP) and comparable to or more than the standard thiourea (THR, Fig. 3). Compounds 3i and 4a were the most potent urease inhibitors revealing IC50 of 58.92 and 73.40 µM, respectively (78.89 µM for thiourea). These compounds can be considered as potential candidates for further more investigation.
Molecular docking
MOE program was utilized to study the docking of the test compounds on the active site of urease enzyme (Fig. 4a–c). Ddocking reliability was validated using the known X-ray structure of Helicobacter pylori urease (PDB: 1E9Y) [36] in complex with acetohydroxamic acid (AHA). Molecular docking study for the most active compounds 3i and 4a showed the ability of such derivatives to bind strongly with the bi-nickel center of the urease enzyme as indicated by their binding energy values. Compounds 3i and 4a revealed binding scores better than that of the standard ligand, AHA (binding energy − 78.70, − 104.16, and − 33.45 kcal/mol, respectively). Figure 4a shows the binding mode of AHA with H. pylori urease, revealing coordination with the bi-nickel center of the enzyme and formation of a hydrogen bond with His221. Binding mode of compound 3i (Fig. 4b) denotes that the carbonyl oxygen of carbamoyl moiety coordinates with Ni3002 and forms two hydrogen bonds with His248 and His274. As a hydrogen bond donor, NH group additionally participates in hydrogen bonding with Asp362. In compound 4a (Fig. 4c), the carbonyl oxygen of urea moiety coordinates with Ni3002 and makes two hydrogen bonds with His248 and His274.
Conclusion
Different N-4 carbamoyl piperazinyl derivatives of ciprofloxacin were synthesized and biologically investigated. However, most of the tested compounds showed a moderate to weak anticancer activity, the 4-bromophenylhydrazone derivative, 3f experienced a potent antitumor activity against the breast cancer BT-549 cell line with a growth percentage of 28.68%. Increasing bulkiness among hydrazone series via introducing a naphthyl moiety (compound 3g) markedly improved activity against breast BT-549 and colon HCT-116 cancer cell lines, where growth percentages of 6.18 and 14.76%, respectively, were recorded. MTT assay indicated cytotoxicity of compounds 3f and 3g comparable to doxorubicin against the breast cancer BT-549 cell line (IC50 = 1.84, 9.83, and 1.29 µM, respectively); however, the test compounds experienced a reduced cytotoxicity toward the non-cancerous MCF10a cells (IC50 = 21.00, 29.1, and 18.7 µM, respectively). The prepared compounds showed reduced antibacterial activity than their parent drug, ciprofloxacin. Meanwhile, among saturated heterocyclic derivatives, compound 4a revealed activity against Klebsiella pneumoniae better than the standard drug used, chloramphenicol (MIC = 100.64 and 217.08 µM, respectively) indicating that a five membered ring cyclic amine is better than six membered ones for antibacterial activity. On the other, the majority of the newly synthesized ciprofloxacin analogs were found to have a urease inhibitory activity more than their parent drug and comparable to thiourea, where the thienyl hydrazone derivative 3i showed a promising activity with IC50 of 58.92 µM (78.89 µM for standard, thiourea). Computational study indicated the newly developed carbamoyl functionality can participate efficiently in binding to Ni ion at the urease active site, which may interpret the positive impact of such modification on the in vitro urease inhibitory activity.
References
Appelbaum P, Hunter P. The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents. 2000;16:5–15.
Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs. 2007;67:2077–99.
Jones RN, Fritsche TR, Sader HS, Stilwell MG. Activity of garenoxacin, an investigational des-F (6)-quinolone, tested against pathogens from community-acquired respiratory tract infections, including those with elevated or resistant-level fluoroquinolone MIC values. Diagn Microbiol Infect Dis. 2007;58:9–17.
Keating GM. Sitafloxacin. Drugs. 2011;71:731–44.
Imramovský A, Polanc S, Vinšová J, Kočevar M, Jampílek J, Rečková Z, et al. A new modification of anti-tubercular active molecules. Biorg Med Chem. 2007;15:2551–9.
Advani RH, Hurwitz HI, Gordon MS, Ebbinghaus SW, Mendelson DS, Wakelee HA, et al. Voreloxin, a first-in-class anticancer quinolone derivative, in relapsed/refractory solid tumors: a report on two dosing schedules. Clin Cancer Res. 2010;16:2167–75.
Harris LM, Merrick CJ. G-quadruplexes in pathogens: a common route to virulence control? PLoS Pathog. 2015;11:e1004562.
Ruggiero E, Richter SN. G-quadruplexes and G-quadruplex ligands: targets and tools in antiviral therapy. Nucleic Acids Res. 2018;46:3270–83.
Abdel-Aal MAA, Abdel-Aziz SA, Shaykoon MSA, Abuo-Rahma GE-DA. Towards anticancer fluoroquinolones: a review article. Arch Pharm. 2019;352:e1800376.
Abdel-Aziz M, Park S-E, Abuo-Rahma GE-DA, Sayed MA, Kwon Y. Novel N-4-piperazinyl-ciprofloxacin-chalcone hybrids: synthesis, physicochemical properties, anticancer and topoisomerase i and ii inhibitory activity. Eur J Med Chem. 2013;69:427–38.
Sissi C, Palumbo M. The quinolone family: from antibacterial to anticancer agents. Curr Med Chem Anticancer Agents. 2003;3:439–50.
Anderson VE, Osheroff N. Type II topoisomerases as targets for quinolone antibacterials turning Dr. Jekyll into Mr. Hyde. Curr Pharm Des. 2001;7:337–53.
Richter S, Parolin C, Palumbo M, Palù G. Antiviral properties of quinolone-based drugs. Curr Drug Targets Infect Disord. 2004;4:111–6.
Shi Z-y, Li Y-q, Kang Y-h, Hu G-q, Huang-Fu C-s, Deng J-b, et al. Piperonal ciprofloxacin hydrazone induces growth arrest and apoptosis of human hepatocarcinoma SMMC-7721 cells. Acta Pharmacol Sin. 2012;33:271–8.
Hu G, Hou L, Wang G, Duan N, Wen X, Cao T. Synthesis and antitumor and antibacterial activities of fluoroquinolone C-3 isosteres I. Norfloxacin C-3 carbonylhydrazone derivatives. J China Pharm Univ. 2012;43:298–301.
Xu Q, Hou L, Wu X. Synthesis and antitumor activity of ciprofloquinolone bis-(C3/C7 hydrazone)s. J China Pharm Univ. 2013;44:35–8.
Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63:424–30.
Rutherford JC. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014;10:e1004062.
Murphy TF, Brauer AL. Expression of urease by Haemophilus influenzae during human respiratory tract infection and role in survival in an acid environment. BMC Microbiol. 2011;11:183.
De Muynck W, De Belie N, Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecol Eng. 2010;36:118–36.
Omoregie AI, Senian N, Li PY, Hei NL, Leong DOE, Ginjom IRH, et al. Screening for urease-producing bacteria from limestone caves of sarawak. Born J Res Sci Technol. 2016;6:37–45.
Zimmer M. Molecular mechanics evaluation of the proposed mechanisms for the degradation of urea by urease. J Biomol Struct Dyn. 2000;17:787–97.
Mollenhauer-Rektorschek M, Hanauer G, Sachs G, Melchers K. Expression of UreI is required for intragastric transit and colonization of gerbil gastric mucosa by Helicobacter pylori. Res Microbiol. 2002;153:659–66.
Collins CM, D’Orazio SE. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol. 1993;9:907–13.
Graham DY, Miftahussurur M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: a mini review. J Adv Res. 2018;13:51–7.
Irwin N, McCoy C, Carson L. Effect of pH on the in vitro susceptibility of planktonic and biofilm-grown P roteus mirabilis to the quinolone antimicrobials. J Appl Microbiol. 2013;115:382–9.
Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–8.
Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, et al. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012;13:789–806.
RhO TC, Bae E-A, Kim D-H, Oh WK, Kim BY, Ahn JS, et al. Anti-Helicobacter pylori acticvity of quinolone alkaloids from evodiae fructus. Biol Pharm Bull. 1999;22:1141–3.
Ramadan M, Tawfik A, El-Kersh T, Shibl A. In vitro activity of subinhibitory concentrations of quinolones on urea-splitting bacteria: effect on urease activity and on cell surface hydrophobicity. J Infect Dis. 1995;171:483–6.
Abdullah MA, El-Baky RMA, Hassan HA, Abdelhafez E-SM, Abuo-Rahma GE-DA. Fluoroquinolones as urease inhibitors: anti-Proteus mirabilis activity and molecular docking studies. Am J Microbiol Res. 2016;4:81–4.
Gul S, Sultana N, Arayne MS, Shamim S, Akhtar M, Khan A. Sparfloxacin-metal complexes as urease inhibitors: their synthesis, characterization, antimicrobial, and antienzymatic evaluation. J Chem. 2013. https://doi.org/10.1155/2013/306385.
Nisar M, Khan S, Qayum M, Khan A, Farooq U, Jaafar H, et al. Robust synthesis of ciprofloxacin-capped metallic nanoparticles and their urease inhibitory assay. Molecules. 2016;21:411.
Abdullah MA, Abuo-Rahma GE-DA, Abdelhafez E-SM, Hassan HA, El-Baky RMA. Design, synthesis, molecular docking, anti-Proteus mirabilis and urease inhibition of new fluoroquinolone carboxylic acid derivatives. Bioorg Chem. 2017;70:1–11.
Abdel-Aal MAA, Abdel-Aziz SA, Shaykoon MSA, Mohamed MF, Abuo-Rahma GE-DA. Antibacterial and urease inhibitory activity of new piperazinyl N-4 functionalized ciprofloxacin-oxadiazoles. J Modern Res. 2019;1:1–7.
Ha N-C, Oh S-T, Sung JY, Cha KA, Lee MH, Oh B-H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Mol Biol. 2001;8:505–9.
Batey RA, Santhakumar V, Yoshina-Ishii C, Taylor SD. An efficient new protocol for the formation of unsymmetrical tri-and tetrasubstituted ureas. Tetrahedron Lett. 1998;39:6267–70.
Weatherburn M. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–4.
Khan M, Khan KM, Parveen S, Shaikh M, Fatima N, Choudhary MI. Syntheses, in vitro urease inhibitory activities of urea and thiourea derivatives of tryptamine, their molecular docking and cytotoxic studies. Bioorg Chem. 2019;83:595–610.
Ayaz M, Lodhi MA, Riaz M, Ul-haq A, Malik A, Choudhary MI. Novel urease inhibitors from Daphne oleoids. J Enzyme Inhib Med Chem. 2006;21:527–9.
Jalisatgi SS, Kulkarni VS, Tang B, Houston ZH, Lee MW Jr, Hawthorne MF. A convenient route to diversely substituted icosahedral closomer nanoscaffolds. J Am Chem Soc. 2011;133:12382–5.
Jayashankar B, Rai KL, Baskaran N, Sathish H. Synthesis and pharmacological evaluation of 1, 3, 4-oxadiazole bearing bis (heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur J Med Chem. 2009;44:3898–902.
Thomas A, Tupe P, Badhe R, Nanda R, Kothapalli L, Paradkar O, et al. Green route synthesis of Schiff’s bases of isonicotinic acid hydrazide. Green Chem Lett Rev. 2009;2:23–7.
Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res. 1995;34:91–109.
El-Ansary SL, Rahman DEA, Ghany LMA. Synthesis and anticancer evaluation of some new 3-benzyl-4, 8-dimethylbenzopyrone derivatives. Open Med Chem J. 2017;11:81–91.
Grever MR, Schepartz SA, Chabner BA. The National Cancer Institute: cancer drug discovery and development program. Semin Oncol. 1992;19:622–38.
Morgan DML. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–84.
Valgas C, Souza SM, Smânia EF, Smânia A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369–80.
Serwar M, Akhtar T, Hameed S, Khan KM. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryl-4-(1-phenylpropyl)-2H-1, 2, 4-triazole-3 (4H)-thiones. Arkivoc. 2009;7:210–21.
Molecular Operating Environment (MOE), Version 2008. 10, Chemical Computing Group, Inc. Montreal, Quebec, Canada. http://www.chemcomp.com.
Acknowledgements
We appreciate the efforts of the Developmental Therapeutics Program of the National Cancer Institute, Bethesda, MD, USA, for performing in vitro anticancer screening. Our thanks also go to Assiut University Mycological Center for evaluation of antibacterial activity.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdel-Aal, M.A.A., Shaykoon, M.S.A., Abuo-Rahma, G.ED.A.A. et al. Synthesis, antitumor, antibacterial and urease inhibitory evaluation of new piperazinyl N-4 carbamoyl functionalized ciprofloxacin derivatives. Pharmacol. Rep 73, 891–906 (2021). https://doi.org/10.1007/s43440-020-00193-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00193-0