Specifications for methods of measuring the concentration of hydrogen in solid samples are analyzed. Multiple discrepancies in the results of comparisons of modern standard samples from different manufacturers are found experimentally and the probable source of these discrepancies is the method used to heat and melt samples in a carrier gas flow for the measurements. It is concluded that this method is unsuitable for metrological support of measurements of the concentration of hydrogen in structural materials, since the metrological characteristics of these means of measurement do not meet the requirements for safe operation in the modern economy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hydrogen is the most “dangerous” element in the composition of structural materials. Its molecules are highly mobile and are redistributed among the local zones of the material by diffusion. The main distinction of hydrogen as an impurity is the very low amount of it in structural materials compared to other impurity elements. The concentration of hydrogen in aluminum alloys and ultrahard steels is typically less than one part per million by mass. Thus, in aluminum alloys, a hydrogen concentration of 0.1 ppm means that there is roughly one H2 molecule per million Al atoms. It has been found by experiment and mathematical analysis that a factor of two excess in the hydrogen content leads to loss of mechanical stability and fracture of a material [1]. Hydrogen is often the major cause for fracture; for example, up to 80% of fractures in oil vessels are associated with a heightened concentration of hydrogen.
Despite the catastrophic consequences of a heightened concentration of hydrogen (rapid brittle fracture, cold brittleness, explosions in gas pipelines), there is no unique metrological system for measuring the concentration of hydrogen. We consider two major problems with metrological support of measurements in this area: there is very little hydrogen, so highly sensitive means of measurement are necessary, and the reproducibility (convergence) of the results of analytic measurements employing different means of measurement should lie within twice the measurement error.
Metallurgists first encountered the destructive influence of hydrogen about 150 years ago. Since then, the task of reducing this influence has been approached in almost every stage of technological development, beginning with high-quality rolling (Krupp’s disease) and ending with the production of modern ultra-high durability steels, titanium, and aluminum-magnesium alloys. The maximum permissible (critical) hydrogen concentrations in a solid sample over the last 100 years have fallen by roughly a factor of 100, from 10 to 0.1 ppm or even 0.05 ppm for certain special aluminum alloys. At present, because of the low “norms” for the hydrogen concentration, these values can be doubled by the amount of moisture present in a cooling steam–gas mixture during rolling or by atmospheric humidity during welding of metals. Thus, technical monitoring is needed during the fabrication stage of ingots and castings, as well as during the subsequent stages involving rolling and forging [2,3,4]. The new measurement methods must provide reliable determinations of the hydrogen content in different materials, ranging from metals and alloys to semiconductors and nanostructured materials.
Hydrogen in solid samples can be subdivided into several groups with different binding energies ranging from 0.2 to 2 eV [5,6,7,8,9,10,11,12,13]. This fact is not taken into account in the existing classification. Diffusively-mobile and bound hydrogen are distinguished in steels. Various means of measurement exist for determining the concentration of diffusively-mobile hydrogen. But there is no base of standard samples for testing these means of measurement. In addition, there is no definition at all of diffusively-mobile and bound hydrogen, or an indication of how they differ from the diffusive hydrogen described in the standard of Ref. 14. For alloys of titanium, nickel, magnesium, and steels, the total hydrogen content is normalized. Two groups of hydrogen are distinguished in aluminum alloys according to the standard of Ref. 15: surface and dissolved. Here only the amount of dissolved hydrogen is normalized.
One of the most acute problems in modern metallurgy is the large fraction of secondary metals used in the production of alloys, which leads to a substantial increase in the hydrogen concentration in the finished product. Today, the raw material for steels from the major producer of ferrous metals (China) contains a high percentage of scrap metal. The European Union has issued a directive on the complete utilization of secondary metals in its territory by 2025. This means that the fraction of scrap metal in the raw material for steel production in Europe may reach 70% (with a current norm of no more than 7–8%). Because of the exhaustion of ore deposits, the fraction of secondary metal in some refractory alloys has already reached 100%.
The question of matching means of measurement with standards documents is decisive in ensuring the military and economic security of Russia. In the State Registry of Means of Measurement of the Russian Federation there are only three standard samples for hydrogen content in the list of available alloys out of a thousand designations. It is necessary to create a new metrological system for support of measurements of the concentration of hydrogen in solid samples over a short time and at a high scientific level.
Results of experimental studies. There are three major methods for measuring the concentration of hydrogen in solid samples: spectral; rapid heating or melting of a sample in a carrier gas flow; and vacuum heating and vacuum melting.
The spectral method is based on detecting the emission intensity of a small part of a solid sample during electrical breakdown of a gas located between the sample surface and an electrode. Hydrogen lines can be found by analyzing the emission spectrum. The sensitivity of this method is limited to hydrogen concentrations at a level of about 1 ppm. This level is an order of magnitude greater than the permissible concentration in modern materials, including in the aerospace industry (except for a number of titanium alloys). In addition, the hydrogen content is determined within a thin surface layer of the sample with a thickness on the order of 100 μm, and a representative sample for technical monitoring of most alloys must have a mass of several grams [15].
Vacuum heating and vacuum melting were the first methods for measuring the amount of hydrogen in solid samples. The important advantage of these methods is their “purity.” There is very little residual gas in the evacuated volume of the extraction system. Even if all of the residual gas were hydrogen, it could not seriously distort the measurement results since a representative sample contains on the order of 1 mm3 hydrogen under standard conditions. When the hydrogen extracted from a sample enters a high vacuum, its volume increases to 1 m3. This volume is a thousand times the volume of an actual measurement vacuum system. The pressure in the vacuum chamber increases by roughly an order of magnitude and during pumpdown to the original pressure, it is possible to determine the amount of extracted hydrogen exactly (with an error of ±10%). During the 1930s, a technique was developed for extracting hydrogen in a vacuum for all the main structural materials, including steels. The total amount of hydrogen is determined by vacuum measurement techniques based on a calibrated volume and pressure increment (volumetric method) or on the use of a mass spectrometer with integration of the time dependence of the hydrogen flow.
The need for technical monitoring in factory laboratories means that vacuum measurement techniques have been completely displaced by “fast” methods, where a sample is heated or melted in a carrier gas flow at atmospheric pressure and a hydrogen analyzer (e.g., LECO (USA) or its European analogs) is used. The fraction of these means of measurement in factory laboratories has gradually reached 99%. The major advantages of the “fast” methods are that the concentration of hydrogen is measured at atmospheric pressure and the analysis time is an order of magnitude less than with vacuum methods. The crucible with the heated sample is placed in a flow of spectrally pure argon or nitrogen (carrier gas). Contactless heating by radio-frequency (rf) pulses takes place in a graphite crucible. Hydrogen released from the sample is mixed with the carrier gas and passes through a sorption filter to remove contaminants and then enters the measurement cell. It is assumed that hydrogen is not sorbed on the sorbent or the harmful impurities trapped in it, and does not enter into chemical reactions with them. The operating principle of the measurement cell is based on the difference (by an order of magnitude) in the heat transfer coefficients of hydrogen and the carrier gas. The measured quantity is the difference in temperature between two heated filaments, one of which is in the flow of carrier gas and hydrogen, while the other is in a flow of pure carrier gas. Almost any impurity (metal vapor, halogens, water) produces contamination of the heat transfer surface of the spiral, so it is impossible to work without a sorbent. Aviation alloys contain significant magnesium and lithium impurities. The vapors of these metals even penetrate through the sorbent and gradually contaminate the surface of the spiral, so that the measurements are systematically distorted.
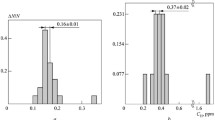
“Fast” analysis techniques are widely used in interlaboratory comparisons of the existing standard samples and for developing new ones. Thus, in 2014, 20 laboratories participated in international comparisons of standard samples of hydrogen in various chemical compositions. 19 of the laboratories used a “fast” analysis technique in a carrier-gas flow and only one (NPK EPT) used the vacuum heating method. A comparison of the vacuum and “fast” methods revealed substantial discrepancies in the measurements for the same standard samples. The vacuum-heating measurements were made with an AV-1 mass spectrometric hydrogen analyzer which underwent tests at VNIIM and is included in the State Register of Means of Measurement of the Russian Federation. The other participants in the comparisons (laboratories and manufacturers of standard samples) used a method of heating and melting in a carrier gas flow. The hydrogen content was determined using LECO (USA) and Bruker Elemental (Germany) analyzers. The results of the comparisons are listed in Tables 1 and 2.
Besides the reference sample (SO) steels, standard samples of pure titanium from the LECO company (LECO SDS 00500 PN 502-024, which are not included in the State Register of Means of Measurement of the Russian Federation) were also compared. The measurements using the vacuum-heating method agree to within the confidence interval, with an average value for the hydrogen concentration of \( \overline{Q} \) = 16 ppm measured by other participants in the comparisons.
The AV-1 hydrogen analyzer was calibrated using a GSO (national reference material) composition for a type 1201 aluminum alloy (SO-0, GSO 6007-91, type certificate No. 1723) with a certified hydrogen content of Qcert = 0.18 ppm and a confidence interval of 2Δconf = 0.02 ppm.
Discussion. The observed discrepancies in the measurements (see Tables 1 and 2) could be explained by calibration error, but the convergence of the measurement results with the certified value for the national reference material from LECO (see rows 4 and 5 of Table 1) and the titanium samples from LECO (\( \overline{Q} \) = 16 ppm) conflicts with this conclusion.
The vacuum-heating method can be used to measure hydrogen flows from a heated sample for tens of hours, until the hydrogen flow reaches the background level. The maximum temperature for extraction of hydrogen in vacuum for titanium and steel samples is 900°C. This value is high enough for complete extraction of hydrogen from solid and nanostructured samples [16,17,18,19].
In the standard of Ref. 20, it is shown that for complete removal of hydrogen from some types of steels it is necessary to use melting at temperatures of 1600–1900°C. But at these temperatures there will be active dissociation of water and hydrogen-containing compounds present in the carrier gas in amounts comparable to the amount of hydrogen in a sample with a mass of 2–5 g. In addition, water and hydrocarbons adsorbed on the inner surfaces of the extraction system will be dissociated and become a source of additional fluxes of hydrogen. The evaporation of zinc and nickel from galvanic coatings on steels can also lead to the release of hydrogen inside the sorbents from substances adsorbed on them. This easily explains the much higher measured hydrogen concentrations in steels with coatings that are observed when heating and melting methods are used in a carrier-gas flow [16].
Recent studies show that the discrepancies in the hydrogen concentration are especially high for comparisons of samples with certified hydrogen contents below 1 ppm [16, 21, 22]. Thus, a fivefold divergence in these values has been obtained [16] for samples with concentrations of 2–6 and 0.6 ppm. Here, all the measurements were made with analyzers from LECO by heating in a flow of carrier gas. Presumably, the main source of these large discrepancies is the technique for heating the samples during the analysis process [21, 22].
In measurements with the vacuum technique, the analytic section of the extractor with the sample to be analyzed is fully heated to the extraction temperature. This makes it possible to keep the sample at the specified temperature for an arbitrary time. The carrier gas, however, cannot be heated to a high temperature (the analysis method in a carrier gas flow) since this leads to distortions in the readouts from the thermal conductivity cell. This makes it necessary to use remote rf heating in which only the crucible with the sample is heated. In order to reduce thermal losses owing to convection, heat transfer, and diffusion, a powerful but short duration heating pulse is used. The carrier gas is at room temperature and a flow of this gas around the crucible cools the sample more rapidly, especially in the 700–200°C range. Hydrogen that is unable to diffuse outward from the sample volume within 3–5 min after heating is not extracted; this leads to large errors in the measurements as well as in calibrating the hydrogen analyzer.
We note that the amplitude, duration, and number of rf heating pulses are arbitrarily adjustable parameters. For example, the company’s setting for the LECO analyzer in studies of aluminum alloys involves the use of a single pulse. In practice, two-pulse heating is used in domestic laboratories.
It can, therefore, be said that the method of heating and melting a sample in a carrier gas flow and the apparatus for this method are not suitable for the development and certification of standards, or for the measurement of relatively low hydrogen concentrations in alloys. The result of the measurements depends on many unstable factors. In addition, heating by rf pulses requires special adjustment for samples of a given type in order to obtain convergence of measurements in samples with approximately the same composition but different concentrations of hydrogen. The measurement results are also affected by the number of times the crucible is reused and by the mass ratio of the crucible and sample [16].
Conclusion. Measurement of the concentration of hydrogen in solid samples is a most important form of technological monitoring for ensuring the quality and operational safety of machines and equipment. Nevertheless, at present the necessary base of standard samples, consistent terminology, and means of measurement do not exist to guarantee reliable measurement results. Standard systems for primary testing of means of measurement and standards for hydrogen content are also lacking.
An analysis of experimental data shows that vacuum techniques for measuring the concentration of hydrogen can provide reliable results and have minimal systematic errors. The use of vacuum techniques ensures a measurement accuracy that is sufficient for detection of levels of hydrogen concentration in solid samples that exceed the critical levels by a factor of two. New standards documents and standard samples have to be developed for these techniques.
Hydrogen analyzers based on the method of heating and melting in a carrier gas flow must be eliminated, especially since the imported instruments of this type and the standards documents for most applications of this method were developed decades ago and do not correspond to the level of current data obtained by domestic and foreign specialists.
References
A. M. Polyanskii, V. A. Polyanskii, and Yu. A. Yakovlev, “System for metrological support of measurements of the concentration of hydrogen in metals – a foundation of safety in the oil and gas industry,” Izmer. Tekhn., No. 3, 56–60 (2013).
A. M. Polyanskii, V. A. Polyanskii, A. N. Pronin, et al., “Metrological support for measurements of the hydrogen content in materials for enhanced safety of objects in the military complex,” Vest. Metrologa, No. 4, 30–36 (2012).
A. K. Belyaev, V. A. Polyanskiy, and Yu. A. Yakovlev, “Stresses in pipeline affected by hydrogen,” Acta Mechan., 224, No. 3–4, 176–186 (2012).
V. Polyanskiy, A. Polyanskiy, and Y. Yakovlev, “The material interaction with the solute hydrogen during fatigue failure,” Lightweight Design: 3rd Fatigue Symp., April 18–19, 2012, Leoben, Austria (2012), pp. 191–201.
G. M. Pressouyre, “A classification of hydrogen traps in steel,” Metallurg. Trans. A., 10, No. 10, 1571– 1573 (1979).
Tomoki Doshida and Kenichi Takai, “Dependence of hydrogen-induced lattice defects and hydrogen embrittlement of cold-drawn pearlitic steels on hydrogen trap state, temperature, strain rate and hydrogen content,” Acta Mater., 79(0), 93–107 (2014).
Hideki Hagi and Yasunori Hayashi, “Effect of dislocation trapping on hydrogen and deuterium diffusion in iron,” Trans. Japan Inst. Metals, 28(5), 368–374 (1987).
A. Kuduzovic. M. C. Poletti, C. Sommitsch, et al., “Investigations into the delayed fracture susceptibility of 34CrNiMo6 steel, and the opportunities for its application in ultra-high-strength bolts and fasteners,” Mater. Sci. Eng. A, 590(0), 66–73 (2014).
Il-Jeong Park, Seo Yeon Jo, Minwoo Kang, et al., “The effect of Ti precipitates on hydrogen embrittlement of Fe–18Mn–0.6C–2Al–xTi twinning-induced plasticity steel,” Corros. Sci., 89(0), 38–45 (2014).
A. M. Polyanskiy, V. A. Polyanskiy, and Yu. A. Yakovlev, “Experimental determination of parameters of multichannel hydrogen diffusion in solid probe,” Int. J. Hydrogen Energy, 39(30), 17381–17390 (2014).
D. Eliezer, E. Tal-Gutelmacher, C. Cross, and T. Boellinghaus, “Irreversible hydrogen trapping in welded beta-21s titanium alloy,” in: Fracture of Nano and Engineering Materials and Structures, E. Gdoutos (ed.), Netherlands, Springer (2006), pp. 985–986.
E. Barel, G. B. Hamu, D. Eliezer, and L. Wagner, “The effect of heat treatment and {HCF} performance on hydrogen trapping mechanism in timetal {LCB} alloy,” J. Alloys Compd., 468(12), 77–86 (2009).
Y. Nie, Y. Kimura, T. Inoue, et al, “Hydrogen embrittlement of a 1500-mpa tensile strength level steel with an ultrafine elongated grain structure,” Metall Mater. Trans. A, 43(5), 1670–1687 (2012).
GOST 23338-91, Welding of Metals. Methods for Determining the Content of Diffuse Hydrogen in Melted Metal and the Metal in a Seam.
GOST 21132.1-98, Aluminum and Aluminum Alloys. Method for Determining Hydrogen in Solid Metals by Vacuum-Heating.
A. Hassel, S. Merzlikin, A. Mingers, et al., Methodology of Hydrogen Measurements in Coated Steels, Publ. Office of the European Union, Luxembourg (2013), https://doi.org/10.2777/10253, acc. Aug. 3, 2017.
GOST 24956-81, Titanium and Titanium Alloys. Method for Determination of Hydrogen.
O. M. Protsenko, F. N. Karachevtsev, and E. A. Mekhanik, “Experience in developing a method for measuring the hydrogen content in titanium alloys,” Electr. Zh. Trudy VIAM, No. 12-8-8 (2014), doi: 10.18577/2307-6046-2014-0-12-8-8.
L. R. Saaknyan, A. P. Efremov, and I. A. Soboleva (eds.), Corrosion Protection of Petroleum Industry Equipment: A Worker’s Handbook, Nedra, Moscow (1985).
GOST 17745-90, Steel and Alloys. Method for Determining Gas Content.
A. M. Polyanskii, V. A. Polyanskii, and Yu. A. Yakovlev, “Investigation of the completeness of specimen degassing in an analysis of the hydrogen content of aluminum alloys,” Metallurgist, 55, No. 3–4, 303–309 (2011).
D. Yu. Andronov, D. G. Arseniev, A. M. Polyanskiy, et al., “Application of multichannel diffusion model to analysis of hydrogen measurements in solid,” Int. J. Hydrogen Energy, 42, No. 1, 699–710 (2017).
This work was supported by the Russian Foundation for Basic Research (Project Nos. 15-08-03112-a and 17-08-00783-a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 12, pp. 46–50, December, 2017.
Rights and permissions
About this article
Cite this article
Konopel’ko, L.A., Polyanskii, A.M., Polyanskii, V.A. et al. New Metrological Support for Measurements of the Concentration of Hydrogen in Solid Samples. Meas Tech 60, 1222–1227 (2018). https://doi.org/10.1007/s11018-018-1343-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-018-1343-3