Abstract
As a potential source of biomass supplies, cassava (Manihot esculenta Crantz) has been studied for bioethanol production, but not for the production of biodiesel. In this study, we used cassava hydrolysate as an alternative carbon source for the growth of microalgae (Chlorella protothecoides) which accumulated oil in vivo, with high oil content up to 53% by dry mass under a 5-L scale fermentation condition. The oils were extracted and converted into biodiesel by transesterification. The biodiesel obtained consisted of mainly unsaturated fatty acids methyl ester (over 82%), cetane acid methyl ester, linoleic acid methyl ester, and oleic acid methyl ester. This work suggests the feasibility of an alternative choice for producing biodiesel from cassava by microalgae fermentation. We report herewith the optimized condition for the fermentation and for the hydrolysis of cassava as the carbon source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In response to the commercialization of transport biofuel amid global petroleum crisis, biodiesel and bioethanol have gained increasing attention (Antoni et al. 2007). As renewable and biodegradable fuel, biodiesel is usually produced from either animal fat or oil crops, such as soybean, corn, rapeseed, palm, and castor bean (Bernardes et al. 2007). Nevertheless, these materials are not economically feasible because of low oil yield and high demands on land, water, and fertilizer.
Biodiesel from microalgae is being considered as promising biofuel, as microalgae have fast growth rate and high photosynthesis efficiency. They can also be industrially cultivated (Miao and Wu 2005). However, biodiesel production from microalgae is not economically feasible since rapid-growing cells contain less oil, whereas those cells accumulating high oil content show little growth ability. To address these biological and technical challenges, we propose a new approach to biodiesel production by a heterotrophic fermentation process which produces maximum amounts of algal biomass rich in oil. This concept is supported by our previous studies with Chlorella protothecoides (Xu et al. 2006), which was able to convert organic carbon into microalgal oil with high oil content (Xiong et al. 2008).
Compared with ethanol production by yeast fermentation, microalgae accumulate oils as intracellular products by utilizing an organic carbon source. In biofuel production, lower price of raw material means less cost of oil production. Accordingly, Jerusalem artichoke, sugar cane, sweet sorghum, and corn powder have been studied as alternative carbon sources for biodiesel production (Cheng et al. 2008, 2009; Gao et al. 2009; Xu et al. 2006). Cassava has been used for producing hydrocyanic acid, ethanol, and feedstuffs (Fujio et al. 1985). It is also well known for its tolerance to abiotic stress (Koch et al. 1994). Its adaptation to industrialization of biofuel and high biological efficiency are as important as reducing procreative cost (Vries et al. 1967). In addition, the tuberous roots of cassava can be harvested within 6 to 24 months depending on cultivating conditions (Cock et al. 1985). These characteristics prevent further occupation of farmlands. Nevertheless, utilizing cassava for large-scale production of biodiesel has not been previously reported.
This study utilized cassava starch as an alternative carbon source in batch and in 5-L fed-batch culture to produce high oil yield in C. protothecoides. Then, the biodiesel from algal oil using cassava as original feedstock was prepared. The application of this approach for large-scale fermentation to produce biodiesel is discussed.
Materials and methods
The strain of Chlorella protothecoides sp. 0710 was originally obtained from the Culture Collection of Algae at the University of Texas (Austin, Texas, USA) and then screened in the Laboratory of Microalgal Fermentation and Bioenergy at Tsinghua University, Beijing, China. The basal culture medium composition was: KH2PO4 0.7 g L−1, K2HPO4 0.3 g L−1, MgSO4·7H2O 0.3 g L−1, FeSO4·7H2O 3 mg L−1, glycine 0.1 g L−1, vitamin B1 0.01 mg L−1, A5 trace mineral solution 1 mL L−1. Different concentrations of glucose or other carbon sources were added to the basal culture medium.
Preparation of hydrolysates
Cassava starch was bought from Roi Et Flour Co., Ltd, Thailand. It was mixed with distilled water to obtain the 5%, 10%, and 20% (w/w) cassava starch solution. These solutions were gelatinized at 60–69°C with continuous stirring until they were fully preheated and dispersed. Based on the dissolution degree observed, we chose the optimal substrate concentration for enzymolysis reaction.
The 32 factorial experiments were designed to find the shortest time of enzymolysis reaction (Table 1). The two factors were liquefaction pH (A, at pH 5.5 (−1), 6.5 (0) and 7.5 (+1)) and saccharification pH (B, at pH 3.5 (−1), 4.5 (0) and 5.5 (+1)). In each parallel experiment, the starch solution was liquefied with alpha-amylase (2,000–5,000 U, Genencor CO, enzyme:substrate = 0.001:1 (w/w)) at 60 ± 2°C and pH 5.5–7.5. Then, it was saccharified by glucoamylase (100,000 U, Genencor CO., enzyme:substrate = 0.02:1 (w/w)) at 60 ± 2°C and pH 3.5–5.5. The iodometric and alcohol methods were used for confirming the end point of liquefaction and saccharification, respectively. All enzymes were inactivated at 100°C at the end. The concentration of reducing sugar in cassava starch hydrolysate (CSH) was measured by the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959) to calculate the hydrolysis rate. The preparation of corn powder hydrolysate (CPH) was as reported previously (Xu et al. 2006).
Shake-flask cultivation
Basal medium with 10 g L−1 glucose was used as control. CSH medium and CPH medium with 10 g L−1 reducing sugar was prepared by adding CSH and CPH solution in basal medium, respectively; 2 g L−1 yeast extract (YE) was added to these media as a nitrogen source and growth factor (Xiong et al. 2008). Chlorella protothecoides in exponential phase was inoculated (2.5%, v/v) into media (200 mL medium in 500 mL shake-flask) and heterotrophically cultivated in shake-flasks at 28 ± 0.5°C, 220 rpm. The biomass concentration and oil content were measured to determine the effects of CSH and CPH on cultivation.
Lab-scale fermentation
CSH was used as substrate with the initial reducing sugar concentration at 30 g L−1 in a 5-L bioreactor and 2 g L−1 YE was mixed with the CSH in both medium and supplement. All of those were added to the basal media and sterilized at 112°C, 0.12 MPa for 30 min. The original conditions of fermentation were: fermentation temperature 28 ± 0.5°C, pH 6.3, pO2 100%, agitation speed 300 rpm. The concentrated CSH and YE solution were batch-fed. During fermentation, all parameters were controlled by computer except the reducing sugar. The concentration of reducing sugar was controlled in the range of 8–25 g L−1 manually by batch-feeding, and the details of the culture conditions were as described previously (Xiong et al. 2008). The dissolved oxygen concentration (pO2) was kept over 20% by modulating agitation speed and airflow.
Monitoring of cell growth, reducing sugar consumption
Algal biomass was harvested and weighed after lyophilization to determine the final biomass. Cell growth was measured by optical density at 540 nm (Becker 1994). Samples were diluted to an appropriate concentration to keep the OD540 between 0.2 and 0.8. The dry cell weight corresponded to OD540 by the regression equation: y = 0.4155x (R 2 = 0.9933, P < 0.05), where y (g L−1) is the dry cell weight, x is the absorbance. The concentration of residual sugar was monitored at regular intervals by the DNS method.
Determination of oil content
Cells were collected by centrifugation and lyophilized. Oil content was analyzed by time-domain nuclear magnetic resonance (NMR) using a Bruker Minispec MQ20 NMR Analyzer (Bruker, Germany) with a 35-mm absolute probe, at resonance frequency of 19.95 MHz (Gao et al. 2008).
Oil extraction and biodiesel preparation by transesterification
Total oils were extracted by the Soxhlet method with n-hexane (Schäfer 1998). The volume of n-hexane used should ensure that liquid could reflux during each cycle by heating bath at 80 ± 2°C for 16 h. Then the solvent–solute combinations were evaporated by rotary evaporation (N-1000, Eyela, Japan) at 40 ± 2°C until no more distilling solvent was collected. Alga-based biodiesel was prepared by transesterification of the algal oil with the following parameters: oil:methanol = 1:56 (by molar), lipid:catalyst = 1:1 (w/w), at 30°C for 4 h (Xu et al. 2006).
Composition analysis of biodiesel
The composition of the biodiesel produced from the extracted algal oil was analyzed by GC-linked mass spectrometry (GC-MS). Dual-stage quadrupoles GC apparatus (Thermo, USA) was equipped with a Varian VF-5ms column (30 m × 0.25 mm ID DF = 0.25 μm) and with a flow rate of 10 mL min−1.
Results
Hydrolysis of cassava starch
As a raw material, cassava starch was gelatinized by pretreatment in heated water so that it can interact with enzyme more directly. Effect of gelatinization was determined by the viscosity of the solution, which was associated with the initial concentration of cassava starch in the solution. It was observed that groups 5% (w/w) and 10% (w/w) were completely gelatinized while there was still some insoluble substrate in the 20% (w/w) group. Thus, the suggested starch substrate concentration for gelatinization in the laboratory is 10% (w/w). The total enzymolysis time was the sum of liquefaction and saccharification time. The shortest total enzymolysis time at liquefaction pH 6.5 and saccharification pH 3.5 was 285 min (Table 2). In summary, the optimal reaction condition was as follows: 10% (w/w) cassava starch solution was incubated at 60–69°C for 30 min, and alpha-amylase (alpha-amylase:cassava starch = 0.001:1, by weight) was added at pH 6.5, maintaining 60 ± 2°C for 45 min, and then glucoamylase (glucoamylase:cassava starch = 0.02:1, by weight) was added at pH 3.5, maintaining 60 ± 2°C for 240 min. The shortest reaction time (285 min) was achieved at a liquefaction pH 6.5 and a saccharification pH 3.5, resulting in a hydrolysis ratio as high as 97.5 ± 1.5%. The results obtained under the optimal reaction condition in lab could supply enough reducing sugar for the cultivation of algal cells in heterotrophic growth.
Shake-flask cultivation of heterotrophic C. protothecoides with CSH
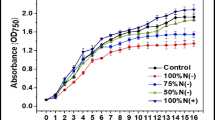
In order to study the effect of using cassava starch for alga-fermentation-based biodiesel production, comparison experiments in shake-flask cultivation using glucose, CPH, and CSH were conducted. It took 128, 120, and 100 h to reach the maximum cell density (concentration of biomass) in glucose, CPH, and CSH medium, respectively. Concentration of biomass both in CPH and CSH medium exceeded 4 g L−1 whereas it was less than 4 g L−1 in glucose medium (Fig. 1, Table 3). Either CPH or CSH medium performed better than glucose medium for heterotrophic growth of C. protothecoides perhaps because glucose is a purified substance with limited nutrition. Our previous study (Xu et al. 2006) and results of the shake-flask experiments also indicate that there are probably some beneficial growth factors in CSH and CPH. The experimental data sets including cell growth rate and oil yield were compared further (Table 3). They showed that the oil yield rate of algal cells in CSH medium was higher than that in glucose and CPH medium. There were also no significant differences in reducing sugar consumption rate among the cells cultivated with glucose, CPH, and CSH. Therefore, the conversion rate of sugar to oil using CSH as feedstock is good, and CSH is a preferable carbon source for alga-fermentation-based biodiesel production.
Cultivation of C. protothecoides in a 5-L bioreactor
To further improve cell density and oil content, a fermentation experiment was conducted in a 5-L bioreactor. Unlike the closed environment in the shake-flask, continuous feeding in the bioreactor provides algal cells with sufficient nutrients to maintain continuous growth during the cultivation cycle. Thus, this strategy has advantages in reducing substrate restriction to a minimum and increasing biomass yield.
The fermentation using C. protothecoides is shown in Fig. 2. The biomass concentration reached a maximum of 53.6 g L−1 after 168 h fermentation. The cell growth rate was 7.66 g L−1 day−1, which is over nine times higher than that in shake-flask cultivation (0.82 g L−1 day−1). In comparison with our previous fermentation studies, both biomass and oil yield using CSH as feedstock in a fed-batch system in this study were even higher than those using glucose (Table 4). We conclude that use of CSH in high-density fermentation of heterotrophic C. protothecoides is probably feasible to realize alga-fermentation-based biodiesel production at an industrial scale.
Composition of biodiesel
Biodiesel was produced from the heterotrophic C. protothecoides oil by acid transesterification. The composition of the biodiesel analyzed by GC-MS showed that there were three main fatty acid methyl esters including cetane acid methyl ester (C17H34O2), linoleic acid methyl ester (C19H34O2), and oleic acid methyl ester (C19H36O2). These components made up more than 90% of the total biodiesel. Some minor methyl esters were also detected (Table 5). The components implied that biodiesel produced from CSH was similar to that of glucose feeding, which had been characterized as having a heating value of 41 MJ kg−1, a density of 0.864 kg L−1, and a viscosity of 5.2 × 10−4 Pa s (at 40°C; Xu et al. 2006). The quality of biodiesel product from heterotrophic microalgal oil using CSH as feedstock is good enough for application in transportation or industry instead of fossil diesel fuel.
Discussion
Heterotrophic algae are currently considered as a promising potential feedstock for biodiesel, since oil is rapidly accumulated in these cells. In the present study, C. protothecoides was fed by CSH for efficient biodiesel production. As shown in Table 3, an average oil yield rate of 0.41 ± 0.02 g L−1 day−1 in shake-flask culture was achieved. This is much higher than that of photoautotrophic algae (eustigmatophyte Nannochloropsis sp.), which ranges from 0.055 to 0.061 g L−1 day−1 in 250-mL flask culture (Rodolfi et al. 2009).
Nevertheless, one of major obstacles for producing biodiesel from heterotrophic algae is the high dependence of carbon substrates, which account for about 80% of the total medium cost (Li et al. 2007). Thus, an economically acceptable and environmentally sustainable carbon source for alga-fermentation-based biodiesel is urgently needed. In the present study, hydrolysis of cassava starch to yield fermentable sugar was investigated as a possible strategy.
As a starch-rich material, cassava can be harvested in infertile soil and cultivated easily in tropical zones. After more than 10 months the yield of its fresh root is 18.9–27.1 t ha−1 and that of root dry matter is 5.10–9.16 t ha−1 by using new technologies (Lenis et al. 2006). Due to these characteristics, limited agricultural resources will not be further occupied during cassava starch production. Moreover, cassava is both a staple crop for over 105 countries and the cheapest source for more than 300 starch-based products. It is reported (United Nations Food and Agriculture Organization 2008) that cassava is the fourth most important cash crop widely grown in tropical Africa, Asia, and Latin America, with an annual production of approximately 238.2 million t (fresh weight) in 2008. Possibly, when there is a fine balance between edible crop and new biofuel, cassava could not only play a vital role for food security but also become an effective source of renewable fuel. Currently, the industrial price of cassava starch is about 240–300 USD−1 in Thailand (data from http://www.thaitapiocastarch.org/price.asp?xyear=2009), which is much lower than that of industrial glucose 320–360 USD−1 in 2009 (Gao et al. 2009). Thus, using cassava to substitute glucose for algal oil production might be feasible and cost-effective.
In this study, the hydrolyzing process of cassava starch was optimized by using 32 factorial experiments. As a result, a high hydrolysis ratio of 97.5 ± 1.5% was achieved in 285 min at pH 6.5/3.5. This enhanced hydrolysis efficiency is of special importance to reduce labor and electric power requirements, which will directly benefit cost control. In order to further reduce costs, the saccharification process can be coupled with fermentation. This integrated strategy has been adopted in bioethanol production. Besides the cost of substrates, cost also comes from maintenance of cultivation conditions, which accounts for about 51.5% of total algal biomass production cost (Li et al. 2007). To address this challenge, efforts range from metabolic engineering to genetic engineering. These studies, to some extent, can help to obtain higher yield of oil and higher conversion efficiency of sugar to oil. In addition, post-processes such as harvesting, drying, oil extraction, and transesterification are also important, since hydrolysis of cassava starch together with these processes constitute an intact technical flow for alga-fermentation-based biodiesel production.
At present, cassava-derived ethanol production has been reported. Our study further suggests cassava is also suitable for integrated production of microalgal biodiesel. Based on this research, further work on fermentation optimization and pilot-scale production will be performed to overcome the commercial barrier of biodiesel industrialization. Overall, utilization of cassava as a feedstock for biodiesel will provide a novel opportunity to boost industrial cassava agro-processing and promote commercial development of algal biodiesel.
References
Antoni D, Zverlov VV, Schwarz WH (2007) Biofuels from microbes. Appl Microbiol Biotechnol 77:23–35
Becker EW (1994) Measurement of algal growth. Microalgae Biotechnology and Microbiology. Cambridge University Press, Cambridge, pp 56–62
Bernardes OL, Bevilaqua JV, Leal MC, Freire DM, Langone MA (2007) Biodiesel fuel production by the transesterification reaction of soybean oil using immobilized lipase. Appl Biochem Biotechnol 137–140:105–114
Cheng Y, Zhou WG, Gao CF, Kenneth L, Gao Y, Wu QY (2008) Biodiesel production from Jerusalem artichoke (Helianthus tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J Chem Technol Biotechnol 84:777–781
Cheng Y, Lu Y, Gao CF, Wu QY (2009) Alga-based biodiesel production and optimization using sugar cane as the feedstock. Energy Fuels 23:4166–4173
Cock JH, Porto MCM, El-Sharkawy MA (1985) Water use efficiency of cassava. III. Influence of air humidity and water stress on gas exchange of field grown cassava. Crop Science 25:265–272
Fujio Y, Ogata M, Ueda S (1985) Ethanol fermentation of raw cassava starch with Rhizopus koji in a gas circulation type fermentor. Biotechnol Bioeng 27:1270–1273
Gao CF, Xiong W, Zhang YL, Yuan WQ, Wu QY (2008) Rapid quantitation of lipid in microalgae by time-domain nuclear magnetic resonance. J Microbiol Methods 75:437–440
Gao CF, Zhai Y, Ding Y, Wu QY (2009) Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy. doi:10.1016/j.apenergy.2009.09.006
Koch BM, Sibbesen O, Swain E, Kahn RA, Liangchen D, Bak S et al (1994) Possible use of a biotechnological approach to optimize and regulate the content and distribution of cyanogenic glycosides in cassava to increase food safety. Acta Hortic 375:45–60
Lenis JI, Calle F, Jaramillo G, Perez JC, Ceballos H, Cock JH (2006) Leaf retention and cassava productivity. Field Crops Res 95:126–134
Li XF, Xu H, Wu QY (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Miao XL, Wu QY (2005) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G et al (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Schäfer K (1998) Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal Chim Acta 358:69–77
United Nations Food and Agriculture Organization (2008) Food lookout. Global Information and Early Warning System on Food and Agriculture. ftp://ftp.fao.org/docrep/fao/011/ai474e/ai474e00.pdf. Accessed 25 October 2009.
Vries CA, Ferweds JD, Flach M (1967) Choice of crops in relation to actual and potential production in tropics. Neth J Agric Sci 15:241–246
Xiong W, Li X, Xiang J, Wu QY (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78:29–36
Xu H, Miao XL, Wu QY (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Acknowledgment
This study was funded by the NSF Guangdong joint project U0633009, NSF project 30670476 and 30970224, the National High Technology Research & Development Program of China (863 Program) 2007AA05Z400, and MOST overseas cooperation project 20070574.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Zhai, Y., Liu, M. et al. Biodiesel production from algal oil using cassava (Manihot esculenta Crantz) as feedstock. J Appl Phycol 22, 573–578 (2010). https://doi.org/10.1007/s10811-009-9496-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9496-8