Abstract
The production of biofuels from microalgae, especially biodiesel, has become a topic of great interest in recent years. However, many of the published papers do not consider the question of scale up and the feasibility of the various processes to be operated at the very large scale required if algal biofuels are to make a meaningful contribution to renewable fuels. All the steps in the process must also be very low cost. This paper discusses the unit processes required for algal biofuels production (i.e., growing the algae, harvesting, dewatering, extraction and conversion to biofuel) and their scalability. In many cases, especially in the lipid extraction step, little is known as yet as to the scalability and economic feasibility of the various processes proposed. We also highlight the key engineering and biological issues which must be resolved for the production of biofuels from microalgae to become an economic reality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The recognition that global oil supplies are finite and that the world is close to reaching ‘peak oil’, combined with the threat of global warming due to anthropogenic CO2 emissions has created enormous interest in the last few years in the development of renewable energy sources, including biofuels (Martinot et al. 2007; Verbruggen and Al Marchohi 2010). Several different biofuels can be produced from plant biomass. These include biodiesel from oil seed plants such as soy bean, rape seed, canola, Jatropha, and oil palm, and ethanol from starch plants such as corn, wheat, sugar cane and cassava or from cellulosic biomass such as eucalypts, Miscanthus and sugar cane. The question of using potential food crops to make biofuels has led to discussions about ‘food vs. fuel’ and the limitations of available agricultural land and fresh water (Hill et al. 2006). Algae have been proposed as an alternative source of plant lipids for the manufacture of biodiesel and possibly also of sugars for fermentation to ethanol. Algae are perceived to have the advantage of requiring less land area to produce an equivalent amount of fuel, and there are also many species which can be grown using saline water. The proposal to use algae for biofuels production is not new and was first suggested in the 1940s, and in the 1990s extensive research was carried out, especially at the Solar Energy Research Institute in Golden, Colorado, USA (Sheehan et al. 1998). Aside from photoautotrophic growth for algal lipid production the mixotrophic and/or heterotrophic culture of algae on sugars from higher plant sources has also been proposed (Lu et al. 2010) although it is hard to see what the advantage is of using these sugars which could be converted directly to ethanol by fermentation.
Currently significant research effort is being focused on developing a viable algae-to-biofuels process around the world, but the major challenge remains to be able to produce such algal biofuels at the scale required and to do this economically (Greenwell et al. 2010; Stephens et al. 2010). The scale of production for significant quantities of algae-derived fuel is extremely large. For example, Borowitzka and Moheimani (2011) have calculated that in order to produce 100,000 bbl of algae oil per year (equivalent to about 10% of Australia’s daily requirement), assuming an areal annual average biomass productivity of 20 g dry weight m−2 d−1 and an 30% total lipid content, some 650 ha of pond and almost 4 GL of water year−1 are required (this calculation assumes a 100% conversion efficiency of the lipid to biodiesel, a figure which is very unlikely to be achieved). The productivity figure used in this calculation is the best achieved in any long-term cultures so far outdoors using natural sunlight; the upper theoretical maximum productivity which could be achieved under ideal conditions is about 30–40 g m−2 d−1 (Grobbelaar 2009b; Walker 2009). High annual lipid productivity is the first essential step in producing biofuels from algae (Griffiths and Harrison 2009), but to achieve an economic process all steps in the production process from algal culture to actual fuel production must be efficient at scale and optimized. Therefore, as previously pointed out by Richmond (1999), the mass culture of microalgae for biofuel production is not an engineering or biological challenge, but is a combination of both.
Here we consider the various steps in the process and their alternatives, as well as aspects of scale-up to the extremely large scale required. Our aim is to highlight some of the key biological and engineering challenges presented by the very large scale of an algae biofuels plant which need to be resolved.
The process of production of biofuels from microalgae requires several steps:
-
production of lipid-rich algal biomass;
-
harvesting of the biomass from the growth medium generally combined with recycling of the medium;
-
dewatering of the harvested biomass (possibly with drying of the biomass);
-
extraction of the lipids (and sugars);
-
conversion of the lipids and sugars (and possibly the remaining biomass) to biofuels;
-
further processing of the remaining biomass to other products of some value.
These process steps are shown diagrammatically in Fig. 1. A variety of culture systems are possible (see section below), but all require an input of water [1], CO2 preferably from a source such as a power station or a cement plant, with the addition controlled by a pH-stat system [2], nutrients (mainly an N and P source, but may also include trace elements and, if diatoms are being cultured, silicon). In a fast growing culture approximately 30–50% of the culture volume would be removed each day [5] for harvesting and dewatering. The harvesting and dewatering steps are depicted as 3 stages depending on the processes used and the degree of dewatering required for the extraction step. If dry biomass is required for extraction then a drying step must also be included.
Process flow diagram of algae to bio-oil. (1) Feed water; (2) CO2; (3) Nutrients; (4) Evaporation water loss; (5) Harvested culture; (6) Concentrated culture; (7) Water removed during harvesting; (8) Recycled water; (9) Stage 1 dewatered culture; (10) Water removed from Stage 1; (11) Stage 2 dewatered culture; (12) Dried culture [<1% water]; (13) Water removed from dryer; (14) Extracted lipids; (15) Remaining biomass; (16) Polar lipids; (17) Nonpolar lipids. Note: Boxes with dashed borders show unit processes which possibly may be excluded depending on the requirements for extraction
2 Algae culture systems
The development of large-scale microalgae culture began approximately 60 years ago (Burlew 1953; Tamiya 1957). Two basic types of culture systems were recognized and developed at that time: ‘open’ pond systems and ‘closed’ photobioreactors. There now is extensive experience in commercial microalgae culture which began with Chlorella production as a health food in the 1950s in Japan and Taiwan (Soong 1980), followed by Spirulina (Arthrospira) in the 1970s in Mexico and the USA (Vonshak 1997), Dunaliella salina for the production of β-carotene in the 1970s in Australia, Israel and the USA (Borowitzka and Borowitzka 1989; Borowitzka 2010b), Haematococcus pluvialis for astaxanthin production in the 1980s in the USA (Lorenz and Cysewski 2000), and Crypthecodinium cohnii for eicosapentaenoic acid production in the USA (Mendes et al. 2009). Commercial-production of these algae is now taking place in many countries, especially in Australia, China, India, Israel, Taiwan and the USA. Almost all commercial producers use open ponds where the algae culture is mixed either by paddle wheels (raceway ponds) or by a centrally pivoted rotating arm. The exceptions are the culture of Haematococcus pluvialis in Israel and Chlorella in Germany, both of which are grown in tubular photobioreactors, the culture of H. pluvialis in Hawaii using dome-shaped photobioreactors, and the heretotrophic production of the thraustrochytrid Crypthecodinium which is grown heterotrophically in fermenters. Dunaliella salina is also grown in very large (>200 ha in area), shallow unmixed ponds in Australia (Borowitzka and Hallegraeff 2007), and Chlorella is also grown on a small scale in a very shallow (as low as 10 mm) cascading system in Trebon, Czech Republic (Doucha et al. 2005). A number of other species of microalgae (e.g., Isochrysis, Pavlova, Nannochloropsis, Tetraselmis, Chaetoceros etc.) are also produced for use a feed in aquaculture at a wide range of scales uses both ‘open’ and ‘closed’ culture systems, mainly large ‘bags’ or tanks (Borowitzka 1997; Fulks and Main 1991; Zmora and Richmond 2004). Aside from the production of microalgae for valuable compounds there is also significant large-scale culture of algae in wastewater treatment, especially in the so-called high rate oxidation ponds (Craggs et al. 2003; Green et al. 1996; Oswald 1988).
The production of algal biomass for biofuels, however, requires significantly larger scale algal culture than is currently practiced commercially. Production costs must also be substantially lower. For example, the lowest production cost in commercial algae production today is about US$4–5 kg−1 algal biomass (Borowitzka 1999b), for algal biomass for biofuel production this cost must be less than US$1 kg−1.
2.1 Open culture systems
The main reason why most commercial producers of microalgae use open pond systems is that these systems are much cheaper to construct and operate at a large scale compared to closed photobioreactors. A variety of open systems with different design exist and they vary in size, shape, material for construction, type of agitation and inclination (Borowitzka 1999a, 2005). These systems can be classified as (a) extensive shallow unmixed ponds; (b) circular ponds mixed with a rotating arm, (c) ‘raceway’ ponds, usually mixed with a paddle wheel, and (d) sloping thin-layer cascade systems. Some of the key characteristics of these systems are summarized in Table 1. The possibility of contamination is often cited as a serious limitation of open systems and it is true that most of the species cultured in such systems currently do grow in selective environments; i.e. Arthrospira (Spirulina) [high alkalinity]; Dunaliella salina [high salinity], Chlorella [high nutrients] (Belay 1997; Borowitzka 2010b; Soong 1980). However, other species with ‘normal’ growth requirements have also been grown successfully in open ponds, either in batch mode [e.g. Haematococcus pluvialis (Cysewski and Lorenz 2004)], or continuously for very long periods [e.g., Phaeodactylum tricornutum; Nannochloropsis and Pleurochrysis carterae (Ansell et al. 1963; Matsumoto et al. 1996; Moheimani and Borowitzka 2006)] without significant contamination problems.
A key limitation to productivity in open pond culture is light. As most open ponds have to be operated a depths of 20–30 cm due to hydraulic limitations this means that at the surface of the pond the algae are exposed to very high light, whereas at the bottom of the ponds the algae are in near darkness (Grobbelaar 1981). The culture needs to be mixed to keep the algae in suspension and move them from the lower (darker) layers to the upper (high light) layers of the ponds to maximize the amount of light the cells receive (see Section 3 below). An alternative approach to overcome this light limitation in raceway ponds was developed by Setlík and co-workers (1970) in the Czech Republic and further improved by Doucha and Livansky (1999, 1995) . In this system the algae culture flows over a sloping surface in a thin (0.5–1 cm) layer before being collected at the bottom and being pumped again to the top of the slope. This ‘cascade’ system can achieve high biomass densities of up to 10 g L−1 for Chlorella and achieves higher productivities compared to a raceway pond (Doucha et al. 2005).
2.2 ‘Closed’ systems
A very wide variety of closed photobioreactors has been developed (Tredici 2004), but fundamentally they can be classified as (a) bags or tanks, (b) towers, (c) plate reactors, or (d) tubular reactors (Borowitzka 1999a). Large bag systems consist of clear plastic bags usually supported by a metal mesh frame or hung from supports and are widely used in the aquaculture industry (Fulks and Main 1991). Similarly the ‘tower’ systems, which are vertical cylinders usually made of fiberglass or acrylic, are also widely used in aquaculture. Plate reactors come in many forms ranging from alveolar panels with internal baffles, to vertical thin plate-like tanks made of glass, Perspex or of metal mesh frames containing a plastic bag (Hu et al. 1998; Tredici and Materassi 1992). The tubular photobioreactors are generally constructed of glass, clear Teflon tubing, or clear PVC tubing arranged either in parallel straight lines (Torzillo et al. 1993) or helically wound around a central support tower (the ‘Biocoil’; Oxley and Startari 1999; Robinson et al. 1988). In the parallel tube photobioreactors the tubes may be arranged vertically (the ‘bio-fence’ types) or the tubes may lay on the ground in one or more layers (Acién Fernández et al. 2001; Torzillo et al. 1993). Table 2 compares some of the properties of these systems. Circulation of the algae culture is achieved by a variety of pumps including airlifts, centrifugal, diaphragm or lobe pumps. Temperature control, which generally means cooling during the day, is by installed heat exchangers or by evaporative cooling by spraying water over the reactor surface. One of the earliest large tubular photobioreactors had the tubes floating in a large pool for temperature control (Chaumont et al. 1988), and new variations of this are being developed. Photobioreactors also have been placed in glasshouses for temperature control, especially in temperate locations, but this increases further the capital costs of the culture system. Furthermore, several lifecycle assessments of closed photobioreactors systems (e.g., Jorquera et al. 2010; Stephenson et al. 2010) have indicated that the energy requirement of operating these is significantly higher than the energy requirement of open raceway-type ponds and can potentially exceed the energy content of the algae produced.
An often cited advantage of ‘closed’ photobioreactors is the ability to eliminate contamination. However, it is also impossible to keep a large ‘closed’ photobioreactor sterile; i.e. to maintain an axenic culture for the relatively long periods required to grow the algae, even in batch mode. The largest steam sterilizable photobioreactors built are a 250 L internally lit photobioreactor (Pohl et al. 1988) and the 100 L tubular glass ‘Medusa’ photobioreactor (Walter et al. 2003). Other reactors need to be chemically sterilized. However, as all photobioreactors need to be supplied with air and CO2 and as the O2 generated by algal photosynthesis must be removed sterility cannot be maintained for longer than about 10–12 days. Our experience with long-term algal culture in 1,000 L ‘closed’ helical tubular photobioreactors (Biocoils) has shown that contamination (including contamination by protozoa) can be managed, but not eliminated.
2.3 Hybrid systems
Hybrid culture systems using a combination of a closed photobioreactor and an open raceway pond have also been proposed. The closed photobioreactor produces a high density inoculum for the open ponds, thus optimising biomass production in the shortest time possible (Grobbelaar 2000). This 2-step concept has been employed, for example, for the production of astaxanthin in Haematococcus pluvialis in Hawaii (Olaizola 2000), but it is generally more expensive in both capital and operating costs as it is a batch rather than a continuous culture process and thus is not suitable for a low cost product such as biofuels.
3 Scaling up of microalgal cultures- constraints and requirements
Production of microalgae for biofuels requires extremely large-scale systems and a major challenge in the development of such systems is the scaling up from the laboratory or small scale to the commercial scale while maintaining high productivity and culture reliability. As illustrated in Table 3, even a relatively small algae biofuel production plant will have a very large number of ponds or photobioreactors and very large volumes of water will be used and processed.
Irrespective of which culture system, either open ponds or closed photobioreactors, is the most suited for large-scale microalgal cultivation (Grobbelaar 2009a; see for example discussion in Pulz 2001), it is very important for any culture system to maintain a balance between turbulence, culture depth (length of light path) and biomass concentration for maximum light capture and optimum biomass productivities with increasing reactor size. High productivities require high cells densities as productivity = cell density × doubling time, and this means a high degree of self-shading by the algal cells (Lee 2001). Therefore mixing is required to continually transfer the algae cells between the surface high light environment and the darker environment deeper within the culture to achieve the optimum average irradiance required for efficient utilization of the available irradiance (Richmond 1996). The cycle of high light-low light to which the cells are exposed to due to the mixing (turbulence) appears also to enhance productivity is some cases due to what is known as the ‘flashing-light effect’ (Kok 1953; Laws et al. 1983). For example, light/dark (L/D) cycles of the order of 0.1 to 10 Hz have been shown to result in a 2-fold increase in photosynthetic rates (Grobbelaar et al. 1996). The theoretical photosynthetic efficiency of algae, like all plants, is about 11% of solar irradiation (Huntley and Redalje 2007; Weyer et al. 2010) but actual efficiencies observed lie between about 2–4%, with cultures grown in tubular photobioreactors generally at the upper end of this range (Del Campo et al. 2001; data from: Laws et al. 1983; Moheimani and Borowitzka 2006; Molina-Grima et al. 1997; Moreno et al. 2003; Pushparaj et al. 1997; Tredici and Zittelli 1998). For example, Hase et al. (2000) observed long-term photosynthetic efficiencies of 6.56% PAR (or 2.8% solar) in Chlorella cultures grown in small raceway ponds in a glasshouse in Sendai, Japan.
Turbulence (mixing) also is an essential prerequisite in the maintenance of high productivity microalgal cultures for other reasons (Bosca et al. 1991; Richmond et al. 1990). Turbulence minimises the development of nutritional, gaseous and thermal gradients by reducing the boundary layer around the cells, which results in better mass transfer of nutrients and cellular metabolites between the cells and the growth medium, and the elimination of unwanted metabolites such as oxygen (Grobbelaar 1994). Heterogeneous mixing throughout the culture also reduces cell settling and the formation of biofilms on the reactor surfaces. However, many algae species, especially the flagellates, are very shear-sensitive and the mixing system used must not damage the cells (Barbosa et al. 2003; Sánchez Mirón et al. 2003).
Algae biofuel plants will also require the transfer of very large amounts of water (see Table 3) and therefore the location of the plant with relation to the water source (i.e. height and distance needed to pump the water) will affect the energy requirements for pumping and the overall economics. Similarly, the distance from the CO2 source will impact on the cost of supplying the CO2 to the algal cultures (Doctor et al. 2005).
3.1 Raceway pond scale-up
The long and extensive use of raceway ponds for commercial algae culture and wastewater treatment means that their characteristics are well understood. In raceway ponds, turbulence is dependent on the culture velocity which in turn is dependent on the dimensions of the ponds, the materials of construction (concrete, plastic lined, clay lined etc.) and the water depth. While culture velocities as low as 5 cm.s−1 are sufficient to maintain many microalgae cells in suspension, velocities between about 20 and 30 cm.s−1 are optimal and are essential for many algae species to prevent the cells from settling (Borowitzka 2005; Oswald 1988). Higher velocities require significantly more energy. As the depth of raceway ponds should not exceed 30 cm for optimum light exposure of the algal cells, the size limit for a raceway pond at these mixing velocities is about 1 ha, with ponds of about 0.5 ha area being the most common. A major limitation to achieving very high productivities in open raceway ponds is that they need to be operated at depths of 20–30 cm and that mixing and turbulence which affect both nutrient availability and the average irradiance received by the cells is relatively low (Borowitzka 1998a; Grobbelaar 2009a; Richmond et al. 1990). Greater mixing and higher productivities can be achieved by faster flow rates, but the energy cost to achieve this is very high (Oswald 1988). The main options to maximize productivity available to the algaeculturalist are to manipulate pond depth and cell density to maximize the effective use of the available irradiance (e.g., Vonshak et al. 1982). However, operating at lower biomass densities will affect the costs of harvesting as larger volumes have to be processed. The use of aeroplane-type ‘wings’ placed in the pond to enhance the generation of turbulence has also been proposed by Laws et al. (1983) to stimulate the ‘flashing light effect’ and thus enhance productivity, but these have never been applied on a large scale.
As the size of raceway ponds increases, the hydrodynamics of the system change and the pattern of water flow, especially at the ends of the ponds, changes significantly and will affect productivity and culture stability. At the ends of the raceway ponds where the direction of flow changes the flow pattern is very uneven. This leads to regions of very high flow, regions of low flow and eddies in different parts of the pond leading to pontential localized settling out of the algal cells. Several methods to create a more even flow pattern to minimize settling of the algal cells have been developed. One early innovation to achieve a more even flow pattern in the ponds was the installation of flow-rectifiers in the pond corners (Shimamatsu 1987). More even flow can also be achieved by having an eccentrically placed curved wall and baffles at the end of the pond furthest away from the paddle wheel (Dodd 1986). This creates a curved zone of accelerating flow followed by a flow expansion zone after the directional change has been made. The rate of constriction of the curved zone needs to be sufficient to avoid eddies and leads to more even water flow.
Since algae cultures generally are also CO2-limited, a supply of CO2 is required to achieve maximum productivity. The source of the CO2 can be flue gas from power stations (coal, gas or oil fired), CO2 from cement plants, or almost any other CO2 source. The CO2 can be supplied by using a diffuser/carbonator and several different designs have been developed. Becker (1994): summarised these as:
-
(1)
active gas transfer by sparging small gas bubbles into the medium or spraying the liquid through the gas phase; and
-
(2)
passive transfer by creation of large contact areas between a CO2-rich atmosphere and the surface of the culture medium.
The simplest, but most effective, type basically consists of a plastic sheet supported by a floating frame made from PVC pipe (Vasquez and Heussler 1985). Diffusers on the pond bottom release CO2 into the water and the gas inflates the plastic dome which prolongs its contact with the water. Spoilers across the injector produce a high turbulence in the running algal suspension for more efficient gas transfer into the liquid. As CO2 addition causes acidification of the medium the CO2 supply is regulated by a pH-stat system thus maintaining a constant pH in the medium.
3.2 Photobioreactor scale-up
The scale-up of closed photobioreactors is much harder to discuss because of the plethora of closed photobioreactor designs. Some of the key design criteria are: surface-to-volume ratio, reactor orientation and inclination, mixing and degassing, cleaning, temperature regulation, transparency and durability of the construction material, and ease of operation (Borowitzka 1996; Richmond 2000; Tredici 2004). Early attempts in scaling-up photobioreactors (see for example Tredici 2004) have met with problems such as reactor instability and premature break-down, inadequate mixing, leading to low productivities, as well as high oxygen build-up and rapid biofouling of internal reactor walls, inadequate temperature control and contamination.
The size (volume) of column/tower reactors is limited by two main factors: the column diameter is limited by the penetration of light into the culture, and the height is limited to a maximum height above which axial mixing rate would decrease and above which the elongated structure will not be able to withstand strong wind loads (Janssen et al. 2002).
Flat panel reactors are limited to a capacity of about 1 m3 (Richmond and Cheng-Wu 2001). This is due to materials constraints to support the culture volume as the thickness of the reactor should not exceed about 5 cm for optimum light for the algae and to achieve adequate mixing of the culture.
The largest closed photobioreactors are the tubular photobioreactors. As with the plate reactors the maximum tube diameter for optimum light supply to the cells is about 4–5 cm. In the period the algae are in the tube the CO2 concentration declines (and pH rises) and the O2 concentration increases due to photosynthesis. Thus the maximum length of the tube depends on the flow rate (usually 30–50 cm.s−1 to prevent cell settling) and is determined by the need to provide CO2 to the cells and to remove the photosynthetically-produced O2 which inhibits photosynthesis due to the oxygenase activity of Rubisco (Raven 1997), and there must be an efficient degasser at the end of the tube (Molina Grima et al. 1999) to remove the excess O2. Even so, if airlifts are used to circulate the medium, the oxygen content of the medium will always be greater than air saturation. Foaming at the top of the degasser can also lead to biomass loss and build-up of a biofilm which can lead to culture instability. Longer tube lengths also lead to loss in turbulence due to frictional loss (Weissmann and Goebel 1988), which translates into a bigger laminar sub-layer on the internal tube surface and longer light/dark cycles (Janssen et al. 2002) as well as a higher energy requirement. Various studies have found that the maximum allowable tube lengths are between 60 and 100 m. Larger reactors are constructed by connecting the tubes to manifolds where gas exchange occurs (Borowitzka 1999a). The largest operational closed photobioreactor to date is the 700 m3 tubular photobioreactor plant in Klötze, Germany, which consists of 20 reactor modules with a total glass tube length of about 500 km and produces about 150 t of Chlorella per year (Moore 2001).
Generation of higher velocities and shorter light/dark cycles close to 10 Hz would be desirable. However, an upper limit is set by the limited pressure tolerance of the typically employed transparent materials of construction and by the sensitivity of many algal species, especially the flagellated species, to hydrodynamic shear, especially shear generated by the pumping system. A possible alternative to improving photosynthetic efficiency is the inclusion of light through optical fibers within the reactor, as suggested by Csögör et al. (2001) and Janssen et al. (2002), or static helical mixers to generate more turbulence (Rosello Sastre et al. 2007). Hydrodynamic shear also significantly limits the species which can be cultivated and is an important factor in the choice of circulation system (Barbosa et al. 2003; Chisti and Moo-Young 1996). The scale-up of the pumping system is especially difficult as the micro-scale of shear within the pumps and its effect on the algae (which is very species dependent) is usually unknown. The types of pumps used to circulate the culture in large photobioreactors include airlifts, centrifugal pumps, lobe pumps and diaphragm pumps. Closed photobioreactors also have a potential problem caused by the formation of biofilms on the inner surface of the reactor which affect light penetration and flow.
As shown above, individual closed photobioreactors are limited in capacity and therefore a production plant will have very many reactors. The arrangement of these reactors is important as shading from adjacent reactors will affect overall areal productivity and the optimum spacing between the reactors needs to be determined (Chini Zittelli et al. 2006).
4 Culture management
Quite often, the success of outdoor cultivation attempts has been dampened and cut short due to an inadequate understanding of the biology and ecology of the algae. Microalgae cultivated outdoors are exposed to daily and annual climatic changes and to non-sterile growth conditions (Note: even the ‘closed’ photobioreactors cannot be maintained sterile for periods longer than about 1–2 weeks at best). As a result, there is always a dynamic shift in the environmental and biological parameters within the culture which, if left uncontrolled, can quickly lead to culture collapse, making reliable outdoor cultivation, let alone on a large scale, quite challenging. Common impediments encountered in outdoor cultures are:
-
reduced photosynthetic activity in the middle of the day due to high light and high O2 (Grobbelaar 2009a) and, on cool mornings, due to sub-optimal culture temperatures (Vonshak et al. 2001), thereby reducing photosynthetic efficiency,
-
photoinhibition and photooxidation due to excessive dissolved oxygen (Richmond and Grobbelaar 1986; Vonshak et al. 1996) [This is especially a problem in closed photobioreactors],
-
Significant biomass loss through respiration at night (Grobbelaar and Soeder 1985) which is higher at suboptimal temperatures (Torzillo et al. 1991), hence leading to additional losses in biomass productivity,
-
Gradual change in phenotypic and genotypic traits of the algae through adaptation (Lakeman et al. 2009),
-
Contamination, competition and/or predation by other organisms (other algae, bacteria, viruses, fungi, zooplankton).
Culture reliability and stability are essential for commercial microalgal cultivation on a large scale and therefore careful selection of species and strains best suited to the culture system and the prevailing environmental conditions plays a crucial role. Some of the desirable characteristics are: (a) broad tolerance to temperature (approx. 0–35°C—i.e., the minimum night temperature and the maximum likely day temperature encountered in dry desert regions), (b) smaller photosynthetic antenna size for more efficient use of light (Melis et al. 1999; Polle et al. 2002), (c) a high light saturation coefficient (Es) so that the algae are less likely to be photoinhibited at high irradiances, (d) a high photosynthetic efficiency (α) (Meyers 1953), (e) higher affinity for carbon dioxide and/or HCO −3 and less sensitivity to high dissolved oxygen levels (Vonshak et al. 1996), (f) algae which display ‘weedy’ features such as more rapid and efficient uptake of nutrients, and (g) fast growth in non-fastidious (cheap) media. Species control/retention is best managed by keeping culture conditions (temperature range, pH, salinity, nutrient type and quantity) optimum for the desired algal species and/or increasing the frequency of dilution to wash out unwanted species and by prophylactic treatment of the water, although the last two methods are generally impractical and too costly on a large scale.
4.1 Culture system control and production facility operation
Given that labour is an important cost factor in an algal production plant (Borowitzka 1992, 1999b), and given the required scale of operation with very many ponds or photobioreactors, the integration of semi-automated and fully automated control systems in the daily monitoring and maintenance of the cultures is essential (Olaizola 2000). A wide array of sophisticated methods is now available combined with process control software and a large commercial algae production plant will need to employ these. For example, cell counting of dead and live cells and bacterial detection can be conveniently determined by flow cytometry (Sosik et al. 2010) and the physiological state of the algae can now be readily and easily determined using fluorescence techniques such as the pulse amplitude modulated fluorometry (PAM) (Kromkamp et al. 2009; Masojidek et al. 2010). The integration of such quick measurement techniques into process control algorithms is highly desirable so that culture ‘health’ can be assessed rapidly and preventive measures can be implemented earlier to maintain optimum growth conditions and avoid culture crashes. Already, the application of intelligent modeling systems (see references in Greenwell et al. 2010) for real time control of system parameters such as pH (including CO2 addition), nutrients, (and light and temperature in closed photobioreactors) and in the harvesting and post-harvesting processes are being used in several commercial algae plants.
5 Harvesting, thickening and dewatering
A key step in the production of algal biofuels is harvesting and dewatering of the algal biomass before extraction or other processing. The high cost of harvesting and dewatering presents major challenges to the development of commercially viable microalgae-based biofuels. For a process to be considered suitable for the recovery of microalgal biomass for biofuel production, it must be able to process very large volumes (see Table 4), be highly reliable, be flexible and it must be cost effective. As microalgae vary greatly in those properties which affect harvesting processes (e.g., size, surface charge, resistance of cell to breakage, compressibility etc.) between species and with growth phase (Danquah et al. 2009; Lee et al. 1998), the biomass recovery processes must be tailored to the species of microalgae and the growth system. As very large amounts of water are used in microalgae culture the medium, which still contains nutrients, must also be able to be recycled after cell harvesting. At the large volumes of an algae biofuels production plant these remaining nutrients represent a valuable resource, and if not recycled this nutrient-containing medium would also represent considerable disposal issues.
The harvesting, thickening and dewatering of microalgae cultures has been extensively reviewed by Shelef (1974); Moraine et al. (1980), Mohn (1988) and Molina Grima et al. (2004). Here we will only outline some of the key aspects.
Key properties of microalgae which influence their separation are: (a) shape: [rods, spheres or chains or filaments], (b) size: [generally between 2 and 30 μm], (c) specific weight: [1.05–1.1], (d) surface charge: [usually negative]. Microalgal cultures to be harvested usually contain between 0.2 to 2 g.L−1 solids and for lipid extraction a concentration of at least 20 g.L−1 solids is required. Filamentous algae such as Spirulina can be harvested by filtration (Belay 1997), but almost all of the algae under consideration as a source of biofuels (e.g., Nannochloropsis or Chlorella) are unicellular and too small for effective filtration. Centrifugation as the only step is too energy intensive (Mohn 1988) and not practical for the extremely high volumes required to be processed for algal biofuels production. Sedimentation is also a possibility, but is generally too slow to be effective (Shelef et al. 1984).
The most commonly considered processes are flocculation followed by flotation or by settling as the first step. Flocculation can be achieved by the use of inorganic flocculants such as alum (Knuckey et al. 2006; Papazi et al. 2009) or organic flocculants such as chitosan (Morales et al. 1985) or starch (Vandamme et al. 2010), although the cost of these flocculants is substantial. The flocculant used must be compatible with the need to recycle the water back to the growth system without complex pretreatment of this recycled water. Other flocculation methods which are being explored include autoflocculation, where the algae are induced to self-flocculate usually by raising the pH due to photosynthetic CO2 uptake (Sukenik and Shelef 1984; Yahi et al. 1994), or bioflocculation by co-cultured bacteria or other algae species (Eisenberg et al. 1981; Lee et al. 2009, 2010a), and alternate methods such as electroflocculation (Poelman et al. 1997; Sandbank et al. 1974) and ultrasound (Bosma et al. 2003). Flocculation of marine and halophilic algae species is generally easier than flocculation of freshwater species because of the high ionic strength of the medium (Ayoub et al. 1986; Sukenik et al. 1988). An alternative process to flocculation may be magnetic separation using magnetic particles such as Fe3O4. The coagulated particles can then be passed through a magnetic field for separation (Bitton et al. 1975; Snook 1983).
Once flocculated the algae then must be concentrated further by settling or by flotation. Flocculation and dissolved air flotation (DAF) are commonly used in wastewater treatment plants to remove the algal/bacterial biomass (Féris and Rubio 1999) and are easily scaled and fairly well understood processes. However, for the production of a relatively low-value product such as biodiesel the cost of the flocculants and the energy cost of a DAF system are significant. Furthermore, an additional thickening step, or steps, such as belt filtration or centrifugation, will probably be required to achieve the solids concentration needed for the next processing step. These steps are illustrated in Fig. 1 and Table 4 gives an indication of the scale-up required to process volumes in each of these steps.
In conclusion, reducing the cost harvesting and dewatering of microalgae cultures for biofuels production with a high volume throughput presents significant challenges. It is most likely that multi-staged separation processes need to be used to amplify the solid content. Due to algae interspecies differences, it is likely that for each microalga species a unique harvesting, thickening and dewatering process will need to be designed (Borowitzka 2010a). The recovery system must (1) be able to handle extremely large volumetric throughputs, (2) be highly reliable, (3) flexible, (4) operate at extremely low cost and have a low energy requirement, and (5) be compatible with recycling of the growth medium and other downstream processes. If microalgae are to be grown in saline water, which is essential for sustainability in most regions (Borowitzka and Moheimani 2011), then the materials of the recovery systems must also be resistant to corrosion. For the recovery of microalgae biomass from the culture medium and before extraction several unit processes are likely to be required (cf. Fig. 1 and Table 4): (1) harvesting (primary concentration) which increases biomass concentration by about 10 fold, (2) thickening (secondary concentration) which thickens the primary concentrate by an additional 10 fold, (3) dewatering (tertiary concentration) which increase the solid content to 15–25% and, if required, (4) drying (quaternary concentration) which removes unbound water (for algae biofuels production drying of the biomass is probably impossible because of the high energy requirement—see below). It is apparent that there is no one unique, ideal, or universal operation, or even sequence of operations, which can be recommended; individual unit operations must be combined in the most suitable, economical and sustainable way for the recovering microalgae and the selection of methods will be strongly influenced by the species of algae.
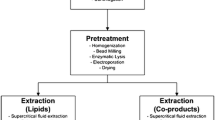
6 Extraction and further downstream processing
Low cost scalable extraction methods for lipids and sugars for subsequent conversion to biodiesel and ethanol at an industrial scale still remain to be developed, optimized and trialed (see Cooney et al. 2009 for a recent review). Many methods are available, but few, if any, have been trialed as yet at scale. Because of the prohibitively high energy requirement that would be needed to dry the algal cells because of the high latent heat of water (Cooney et al. 2009; Yang et al. 2004), the extraction process must be able to work with wet biomass. Solar drying has been proposed, but the large volumes which need to be processed mean that a very large land area is required and the capital and labor costs are likely to be prohibitive. Alternatively, it may be possible to integrate waste heat (i.e., from a power station) into a drying process (Katchanov 2010) in some cases.
The extraction process will determine the degree of dewatering required in the preceding harvesting and dewatering steps. The extraction process also will be species-dependent, mainly because of the variety of cell coverings of different microalgae species; in many algae the cell walls need to be broken to disrupt the cells in order to achieve efficient extraction.
6.1 Cell disruption
Breaking of the algal cell covering is essential for efficient extraction and negates the need to use elevated temperature and pressure extraction processes. Microalgae have a variety of cell coverings, ranging from the ‘naked’ cells of Dunaliella which only have a thin outer glycocalyx, to the highly-resistant algenin containing cell walls of Nannochloropsis and Chlorella, and the silica frustules of diatoms (Atkinson et al. 1972; Corre et al. 1996; Hatanaka et al. 1998; Schmid et al. 1981). Methods which have been used to break the cells include mechanical processes such as bead mills, sonication, cavitation and autoclaving. Non-mechanical methods include freezing, osmotic shock, enzymatic digestion, use of organic solvents, and acid or base reactions. (Cooney et al. 2009; Cravotto et al. 2008; Kanel and Guelcher 1998; Lee et al. 2010b; Pernet and Tremblay 2003; Ranjan et al. 2010). Because of the wide variety of cell wall types and chemistry between microalgae, different methods will be required for different species, but so far none have been applied on a large scale, nor has the energy requirement and cost impact on the whole process been evaluated as yet.
6.2 Extraction of lipids
Lipids are mostly hydrophobic molecules (e.g., neutral lipids) which interact with relatively non-polar solvents such as ethyl ether, chloroform and benzene, while membrane-associated polar lipids require polar solvents such as ethanol and methanol to disrupt the hydrogen bonding and electrostatic forces between the lipids and proteins. The most common laboratory methods for lipid extraction are the Soxhlet extraction (usually with n-hexane as solvent), and the Folch (Folch et al. 1951) and Bligh and Dyer (Bligh and Dyer 1959; Kates and Volcani 1966) methods which use chloroform and methanol in varying ratios as solvents. Because of the toxicity of methanol and chloroform, and the fact that chloroform extracts more than just saponifiable lipids (e.g., pigments and other lipids and non-lipid contaminants), methods using other alcohols such as ethanol, 1-butanol and isopropanol are being developed to replace the methanol (Nagle and Lemke 1990). Other combinations of co-solvents also have been proposed for the extraction of lipids from microalgae are hexane/ethanol (Cartens et al. 1996), and hexane/isopropanol (Nagle and Lemke 1990). In the hexane system the hexane and alcohol will readily separate into two separate phases when water is added, thereby improving downstream separations (Schaefer 1998). Furthermore, the combination of ethanol and hexane at a 5:1 v/v ratio in a 2-step extraction procedure reduces the solvent requirement for the same amount of biomass by approximately 10-times compared to the chloroform/methanol extraction procedure (Fajardo et al. 2007). It also eliminates the use of the toxic chloroform. This is advantageous in terms of organic solvent utilization in a very large scale production of biodiesel from microalgae as it reduces the solvent requirement and where the ethanol could be produced as a byproduct by fermentation of the microalgae sugars. Solvent extraction can be accelerated by the use of microwave or ultrasound energy (Cravotto et al. 2008; Lee et al. 2010b) but so far the energy costs at scale have not been evaluated.
An interesting new development is the use of switchable solvents. These solvents are capable of turning from a non-ionic form to an ionic liquid by the bubbling of CO2, and can be reconverted to the non-ionic form by bubbling N2. Switchable polarity solvents are lipophilic in the non-ionic form and hydrophilic in the ionic form (Handy 2003; Phan et al. 2009). The application of switchable solvents to algae has been limited so far. Samori et al. (2010) have used switchable solvents to extract hydrocarbons from Botryococcus braunii. Switchable solvents are part of the so-called ‘green solvents’ which have lower toxicity and flammability than more conventional solvents. Other ‘green’ solvent systems include subcritical water extraction and supercritical fluid extraction with CO2 (Herrero et al. 2006), or co-solvent mixtures of ionic liquids and polar covalent molecules such as ethanol as has been used to extract lipids from Chlorella and Dunaliella by Young et al. (2010). A potential disadvantage of some of these ‘green’-solvent extractions is the generally high energy requirement compared to conventional organic solvents.
Despite the wide range of lipid extraction methods available little is known about the scalability of the processes and the economics of their application on an industrial scale with microalgal biomass (Cooney et al. 2009).
7 Conversion into biofuel
The energy contained in the algal biomass can be recovered in a number of ways including direct thermochemical liquefaction (Demirbas 2010; Yang et al. 2004). However the current main interest in algae is the production of liquid fuels, especially biodiesel and jet fuel, from the algal lipids.
The oils or lipids from microalgae are very similar to plant oils which usually contain free fatty acids, phospholipids, sterols, water, and other impurities which cannot directly be used as fuel and which affect the efficiency of the transesterification reaction. Compared to higher plant lipids the lipids of many algal species are also relatively rich in polyunsaturated fatty acids which negatively affect the properties and quality of the biodiesel. These oils require chemical modification such as transesterification, pyrolysis or hydrogenation to produce fuel of acceptable quality (Murugesan et al. 2008). The current standards for biodiesel which must be met are discussed by Knothe (2006, 2010) Methods for production of biodiesel and the quality of the biodiesel produced from algal oils have been reviewed extensively recently (Amin 2009; Borowitzka 2010a; Brennan and Owende 2010; Demirbas 2009; Meher et al. 2006; Vasudevan and Briggs 2008), and will not be covered in detail in this paper. The three main approaches currently used for biodiesel production are base catalyzed transesterification, acid catalyzed transesterification (with simultaneous esterification of free fatty acids) and noncatalytic conversion (Demirbas 2003; McNeff et al. 2008).
Transesterification is currently the main process by which fuel is produced from different natural oil sources such as vegetable oil or cooking waste oil. Transesterification or alcoholysis is the reaction of a lipid with an alcohol to form esters and a by-product, glycerol. The type of catalyst is the most important variable in the conversion process. Nagle and Lemke (1990), using algal lipids, achieved a maximum yield of 68% with a hydrochloric acid-methanol catalyst compared to a yield of 32% with sodium hydroxide as the catalyst. Although catalyst-free processes for biodiesel production exist, such as a supercritical methanol method with a reaction temperature of 350°C and a molar ratio of methanol of 42 (Kudsiana and Saka 2001), they have a large requirement of methanol and energy, as well as a requiring more costly equipment, probably making them uneconomical for large scale biodiesel production.
Methanol (methanolysis) is commonly used in the conventional biodiesel production process, and although ethanol (ethanolysis) has been shown to produce a more environmentally friendly fuel and is less toxic than methanol, however ethanolysis is more expensive. Both alcohols are not miscible with triglycerides under normal room temperature condition and so the mass transfer in the reaction mixture is usually increased by mechanical stirring and emulsions are formed. In methanolysis, the emulsion formed is unstable and will break down quickly and easily to form a lower glycerol-rich layer and upper methyl-rich layer, whereas the emulsion formed by ethanolysis is more stable and the separation and purification of ester is more complicated (Anastopoulos et al. 2009; Ehimen et al. 2010).
The lipids used for the transesterification reaction must be free of water as the presence of even small amounts of water reduces the efficiency of the reaction (Bikou et al. 1999; Canakci and Van Gerpen 1999). This means that the algal biomass must be dried before lipid extraction (a very energy intensive step), or the extracted lipids must be dried before transesterification. The presence of free fatty acids also reduces the efficiency of the reaction (Ma et al. 1998) and the extracted algal lipids may need pretreatment to reduce the free fatty acid content. Alternatively, acid catalyzed transesterification with simultaneous esterification of free fatty acids can be carried out using sulphuric, hydrochloric, phosphoric or sulphonic acid (Meher et al. 2006). However, acid catalyzed transesterification has a slower reaction rate than alkali catalyzed transesterification, and the acids are more corrosive, thus making the process more expensive.
Recently there have been several studies of biodiesel production from algae by direct (in situ) transesterification of the algal biomass (Ehimen et al. 2010; Johnson and Wen 2009; Koberg et al. 2010; Wahlen et al. 2011; Xu and Mi 2011) including one patent (Machacek and Smith 2009). In situ transesterification negates the need for the lipid extraction step which has a high energy requirement for the vacuum evaporation to recover the solvent (usually hexane), process heating and stirring during transesterification. Cooney et al. (2009) have compared several direct transesterification methods using various solvents (methanol or the ionic liquid 1-ethyl-3-methylimidazolium methyl sulphate) and catalysts (hydrochloric acid, sulphuric acid, acetyl chloride) on a number of species of microalgae. Koberg et al. (2010) used direct transesterification of Nannochloropsis biomass suspended in a mixture of methanol-chloroform (1:2 v/v) using microwaves and a SrO catalyst. Similarly, Wahlen et al. (2011) also used a microwave, but used methanol with sulphuric acid in various ratios as catalyst. Two of these studies (Koberg et al. 2010; Wahlen et al. 2011) present some evidence that direct transesterification produces significantly more biodiesel than would be expected from available tryglycerides alone, indicating conversion of fatty acids in other molecules such as phospholipids.
However, almost all of these in situ transesterification studies have used dried algal biomass thereby still requiring the energy intensive drying step of the harvested algal biomass which is probably impractical. In the few studies with wet algal biomass the presence of water has been found to significantly reduce the yield of the fatty acid methyl esters (FAME) (Wahlen et al. 2011) However, by increasing the amount of methanol, the effect of the water could be reduced but this would increase costs. So far all of these studies have only been carried out at the laboratory scale only and no comparative economic studies between the various methods have been carried out. An important question to be answered is the economics of drying the algae and then using direct transesterification as compared to the more conventional extraction of the wet biomass, drying of the extract and then transesterifying the lipids.
8 Summary
In order to achieve the significant production of algal oils for biofuels required if these fuels are to partially replace fossil fuels requires the production of the algal biomass, harvesting and extraction of the biomass, and the efficient conversion of the lipids to fuels, all on a very large scale and at low cost. All the unit process steps shown in Fig. 1 need to be optimized and optimally integrated to minimize costs. As pointed out by Cooney et al. (2009), there is still a wide gap between existing technologies (especially in the extraction/fractionation process) and an industrial scale economic microalgae-based biofuel process.
References
Acién Fernández FG, Fernández Sevilla JM, Sánchez Pérez JA, Molina Grima E, Chisti Y (2001) Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: assessment of design and performance. Chem Eng Sci 56:2721–2732
Amin S (2009) Review of biofuel oil and gas production processes from microalgae. Energy Convers Manag 50:1834–1840
Anastopoulos G, Zannikou Y, Stournas S, Kalligeros S (2009) Transesterification of vegetable oils with ethanol and characterization of the key fuel property of ethyl esters. Energies 2:362–376
Ansell AD, Raymont JEG, Lauder KF, Crowley E, Shackley P (1963) Studies on the mass culture of Phaeodactylum. II. The growth of Phaeodactylum and other species in outdoor tanks. Limnol Oceanogr 8:184–206
Atkinson AW, Gunning BES, John PCL (1972) Sporopollenin in the cell wall of Chlorella and other algae: ultrastructure, chemistry, and incorporation of 14C-acetate, studied in synchronous culture. Planta 107:1–32
Ayoub GM, Lee S, Koopman B (1986) Seawater induced algal flocculation. Water Res 20:1265–1271
Barbosa MJ, Albrecht M, Wijffels RH (2003) Hydrodynamic stress and lethal events in sparged microalgae cultures. Biotechnol Bioeng 83:112–120
Becker EW (1994) Microalgae. Biotechnology and microbiology. Cambridge University Press, Cambridge, p 293
Belay A (1997) Mass culture of Spirulina outdoors—the Earthrise Farms experience. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biochemistry. Taylor & Francis, London, pp 131–158
Bikou E, Louloudi A, Papayannakos N (1999) The effect of water on the transesterification kinetics of cotton seed oil with ethanol. Chem Eng Technol 22:70–75
Bitton G, Fox JL, Strickland HG (1975) Removal of algae from Florida lakes by magnetic filtration. Appl Environ Microbiol 30:905–908
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1992) Algal biotechnology products and processes: matching science and economics. J Appl Phycol 4:267–279
Borowitzka MA (1996) Closed algal photobioreactors: design considerations for large-scale systems. J Mar Biotechnol 4:185–191
Borowitzka MA (1997) Algae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (1998a) Limits to growth. In: Wong YS, Tam NFY (eds) Wastewater treatment with algae. Springer, Berlin, pp 203–226
Borowitzka MA (1998b) Tubular photobioreactors for large-scale algal culture. In: Subramanian G, Kaushik BD, Venkatamaran GS (eds) Cyanobacterial biotechnology. Oxford &IBH Publishing Co, New Delhi, pp 249–257
Borowitzka MA (1999a) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (1999b) Economic evaluation of microalgal processes and products. In: Cohen Z (ed) Chemicals from microalgae. Taylor & Francis, London, pp 387–409
Borowitzka MA (2005) Culturing microalgae in outdoor ponds. In: Anderson RA (ed) Algal culturing techniques. Elsevier Academic, London, pp 205–218
Borowitzka MA (2010a) Algae oils for biofuels: chemistry, physiology, and production. In: Cohen Z, Ratledge C (eds) Single cell oils. Microbial and algal oils. AOCS, Urbana, pp 271–289
Borowitzka MA (2010b) Carotenoid production using microalgae. In: Cohen Z, Ratledge C (eds) Single cell oils. Microbial and algal oils. AOCS, Urbana, pp 225–240
Borowitzka LJ, Borowitzka MA (1989) ß-Carotene (Provitamin A) production with algae. In: Vandamme EJ (ed) Biotechnology of vitamins. Pigments and growth factors. Elsevier Applied Science, London, pp 15–26
Borowitzka MA, Hallegraeff G (2007) Economic importance of algae. In: McCarthy PM, Orchard AE (eds) Algae of Australia: introduction. ABRS, Canberra, pp 594–622
Borowitzka MA, Moheimani NR (2011) Sustainable biofuels from algae. Mitig Adapt Strateg Glob Change. doi:10.1009/s11027-010-9271-9
Bosca C, Dauta A, Marvalin O (1991) Intensive outdoor algal cultures—how mixing enhances the photosynthetic production rate. Bioresour Technol 38:185–188
Bosma R, van Spronsen WA, Tramper J, Wijffels RH (2003) Ultrasound, a new separation technique to harvest microalgae. J Appl Phycol 15:143–153
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extraction of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Burlew JS (1953) Algae culture. From laboratory to pilot plant. Carnegie Institution of Washington, Washington, pp 1–357
Canakci M, Van Gerpen J (1999) Biodiesel production via acid catalysis. Trans ASAE 42:1203–1210
Cartens M, Molina-Grima E, Robles-Medina A, Giminez Giminez A, Ibanez Gonzalez J (1996) Eicosapentaenoic acid (20:5n-3) from the marine microalgae Phaeodactylum tricornutum. JAOCS 73:1025–1031
Chaumont D, Thepenier C, Gudin C, Junjas C (1988) Scaling up a tubular photoreactor for continuous culture of Porphyridium cruentum from laboratory to pilot plant (1981–1987). In: Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D (eds) Algal biotechnology. Elsevier Applied Science, London, pp 199–208
Cheng-Wu Z, Zmora O, Kopel R, Richmond A (2001) An industrial-size flat plate glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture 195:35–49
Chini Zittelli G, Rodolfi L, Biondi N, Tredici MR (2006) Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 261:923–943
Chisti Y, Moo-Young M (1996) Bioprocess intensification through bioreactor engineering. Trans IChemE 74:575–583
Cooney M, Young G, Nagle N (2009) Extraction of bio-oils from microalgae. Sep Purif Rev 38:291–325
Corre G, Templier J, Largeau C, Rousseau B, Berkaloff C (1996) Influence of cell wall composition on the resistance of two Chlorella species (Chlorophyta) to detergents. J Phycol 32:584–590
Craggs RJ, Davies-Colley RJ, Tanner CC, Sukias JP (2003) Advanced pond system: performance with high rate ponds of different depths and areas. Water Sci Technol 48:259–267
Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, Cintas P (2008) Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem 15:898–902
Csögör Z, Herrebauer M, Schmidt K, Posten C (2001) Light distribution in a novel photobioreactor—modelling for optimisation. J Appl Phycol 13:325–333
Cysewski GR, Lorenz RT (2004) Industrial production of microalgal cell-mass and secondary products—species of high potential: Haematococcus. In: Richmond A (ed) Microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 281–288
Danquah MK, Gladman B, Moheimani N, Forde GM (2009) Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem Eng J 151:73–78
Del Campo JA, Rodríguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 85:289–295
Demirbas A (2003) Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers Manag 44:2093–2109
Demirbas A (2009) Production of biodiesel from algae oils. Energy Sources A 31:163–168
Demirbas A (2010) Use of algae as biofuels. Energy Convers Manag 51:2738–2749
Doctor R, Palmer A, Coleman D, Davidson J, Hendricks C, Kaarstad O, Ozaki M, Austell M (2005) Transport of CO2. In: Metz B, Davidson O, de Coninck H, Loos M, Meyer L (eds) IPCC special report on carbon dioxide capture and storage. Cambridge University Press, Cambridge, pp 179–193
Dodd JC (1986) Elements of pond design and construction. In: Richmond A (ed) CRC handbook of microalgal mass culture. CRC, Boca Raton, pp 265–283
Doucha J, Livansky K (1995) Novel outdoor thin-layer high density microalgal culture system: productivity and operational parameters. Algol Stud 76:129–147
Doucha J, Livansky K (1999) Process of outdoor thin-layer cultivation of microalgae and blue-green algae and bioreactor for performing the same. USA Patent 5,981,271
Doucha J, Straka F, Livansky K (2005) Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol 17:403–412
Ehimen EA, Sun ZF, Carrington CG (2010) Variables affecting the in situ transesterification of microalgae lipids. Fuel 89:677–684
Eisenberg DM, Koopman B, Benemann JR, Oswald WJ (1981) Algal bioflocculation and energy conservation in microalgal sewage ponds. Biotechnol Bioeng Symp 11:429–448
Fajardo AR, Esteban-Cerdan L, Robles-Medina A, Acién Fernández FG, Gozalez-Moreno PA, Molina-Grima E (2007) Lipid extraction from the microalga Phaeodactylum tricornutum. Eur J Lipid Sci Technol 109:120–126
Féris LA, Rubio J (1999) Dissolved air flotation (DAF) at low saturation pressures. Filtr Sep 36:61–65
Folch J, Lees M, Stanley GHS (1951) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Fulks W, Main KL (1991) The design and operation of commercial-scale live feeds production systems. In: Fulks W, Main KL (eds) Rotifer and microalgae culture systems. The Oceanic Institute, Honolulu, pp 3–52
Garcia-Camacho F, Contreras Gomez A, Acién Fernández FG, Fernandez Sevilla JM, Molina Grima E (1999) Use of concentric-tube airlift photobioreactors for microalgal outdoor mass cultures. Enzyme Microb Technol 24:165–172
Green FB, Bernstone LS, Lundquist TJ, Oswald WJ (1996) Advanced integrated wastewater pond systems for nitrogen removal. Water Sci Technol 33:207–217
Greenwell HG, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface. doi:10.1098/rsif.2009.0322
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Grobbelaar JU (1981) Deterministic production model for describing algal growth in large outdoor mass algal cultures. U O F S Publ No. 3:173–181
Grobbelaar JU (1994) Turbulence in mass algal cultures and the role of light dark fluctuations. J Appl Phycol 6:331–335
Grobbelaar JU (2000) Physiological and technical considerations for optimising algal cultures. J Appl Phycol 12:201–206
Grobbelaar JU (2009a) Factors governing algal growth in photobioreactors: the “open” versus “closed” debate. J Appl Phycol 21:489–492
Grobbelaar JU (2009b) Upper limits of productivity and problems of scaling. J Appl Phycol 21:519–522
Grobbelaar JU, Soeder CJ (1985) Respiration losses in planktonic green algae cultivated in raceway ponds. J Plankton Res 7:497–506
Grobbelaar JU, Nedbal L, Tichy V (1996) Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photo acclimated to different light intensities and implications for mass algal cultivation. J Appl Phycol 8:335–343
Hall DO, Acién Fernández FG, Cañizares Guerrero E, Rao KK, Molina Grima E (2003) Outdoor helical tubular photobioreactors for microalgal production: modeling of fluid-dynamics and mass transfer and assessment of biomass productivity. Biotechnol Bioeng 82:62–73
Handy ST (2003) Greener solvents: room temperature ionic liquids from biorenewable resources. Chem Eur J 9:2938–2944
Hase R, Oikawa H, Sasao C, Morita M, Watanabe Y (2000) Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai City. J Biosci Bioeng 89:157–163
Hatanaka Y, Inaoka K, Kobayashi O, Higashihara M, Hiyama K (1998) Sensitivity of the surface coat of the halotolerant green alga Dunaliella parva (Volvocales, Chlorophyceae) to lysozyme. Phycol Res 46:1–147
Herrero M, Cifuentes A, Ibanez E (2006) Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae. A review. Food Chem 98:136–148
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Nat Acad Sci USA 103:11206–11210
Hu Q, Kurano N, Kawachi M, Iwasaki I, Miyachi S (1998) Ultrahigh-cell-density culture of a marine green alga Chlorococcum littorale in a flat-plate photobioreactor. Appl Microbiol Biotechnol 49:655–662
Huntley M, Redalje D (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strateg Glob Chang 12:573–608
Janssen M, Tramper J, Mur LR, Wijffels RH (2002) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol Bioeng 81:193–210
Jiménez C, Cossío BR, Labella D, Niell FX (2003) The feasibility of industrial production of Spirulina (Arthrospira) in southern Spain. Aquaculture 217:179–190
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizotrychium limacinum by direct transesterification of algal biomass. Energy Fuels 23:5179–5183
Jorquera O, Kiperstock A, Sales EA, Embirucu M, Ghirardi ML (2010) Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour Technol 101:1406–1413
Kanazawa T, Yuhara T, Sasa T (1958) Mass culture of unicellular algae using the “open circulation method”. J Gen Appl Microbiol 4:135–152
Kanel JS, Guelcher SA (1998) Method for rupturing microalgae cells. PCT Patent 9828407
Katchanov EH (2010) Energy production from algae in photobioreactors enriched with carbon dioxide. US Patent Application 2010/0233787
Kates M, Volcani BE (1966) Lipid components of diatoms. Biochim Biophys Acta 116:264–278
Knothe G (2006) Analyzing biodiesel: standards and other methods. JAOCS 83:823–833
Knothe G (2010) Biodiesel: current trends and properties. Top Catal 53:714–720
Knuckey RM, Brown MR, Robert R, Frampton DMF (2006) Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquac Eng 35:300–313
Koberg M, Cohen M, Ben-Amotz A, Gedanken A (2010) Bio-diesel production directly from the algae biomass of Nannochloropsis by microwave and ultrasound radiation. Bioresour Technol. doi:10.1016/j.biortech.2010.12.004
Kok B (1953) Experiments on photosynthesis by Chlorella in flashing light. In: Burlew JS (ed) Algal culture. From laboratory to pilot plant. Carnegie Institution of Washington, Washington, pp 63–75
Kromkamp JC, Beardall J, Sukenik A, Kopecky J, Masojidek J, Van Bergeijk S, Gabai S, Shaham E, Yamshon A (2009) Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems. Aquat Microb Ecol 56:309–322
Kudsiana D, Saka S (2001) Methyl esterification of free fatty acids of rapeseed oil as treated in supercritical methanol. Fuel 80:225–231
Lakeman MB, von Dassow P, Cattolico RA (2009) The strain concept in phytoplankton ecology. Harmful Algae 8:746–758
Laws EA, Terry KL, Wickman J, Chalup MS (1983) A simple algal production system designed to utilize the flashing light effect. Biotechnol Bioeng 25:2319–2335
Lee YK (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:307–315
Lee YK, Ding SY, Low CS, Chang YC, Forday WL, Chew PC (1995) Design and performance of an α-type tubular photobioreactor for mass cultivation of microalgae. J Appl Phycol 7:47–51
Lee SJ, Kim SB, Kim JE, Kwon GS, Yoon BD, Oh HM (1998) Effects of harvesting method and growth stage on the flocculation of the green alga Botryococcus braunii. Lett Appl Microbiol 27:14–18
Lee AK, Lewis DM, Ashman PJ (2009) Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J Appl Phycol 21:559–567
Lee AK, Lewis DM, Ashman PJ (2010a) Energy requirements and economic analysis of a full-scale microbial flocculation system for microalgal harvesting. Chem Eng Res Des 88:988–996
Lee JY, Jun SY, Ahn CY, Oh HM (2010b) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S71–S74
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Lu Y, Zhai Y, Liu M, Wu Q (2010) Biodiesel production from algal oil using cassava (Manihot esculenta Crantz) as feedstock. J Appl Phycol 22:573–578
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, freee fatty acids and water on transesterificatioon of beef tallow. Trans ASAE 41:1261–1264
Machacek MT, Smith TG (2009) Continuous algal biodiesel production facility. USA Patent US 2009/0071064
Martinot E, Dienst C, Weilang L, Qimin C (2007) Renewable energy futures: targets, scenarios, and pathways. Annu Rev Environ Resour 32:205–239
Masojidek J, Vonshak A, Torzillo G (2010) Chlorophyll fluorescence applications in microalgal mass cultures. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic science: methods and applications. Springer, Dordrecht, pp 277–292
Matsumoto H, Shioji N, Hamasaki A, Ikuta Y (1996) Basic study on optimization of raceway-type algal cultivator. J Chem Eng Jpn 29:541–543
McNeff CV, McNeff LC, Yan B, Nowlan DT, Rasmussen M, Gyberg AE, Krohn BJ, Fedie RL, Hoye TR (2008) A continuous catalytic system for biodiesel production. Appl Catal A 343:39–48
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sustain Energy Rev 10:248–268
Melis A, Neidhardt J, Benemann J (1999) Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol 10:515–525
Mendes A, Reis A, Vasconcelos R, Guerra P, Lopes da Silva T (2009) Crypthecodinium cohnii with emphasis on DHA production: a review. J Appl Phycol 21:199–214
Meyers J (1953) Growth characteristics of algae in relation to the problems of mass culture. In: Burlew JS (ed) Algae culture. From laboratory to pilot plant. Carnegie Institution of Washington, Washington, pp 37–54
Moheimani NR, Borowitzka MA (2006) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 18:703–712
Mohn FH (1988) Harvesting of micro-algal biomass. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 395–414
Molina-Grima E, Garcia-Camacho F, Sanchez Perez JA, Acién Fernández FG, Fernandez Sevilla JM (1997) Evaluation of photosynthetic efficiency in microalgal cultures using averaged irradiance. Enzyme Microb Technol 21:375–381
Molina Grima E, Ácien Fernandez FG, Garcia Camacho F, Chisti Y (1999) Photobioreactors: light regieme, mass transfer, and scaleup. J Biotechnol 70:231–247
Molina Grima E, Acién Fernández FG, Robles Medina A (2004) Downstream processing of cell-mass and products. In: Richmond A (ed) Microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 215–251
Moore A (2001) Blooming prospects? EMBO Rep 2:462–464
Moraine R, Shelef G, Sandbank E, Bar-Moshe Z, Shvartzbund A (1980) Recovery of sewage-borne algae: flocculation, flotation, and centrifugation techniques. In: Shelef G, Soeder CJ (eds) Algae biomass. Elsevier, Amsterdam, pp 531–545
Morales J, de la Noüe J, Picard G (1985) Harvesting marine microalgae species by chitosan flocculation. Aquac Eng 4:257–270
Moreno J, Vargas MA, Rodriguez H, Rivas J, Guerrero MG (2003) Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomol Eng 20:191–197
Murugesan A, Umarani C, Chinnusamy TR, Krishnan M, Subramanian R, Neduzchezhain N (2008) Production and analysis of bio-diesel from non-edible oils—a review. Renew Sustain Energy Rev 13:825–834
Nagle N, Lemke P (1990) Production of methyl ester fuel from microalgae. Appl Biochem Biotechnol 24(25):355–361
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12:499–506
Oswald WJ (1988) Large-scale algal culture systems (engineering aspects). In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 357–394
Oxley J, Startari JF (1999) Modified bioreactor. PCT Patent 99/15620
Papazi A, Makridis P, Divanach P (2009) Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol 22:349–355
Pernet F, Tremblay R (2003) Effect of ultrasonication and grinding on the determination of lipid class content of microalgae harvested on filters. Lipids 38:1191–1195
Phan L, Brown H, White J, Hodgson A, Jessop PG (2009) Soybean oil extraction and separation using switchable or expanded solvents. Green Chem 11:53–59
Poelman E, De Pauw N, Jeurissen B (1997) Potential of electrolytic flocculation for recovery of microalgae. Resour Conserv Recycl 19:1–10
Pohl P, Kohlhase M, Martin M (1988) Photobioreactors for the axenic mass cultivation of microalgae. In: Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D (eds) Algal biotechnology. Elsevier Applied Science, London, pp 209–217
Polle JEW, Kanakagiri S, Jin ES, Masuda T, Melis A (2002) Truncated chlorophyll antenna size of the photosystems—a practical method to improve microalgal productivity and hydrogen production in mass culture. Int J Hydrogen Energy 27:1257–1264
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Pushparaj B, Pelosi E, Tredici MR, Pinzani E, Materassi R (1997) An integrated culture system for outdoor production of microalgae and cyanobacteria. J Appl Phycol 9:113–119
Ranjan A, Patil C, Moholkar VS (2010) Mechanistic assessment of microalgal lipid extraction. Ind Eng Chem Res 49:2979–2985
Raven JA (1997) Putting the C in phycology. Eur J Phycol 32:319–333
Richmond A (1996) Efficient utilization of high irradiance for production of photoautotrophic cell mass: a survey. J Appl Phycol 8:381–387
Richmond A (1999) Physiological principles and modes of cultivation in mass production of photoautotrophic microalgae. In: Cohen Z (ed) Chemicals from microalgae. Taylor & Francis, London, pp 353–386
Richmond A (2000) Microalgal biotechnology at the turn of the millenium: a personal view. J Appl Phycol 12:441–451
Richmond A, Cheng-Wu Z (2001) Optimisation of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85:259–269
Richmond A, Grobbelaar JU (1986) Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 10:253–264
Richmond A, Lichtenberg E, Stahl B, Vonshak A (1990) Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways. J Appl Phycol 2:195–206
Robinson LF, Morrison AW, Bamforth MR (1988) Improvements relating to biosynthesis. European Patent 261:872
Rodolfi L, Zitelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rosello Sastre R, Csögör Z, Perner-Nochta I, Fleck-Schneider P, Posten C (2007) Scale-down of microalgae cultivations in tubular photo-bioreactors—a conceptual approach. J Biotechnol 132:127–133
Samori C, Torri C, Samori G, Fabbri D, Galletti P, Guerrini F, Pistocchi R, Tagliavini E (2010) Extraction of hydrocarbons from microalga Botryococcus braunii with switchable solvents. Bioresour Technol 101:3274–3279
Sánchez Mirón A, Cerón García MC, Contreras Gómez A, García Camacho F, Molina Grima E, Chisti Y (2003) Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem Eng J 16:287–297
Sandbank E, Shelef G, Wachs AM (1974) Improved electroflotation for the removal of suspended solids from algae pond effluents. Water Res 8:587–592
Schaefer K (1998) Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal Chim Acta 358:69–77
Schmid AM, Borowitzka MA, Volcani BE (1981) Morphogenesis and biochemistry of diatom cell walls. In: Kiermayer O (ed) Cytomorphogenesis in plants. Springer Verlag, Vienna, pp 61–97
Scragg AH, Illman AM, Carden A, Shales SW (2002) Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 23:67–73
Setlík I, Veladimir S, Malek I (1970) Dual purpose open circulation units for large scale culture of algae in temperate zones. I. Basic design considerations and scheme of pilot plant. Algol Stud (Trebon) 1:111–164
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program—biodiesel from algae. National Renewable Energy Laboratory: Golden, Colorado. NREL/TP-580-24190, pp 1–328
Shelef G (1974) Process and apparatus for sewage treatment and wastewater reclamation. Great Britain Patent GB1358244
Shelef G, Sukenik A, Green M (1984) Microalgal harvesting and processing: a literature review. US Department of Energy: Golden Colorado. SERI/STR-231-2396, pp 1–65
Shimamatsu H (1987) A pond for edible Spirulina production and its hydraulic studies. Hydrobiologia 151/152:83–89
Snook H (1983) Apparatus and method for magnetic separation. PCT Patent WO83/01398
Soong P (1980) Production and development of Chlorella and Spirulina in Taiwan. In: Shelef G, Soeder CJ (eds) Algae biomass. Elsevier/North Holland Biomedical, Amsterdam, pp 97–113
Sosik HM, Olson J, Armbrust EV (2010) Flow cytometry in phytoplankton research. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 171–185
Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B (2010) An economic and technical evaluation of microalgal biofuels. Nature Biotechnol 28:126–128
Stephenson AL, Kazamia E, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy Fuels 24:4062–4077
Sukenik A, Shelef G (1984) Algal autoflocculation—verfication and proposed mechanism. Biotechnol Bioeng 26:142–147
Sukenik A, Bilanovic D, Shelef G (1988) Flocculation of microalgae in brackish and sea waters. Biomass 15:187–199
Tamiya H (1957) Mass culture of algae. Annu Rev Plant Physiol 8:309–344
Torzillo G, Sacchi A, Materassi R, Richmond A (1991) Effect of temperature on yield and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. J Appl Phycol 3:103–109
Torzillo G, Carlozzi P, Pushparaj B, Montaini E, Materassi R (1993) A 2-plane tubular photobioreactor for outdoor culture of Spirulina. Biotechnol Bioeng 42:891–898
Tredici MR (2004) In: Richmond A (ed) Mass production of microalgae: photobioreactors. Blackwell Science, Oxford, pp 178–214
Tredici MR, Materassi R (1992) From open ponds to vertical alveolar panels—the Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. J Appl Phycol 4:221–231
Tredici MR, Zittelli GC (1998) Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnol Bioeng 57:187–197
Tredici MR, Carlozzi P, Zittelli GC, Materassi R (1991) A vertical alveolar panel (VAP) for outdoor mass cultivation of microalgae and cyanobacteria. Bioresour Technol 38:153–159
van Bergeijk SA, Salas-Leiton E, Cañavate JP (2010) Low and variable productivity and low efficiency of mass cultures of the haptophyte Isochrysis aff. galbana (T-iso) in outdoor tubular photobioreactors. Aquac Eng 43:14–23
Vandamme D, Foubert I, Meesschaert B, Muylaert K (2010) Flocculation of microalgae using cationic starch. J Appl Phycol 22:525–530
Vasquez V, Heussler P (1985) Carbon dioxide balance in open air mass culture of algae. Arch Hydrobiol Ergebn Limnol, Beih 20:95–113
Vasudevan PT, Briggs M (2008) Biodiesel production—current state of the art and challenges. J Ind Microbiol 35:421–430
Verbruggen A, Al Marchohi M (2010) Views on peak oil and its relation to climate change policy. Energy Policy 38:5572–5581
Vonshak A (1997) Outdoor mass production of Spirulina: the basic concept. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biochemistry. Taylor & Francis, London, pp 79–99
Vonshak A, Abeliovich A, Boussiba S, Arad S, Richmond A (1982) Production of Spirulina biomass: effects of environmental factors and population density. Biomass 2:175–185
Vonshak A, Torzillo G, Accolla P, Tomaselli L (1996) Light and oxygen stress in Spirulina platensis (cyanobacteria) grown outdoors in tubular reactors. Physiol Plant 97:175–179
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Wahlen BD, Willis RM, Seefeldt LC (2011) Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour Technol 102:2724–2730
Walker DA (2009) Biofuels, facts, fantasy and feasibility. J Appl Phycol 21:508–517
Walmsley RD, Shillinglaw SN (1984) Mass algal culture in outdoor plastic-covered miniponds. Ann Appl Biol 104:185–197
Walter C, Steinau T, Gerbsch N, Buchholz R (2003) Monoseptic cultivation of phototrophic organisms—development and scale-up of a photobioreactor system with thermal sterilization. Biomol Eng 20:261–271
Weissmann JC, Goebel RP (1988) Photobioreactor design: mixing, carbon utilisation, and oxygen accumulation. Biotechnol Bioeng 31:336–344
Weyer KM, Bush DR, Darzins A, Willson BD (2010) Theoretical maximum algal oil production. Bioenergy Res 3:204–213
Xu R, Mi Y (2011) Simplifying the process on microalgal biodiesel production through in situ transesterification technology. JAOCS 88:91–99
Yahi H, Elmaleh S, Coma J (1994) Algal flocculation-sedimentation by pH increase in a continuous reactor. Water Sci Technol 30:259–267
Yang YF, Feng CP, Inamori Y, Maekawa T (2004) Analysis of energy conversion characteristics in liquefaction of microalgae. Resour Conserv Recycl 43:21–33
Young G, Nippgen F, Titterbrandt S, Cooney MJ (2010) Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules. Sep Purif Technol 72:118–121
Zittelli GC, Lavista F, Bastianini A, Rodolfi L, Vincencini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70:299–312
Zmora O, Richmond A (2004) Microalgae for aquaculture. Microalgae production for aquaculture. In: Richmond A (ed) Microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 365–379
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fon Sing, S., Isdepsky, A., Borowitzka, M.A. et al. Production of biofuels from microalgae. Mitig Adapt Strateg Glob Change 18, 47–72 (2013). https://doi.org/10.1007/s11027-011-9294-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11027-011-9294-x