Abstract
Maintaining the balance of mitochondrial fission and mitochondrial autophagy on seizures is helpful to find a solution to control seizures and reduce brain injuries. The present study is to investigate the protective effect of inhibiting mitochondrial fission on brain injury in juvenile rat epilepsy induced by pentatetrazol (PTZ) by inhibiting the BCL2L13/LC3-mediated mitophagy pathway. PTZ was injected (40 mg/kg) to induce kindling once every other day, for a total of 15 times. In the PTZ + DMSO (DMSO), PTZ + Mdivi-1 (Mdivi-1), and PTZ + WY14643 (WY14643) groups, rats were pretreated with DMSO, Mdivi-1 and WY14643 for half an hour prior to PTZ injection. The seizure attacks of young rats were observed for 30 min after model establishment. The Morris water maze (MWM) was used to test the cognition of experimental rats. After the test, the numbers of NeuN(+) neurons and GFAP(+) astrocytes were observed and counted by immunofluorescence (IF). The protein expression levels of Drp1, BCL2L13, LC3 and caspase 3 in the hippocampus of young rats were detected by immunohistochemistry (IHC) and Western blotting (WB). Compared with the PTZ and DMSO groups, the seizure latency in the Mdivi-1 group was longer (P < 0.01), and the severity degree and frequency of seizures were lower (P < 0.01). The MWM test showed that the incubation periods of crossing the platform in the Mdivi-1 group was significantly shorter. The number of platform crossings, the platform stay time, and the ratio of residence time/total stay time were significantly increased in the Mdivi-1 group (P < 0.01). The IF results showed that the number of NeuN(+) neurons in the Mdivi-1 group was greater, while the number of GFAP(+) astrocytes was lower. IHC and WB showed that the average optical density (AOD) and relative protein expression levels of Drp1, BCL2L13, LC3 and caspase 3 in the hippocampi of rats in the Mdivi-1 group were higher (P < 0.05). The above results in the WY14643 group were opposite to those in the Mdivi-1 group. Inhibition of mitochondrial fission could reduce seizure attacks, protect injured neurons, and improve cognition following PTZ-induced epilepsy by inhibiting mitochondrial autophagy mediated by the BCL2L13/LC3 mitophagy pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is the most common neurological disease in childhood. The prevalence of epilepsy is approximately 70 million people worldwide (Fiest et al. 2017). Sixty percent of epilepsy cases originate from childhood. Recurrent seizures cause neural damage and even necrosis, which affect children’s intellectual and motor development and contribute to children’s memory decline and intellectual and mental disorders (Shlobin and Sander 2022). Therefore, it is particularly important to understand the mechanisms underlying the process of epileptogenesis. At present, it is considered that the damage to brain neural mitochondria caused by frequent seizures leads to mitochondrial dysfunction, which is a major cause of recurrent seizures (Khurana et al. 2013). Mitochondrial homeostasis is a dynamically regulated process, and its balance can be mainly regulated by the key balance between mitochondrial dynamics and mitochondrial autophagy (Dulac et al. 2020). Further study of the effect of mitochondrial dynamics and mitochondrial autophagy on seizures is helpful to find a solution to control seizures and reduce brain injuries.
Mitochondrial function depends on mitochondrial fission and fusion to maintain dynamic balance, and mitochondrial fission may play a greater role (Parone et al. 2008). Dynamic related protein 1 (Drp1), mainly located in the cytoplasm, is a key protein in the regulation of mitochondrial fission and is transposed to the potential division site of mitochondria by the recruitment of Fis1, where it forms a ring structure and is gradually compressed until mitochondrial fission occurs. After mitochondrial fission, Drp1 returns to the cytoplasm (Smirnova et al. 2001). Seizures cause excessive mitochondrial fission. Drpl can interact with the proapoptotic protein Bax/Bak, participate in the induction process of neuronal apoptosis, and inhibit mitochondrial fusion (Oettinghaus et al. 2016). The above process aggravates the abnormal function of the mitochondrial electron transport chain, affects the processes of oxidative phosphorylation and ATP production, causes dysfunction of the sodium and potassium pumps on the cell membrane, aggravates cell damage and even causes cell death (Landes and Martinou 2011).
Autophagy is a regulated process that aims to maintain cell integrity and intracellular homeostasis (Galluzzi et al. 2014). Hyperactivity of autophagy may have toxic effects on cells and participate in the pathogenic process of neurodegenerative diseases (Palikaras et al. 2016; Zapata-Munoz et al. 2021). Damaged mitochondria are engulfed and degraded in cells through a selective autophagy mechanism to maintain mitochondrial network homeostasis, which plays an important role in maintaining mitochondrial homeostasis of quality and quantity (Vives-Bauza et al. 2010; Zhang et al. 2019). However, excessive activation of autophagy can lead to programmed cell apoptosis, thereby damaging normal cells (Su et al. 2019). Previous studies have found that excessive autophagy activation can lead to neuronal death and aggravate hippocampal injury under oxidative stress in epilepsy. Pretreatment with the autophagy inhibitor wortmannin (WM) can reduce the damage to hippocampal neurons in epileptic rats, increase the number of surviving neurons and inhibit the activation of autophagy (Xue et al. 2011; Carter et al. 2017).
Drp1 is a key protein in mitochondrial fission and is closely related to autophagy. Microtubule-associated protein 1 light chain 3 (MAP1LC3) is a marker molecule of autophagy (Heckmann et al. 2017). Drp1 can regulate the process of mitochondrial autophagy through interaction with LC3 connector protein or LC3 receptor (Shirihai et al. 2015). Drp1 can bind to the mitochondrial membrane protein Bcl-2-like protein 13 (BCL2L13), which is the LC3 receptor, and induce mitochondrial autophagy (Wu et al. 2016). A previous study showed that the protein expression of LC3-II was also downregulated following a decrease in the expression of Drp1, which can inhibit abnormal autophagy in mitochondria (Wu et al. 2022). In vitro studies of animal and cellular epilepsy models have demonstrated that the reduction in mitochondrial fission mediated by Drp1 can reduce the mitochondrial oxidative stress response, alleviate the inflammatory response and apoptosis, and protect damaged neuron (Kim and Kang 2017). Dysfunctions of the process of autophagy have been associated with epileptogenesis (Harris and Rubinsztein 2011). Some studies have investigated the potential role of autophagy in epilepsy, including the mammalian target of rapamycin (mTOR) pathway and Atg7 as essential promoters of autophagy (Wong 2010; Wong 2013). Thus, Drp1-mediated mitochondrial fission and the mitochondrial autophagy pathway play significant roles in epileptogenesis. The latest research found that regulation of Drp1 mediated mitochondrial fission and inhibition of autophagy might be a novel therapeutic treatment for brain injury associated with epilepsy (Xie et al. 2021; Lee et al. 2022). Furthermore, the specific molecular mechanism of mutual regulation between mitochondrial dynamics and mitochondrial autophagy remains to be further studied in the epilepsy. Therefore, in the present study, we investigated the possible antiepileptic effect of inhibiting mitochondrial fission on young rats with epilepsy induced by PTZ and its possible underlying mechanisms by the Drp1-mediated mitochondrial autophagy pathway.
Materials and methods
Experimental animals
Healthy young male Sprague–Dawley (SD) rats (3 ~ 4 weeks old), weighing 50 ~ 80 g, were obtained from and housed at the Center of Laboratory Animal Science, Fujian Medical University (SCXK-2016-0002). The rats were maintained and treated at a temperature of 21 ~ 25 °C and 60 ~ 70% humidity with a 12-h/12-h light/dark cycle. The rats ate and drank freely during the entire experiment. All experiments were conducted between 8 am and 10 am. All of the experimental animal procedures were performed according to international guidelines and based on the animal ethics committe of Fujian Medical University (2018–014).
Experimental groups and drug intervention
The chronic model of seizures was used according to the previous study (Dhir 2012). The young rats were intraperitoneally injected with drugs for intervention every other day for a total of 15 times (total 29 days) (Taskiran et al. 2020; Filiz et al. 2021). Pentylenetetrazole (PTZ) was dissolved in physiological saline. A mitochondrial division inhibitor (Mdivi-1) and WY14643 were dissolved in dimethyl sulfoxide (DMSO). The animals were randomly divided into control, PTZ, PTZ + DMSO (DMSO), PTZ + Mdivi-1 (Mdivi-1), and PTZ+ WY14643 (WY14643) groups, with ten rats in each group. The control group was treated with the same dose of physiological saline. In the PTZ group, physiological saline (1 mL/kg) was given 30 min before PTZ (40 mg/kg) injection every other day (Kaur et al. 2015). The DMSO group received 0.1% DMSO. The Mdivi-1 group received Mdivi-1 (Qiu et al. 2013) (1.2 mg/kg). The WY14643 group was given WY14643 (3.6 mg/kg) (Ko et al. 2016) before stimulation.
Behavioral observation
The juvenile rats were observed continually for 30 min after each intervention, and then the latency, degree and frequency of seizure attacks were recorded. Racine’s scale was used for measuring seizures, and the specific scores included grade I: facial clonus, staring and wet dog shakes; grade II: vertical movement of the head; grade III: rhythmic contraction of forelimb; grade IV: rhythmic contraction along with upright raising of the forelimbs; and grade V: rearing, jumping, falling and recurrent seizure (Hernandez-Ceron et al. 2017). When the rats attained a seizure score of more than phase 3 for at least three consecutive days, they was defined as “kindling”(Filiz et al. 2021).
Morris water maze test
The cognitive function of young rats was evaluated using the Morris water maze (MWM) test (Hu et al. 2017). Fifty experimental rats (10 in each group) were subjected to the above experiment for 5 days after 15 days of drug intervention. The swimming path of the rats was accurately tracked by video and identified through software (Yiyan Co., Shanghai, China). The device was a circular water tank with a diameter of 120 cm and a height of 50 cm that was filled with water (23 ± 2 °C). The escape platform was a transparent disc with a diameter of 10 cm and a height of 30 cm, which was placed 2 cm below the water surface. The water in the pool became opaque by adding edible bamboo charcoal powder, which made the platform invisible.
The MWM test was divided into two parts: positioning navigation and space exploration. The directional navigation experiment was conducted continuously for 4 days. The rats were trained 4 times in each time period for a fixed time every day. At the beginning of the training, the rats were placed in the pool from any point in the four quadrants to find the platform hidden under the water. If they stayed on the platform for more than 2 s, it was judged as a successful platform search. The escape latency was recorded as the time from entering the water to succeeding in finding the platform. If the rat could not find the platform within 60 s, the escape latency was counted as 60 s.
After the test, the rats were allowed to rest for 10 s before the next experiment. The escape latency (average value) every day was recorded to evaluate the spatial learning ability of the animals. The spatial exploration experiment was conducted on the 5th day. The platform was removed, and the animals entered the water from the opposite quadrant of the original platform. The number of times the animal passed through the original platform position within 60 s, the platform quadrant dwell time, and the ratio of the platform dwell time/total time were recorded to evaluate the spatial memory ability of the animal.
Immunofluorescence (IF) staining
After the MWM test, 25 rats (5 in each group) were transcardially perfused with 4% paraformaldehyde (PFA) under anesthesia using 1% pentobarbital sodium (50 mg/kg) by intraperitoneal injection. Dissected brains were embedded in paraffin and then cut into 5 μm thick coronal sections, and then were performed for IF assay and immunohistochemical staining (IHC). Briefly, tissue sections underwent dehydration through an ethanol gradient. Then, the sections were blocked and treated with 0.5% Triton X-100 for 15 min and 5% bovine serum albumin (BSA) for 1 h at 37 °C. The sections were incubated with GFAP polyclonal antibody (Cat. No. 16825–1-AP, 1:500, Proteintech, China) and NeuN polyclonal antibody (Cat. No. 26975–1-AP, 1:500, Proteintech) overnight at 4 °C. After washing with PBS three times, the sections were incubated with secondary antibodies for 30 min at 37 °C. The secondary antibodies were tetramethyl rhodamine isothiocyanate (TRITC)-conjugated anti-rabbit IgG (Cat. SA00013–2, 1:200; Proteintech) at room temperature for 1 h to detect the expression of GFAP and NeuN. Subsequently, the sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescent images were obtained under a fluorescence microscope (DMI8, Leica, Wetzlar, Germany). The image data were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA) to obtain quantitative information.
IHC staining
Hippocampus tissue sections were dewaxed, hydrated, and then blocked with 3% H2O2 at room temperature for 10 min. The specimens were incubated with Drp1 polyclonal antibody (Cat. No. 12957–1-AP, 1:200, Proteintech, China), BCL2L13 polyclonal antibody (Cat. No. 16612–1-AP, 1:200, Proteintech), LC3 polyclonal rabbit antibody (Cat. No. 14600–1-AP,1:200, Proteintech), and caspase 3 polyclonal antibody (Cat. No. 19677–1-AP, 1:200 Proteintech) at 4 °C overnight and then incubated with secondary goat anti-rabbit IgG (Cat. No. RS0011, 1:500, ImmunoWay, China) at 37 °C for 30 min. The slides were cultured in streptavidin-horseradish peroxidase (SA-HRP) (Boster, Science and Culture Center, CA, USA) at 37 °C for 30 min, observed with DAB for 15 s, and then stained with hematoxylin for 30 s. Images were obtained under an inverted microscope. The average optical density (AOD) values of Drp1, BCL2L13, LC3, and caspase 3 protein expression in the hippocampal areas were analyzed using ImageJ.
Western blotting (WB) analysis
Twenty five rats (5 in each group) were decapitated after function test. The brains were removed and dissected on cold glass plates. When the cortical hemisphere was completely detached from the side, the hippocampus at the bottom of the cerebral cortex was exposed and isolated immediately. Frozen hippocampal tissues of were dissolved in 0.5 ml lysis buffer containing protease and phosphatase inhibitors and then centrifuged at 12000 rpm for 20 min at 4 °C to extract protein. The extracted protein was determined by the Bradford method. According to molecular weight, the protein samples (50 μg/μl each) were electrophoretically separated on SDS polyacrylamide gels and then transferred onto polyvinylidene fluoride (PVDF) membranes.
After blocking with 5% skimmed milk for 1 h at room temperature, the PVDF membranes containing the proteins were incubated with Drp1 (1:500), BCL2L13 (1:500), LC3 (1:500), caspase 3 (1:500), and β-actin (Cat. No. 23660–1-AP, 1:1000, Proteintech) antibodies overnight at 4 °C. The membranes were washed in TBST buffer (10 mM Tris-HCl and 0.05% Tween 20) three times and incubated with goat anti-rabbit IgG (Cat. 04–15-06, 1:5000, KPL, USA) for 1 h at room temperature. The protein bands were observed by enhanced chemiluminescence (ECL) solution (Amersham, Piscataway, NJ, USA) and analyzed by the Bio-Image Analysis System (Bio–Rad, Hercules, CA, USA). Target protein expression using the densitometric ratio of the protein of interest to β-actin was quantified by ImageJ software.
Statistical analysis
SPSS 25.0 software (SPSS, Armonk, NY, USA) was used to analyze the experimental data. The mean ± standard deviation (SD) was used to express quantitative data. The homogeneity of variance was assessed by Levene’s test. If the variance was homogeneous, one-way ANOVA was performed, and the post hoc Tukey test was used for paired comparisons. A p value <0.05 was considered statistically significant.
Results
Epileptogenesis following drug intervention
The seizure attacks of half an hour in young rats were recorded at a fixed time after drug intervention. The young rats in the control group had no seizures. The rats in other groups induced by PTZ reached the kindling criterion on the 9th day, and continued with the same intervention until the 15th day. Compared to the PTZ and DMSO group, the Racine scale of young rat in the Mdivi-1 group was lower from the 8th day to the 15th day (P < 0.05). The Racine scale in the WY14643 was higher after the 11th day (P < 0.05). Compared with the PTZ and DMSO group, the number of seizure in the Mdivi-1 group was less from the 8th day to the 15th day (P < 0.05), and was more in the WY14643 group later than 10th day (P < 0.05). The seizure latency was compared on the 1st, 5th, 10th, and 15th days of PTZ injection. In comparison to the PTZ and DMSO group, seizure latency was increased in the Mdivi-1 group on the 10th and 15th days (P < 0.05), and decreased in the WY14643 group at the same time (P < 0.05). The above behavioral results demonstrated that Mdivi-1 effectively suppressed PTZ-induced epileptogenesis which could be aggravated by WY14643 (Fig. 1, Table S-1, 2, 3).
Epileptogenesis in young rats following PTZ kindling decreased by inhibiting mitochondrial fission. (A) Racine scale of seizures in the PTZ, PTZ + DMSO (DMSO), PTZ + Mdivi-1 (Mdivi-1), and PTZ + WY14643(WY14643) groups. (B) Frequency of seizure in the above four groups. (C) Lantency of seizures in the above four groups. Seizure attacks of the rats within 30 minutes were recorded after intraperitoneal injection of drugs. Mdivi-1 (a mitochondrial fission inhibitor) reduced seizure grade, decreased seizure frequency, and prolonged seizure latency in young rats induced by PTZ. WY14643 (a mitochondrial fission stimulator) increased seizure attacks. *P < 0.05, **P < 0.01, ***P < 0.001
Results of the Morris water maze (MWM) test
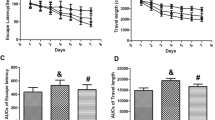
After successful modeling, the cognition of young rats was tested using the MWM test. Compared with the control group, the incubation periods of crossing the platform in the PTZ, DMSO and WY14643groups were significantly longer (P < 0.05). The crossing platform times, platform residence times and the ratios of residence time/total time in the above three groups were significantly shorter (P < 0.05). The above results in the Mdivi-1 group were close to those in the control group (P > 0.05). Compared with the PTZ group, the incubation period was longer in the WY14643 group (P < 0.05). The crossing platform time, platform residence time and ratio of residence time/total time were significantly shorter in the WY14643 group (P < 0.05). The above results showed that the cognition level of epileptic young rats was injured and was improved by Mdivi-1 but was decreased by WY14643 (Fig. 2, Table S-4).
Cognition of young rats following PTZ kindling increased by inhibiting mitochondrial fission. (A) Localization and navigation trajectories of young rats in the Morris water maze test. (B) The incubation period of crossing the platform of young rats in the control, PTZ, DMSO, Mdivi-1, and WY14643 groups. (C) The number of platform crossings of young rats in the above five groups. (D) The platform residence time of young rats in the above five groups. (E) The residence time/total time ratio of young rats in the above five groups. The incubation period of crossing the platform was prolonged, and the crossing platform time, the platform residence time and the residence time/total time ratio decreased in young rats following PTZ kindling. Mdivi-1 shortened the incubation period of crossing the platform and increased the number of platform crossings, the platform residence time and the residence time/total time ratio of young rats with PTZ-induced epilepsy. However, WY14643 resulted in the opposite effects. *P < 0.05, **P < 0.01, ***P < 0.001
Quantification of hippocampal neurocytes by IF
Neural cells were counted under an inverted fluorescence microscope after the MWM test. Compared with the control group, the numbers of NeuN (+) neurons in the rat hippocampus in the PTZ, DMSO, Mdivi-1 and WY14643 groups were lower (P < 0.05), and the numbers of GFAP (+) astrocytes were higher (P < 0.05). Compared with the PTZ group, the number of NeuN (+) neurons was significantly higher in the Mdivi-1 group (P < 0.05) and lower in the WY14643 group (P < 0.05). However, compared with the PTZ group, the number of GFAP (+) astrocytes was significantly lower in the Mdivi-1 group (P < 0.05) but higher in the WY14643 group (P < 0.05). These results showed that PTZ caused the loss of neurons and the proliferation of glial cells in the young rat hippocampus. Mdivi-1 could properly protect the injured NeuN (+) neurons and prevent gliosis of the hippocampus in PTZ-induced epilepsy, while WY14643 had the opposite effect (Figs. 3 and 4, Table S-5).
The number of NeuN(+) neurons in the young rat hippocampus following PTZ kindling increased by inhibiting mitochondrial fission. (A) Immunofluorescence against NeuN(+) neurons in coronal hippocampal sections in the control, PTZ, DMSO, Mdivi-1 and WY14643 groups. The neurons were stained for NeuN (red, a neuron marker) and with DAPI (blue, nuclei) and visualized by fluorescence microscopy. Scale bar: 50 μm. (B) Quantitative determination of the mean number of NeuN(+) cells in the hippocampal CA1 region under a microscope at 400× magnification. The results showed that the number of NeuN(+) neurons in the hippocampi of young rats kindled by PTZ was decreased but was increased following Mdivi-1 supplementation. However, WY14643 decreased the number of NeuN(+) neurons. *P < 0.05, **P < 0.01, ***P < 0.001
The number of GFAP(+) astrocytes in the hippocampus of young rats following PTZ kindling was decreased by inhibiting mitochondrial fission. (A) Immunofluorescence images of GFAP(+) astrocytes in the control, PTZ, DMSO, Mdivi-1 and WY14643 groups. The astrocytes were stained for GFAP (red, a glial marker) and with DAPI (blue, nuclei) and visualized by fluorescence microscopy. Scale bar: 50 μm. (B) Quantitative determination of the mean number of GFAP-positive cells under a microscope at 400× magnification. The results showed that Mdivi-1 supplementation decreased the number of GFAP(+) cells in the young rat hippocampus following PTZ kindling, while WY14643 increased the number of GFAP(+) cells. *P < 0.05, **P < 0.01, ***P < 0.001
Protein expression of the Drp1-mediated mitophagy pathway in the hippocampi of young rats evaluated by IHC
After the MWM test of young rats in each group, the hippocampal tissue was taken for immunohistochemical analysis. The positive signals were brown particles. Compared with the control group, the AOD values of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampal regions in the other four groups were higher (P < 0.05). Compared with the PTZ group, the AOD values of the above proteins were lower in the Mdivi-1 group (P < 0.05) but higher in the WY14643 group (P < 0.05). Immunohistochemical results suggested that mitophagy and neural apoptosis of the hippocampus in young epileptic rats were enhanced, Mdivi-1 inhibited Drp1-mediated autophagy activity in the hippocampus in young epileptic rats and ultimately alleviated neuronal apoptosis, and WY14643 aggravated mitophagy and increased neuronal apoptosis (Fig. 5, Table S-6).
The distribution of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampus of young rats following PTZ kindling was decreased by inhibiting mitochondrial fission. (A) Immunostaining for Drp1, BCL2L13, LC3, and caspase 3 in coronal hippocampal sections in the control, PTZ, DMSO, Mdivi-1 and WY14643 groups. Scale bar = 50 μm. (B) Quantitative analysis of the immunohistochemistry results showed that Mdivi-1 supplementation decreased the AOD values of Drp1, BCL2L13, LC3, and caspase 3 of young rat hippocampus following PTZ kindling, which were increased by WY14643 supplementation. *P < 0.05, **P < 0.01, ***P < 0.001
Protein expression of the Drp1-mediated mitophagy pathway evaluated by WB
After the MWM test of young rats, the hippocampal tissue was taken for protein analysis. The results of WB showed the same trend as those of IHC. Compared with the control, the protein expression levels of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampi of rats in the PTZ, Mdivi-1 group and WY14643 groups were higher (P < 0.05). Compared with the PTZ group, the above protein expression levels were lower in the Mdivi-1 group (P < 0.05) but were higher in the WY14643 group (P < 0.05). WB results also suggested that mitophagy was enhanced in the hippocampi of young epileptic rats, while Mdivi-1 could inhibit Drp1-mediated mitochondrial autophagy and neural apoptosis, which could be enhanced by WY14643 (Fig. 6, Table S-7).
The protein expression levels of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampus of young rats following PTZ kindling were decreased by inhibiting mitochondrial fission. (A) Representative protein expression bands of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampi of young rats measured by WB. (B) WB analysis was performed for the above proteins in the hippocampus of young rats from the five groups. Mdivi-1 decreased the protein expression levels of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampi of young rats following PTZ kindling. WY14643 increased the expression levels of the above proteins. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In this study, we demonstrated that inhibition of Drp1-mediated mitochondrial fission alleviated seizure attack, decreased the number of injured neurons and improved cognition in young epileptic rats induced by PTZ. At the same time, the protein expression levels of Drp1, BCL2L13, LC3, and caspase 3 in the hippocampi of PTZ-kindled rats were decreased following the inhibition of mitochondrial fission. Therefore, inhibiting mitochondrial fission also decreased the protein expression of mitochondrial autophagy and neuron necrosis in juvenile rats. Overall, our study suggested that mitochondrial fission and mitophagy might play vital roles in PTZ kindling-induced epilepsy. Inhibiting Drp-1-mediated mitochondrial fission may effectively play a role in antiepileptic treatment.
Neuronal loss is not only the pathological result of epilepsy but also can induce seizure recurrence (Costa-Ferro et al. 2012; Suchomelova et al. 2015; Wang et al. 2019). In the present study, the number of neural cells in the hippocampal area was counted using IF. The study showed that more NeuN(+) neurons in the young rat hippocampus following PTZ kindling were lost, and then GFAP(+) astrocytes proliferated. The results were consistent with previous reports that showed apoptosis and necrosis of neurons and proliferation of glial cells in the epileptic area, not only in an epileptic animal model (Loewen et al. 2016; Matyas et al. 2021) but also in clinical epileptic surgery patients (Wong and Crino 2012; Schurr et al. 2017). The hippocampus is an important brain area that is responsible for memory and cognition. Hippocampal neuron damage is bound to affect the cognition of young rats. Therefore, in the MWM experiment, we found that after seizures, the latency of crossing the platform was prolonged, and the frequency of crossing the platform, the residence time and the ratio of residence time/total time were reduced, which suggested that seizures caused hippocampal damage and affected cognition. These findings were similar to previous studies that showed that cognitive impairment was often detected in animal models of epilepsy or in patients with temporal lobe epilepsy, whose cognitive decline may be related to the occurrence of focal brain injury, especially in the hippocampus (Gelfuso et al. 2020; Mukheem et al. 2021).
Recent studies have revealed that excessive mitochondrial fission is an underlying mechanism leading to neuronal damage in seizure attacks, and anti-mitochondrial fission has been proven to have neuroprotective effects in animal models (Qiu et al. 2013; Kim and Kang 2017). In our study, the results indicated that Mdivi-1, as a specific inhibitor of Drp1(Rexius-Hall et al. 2020), protected injured neurons and reduced microgliosis in the hippocampi of young rats with epilepsy induced by PTZ. WY14643, as an activator of Drp1 (Ko et al. 2016), decreased the number of neurons and increased gliosis in the hippocampus following PTZ kindling. Drp1 is a key protein in mitochondrial fission. The above results proved that inhibiting mitochondrial fission could protect against brain damage.
The animal behaviors, consisting of seizure attacks and MWM tests in rats, also confirmed the above results. Mdivi-1 supplementation prolonged the seizure latency, alleviated the seizure grades, and reduced the seizure frequency of young rats with seizure attacks induced by PTZ. In the MWM test, Mdivi-1 reduced the latency to cross the platform and increased the number of platform crossings, the platform residence time and the ratio of residence time/total time. However, WY14643 increased the degree of seizure attacks following PTZ kindling and decreased the cognition of young epileptic rats. The above animal behavior studies showed that inhibition of mitochondrial fission mediated by Drp1 could ease seizure attacks and improve cognition in young epileptic rats induced by PTZ. However, the role of inhibiting mitochondrial fission in seizures, which could reduce recurrent epilepsy, protect injured brains, and improve cognition, remains unknown. Furthermore, more experiments are needed to study the specific mechanism.
The interaction and regulation between mitochondrial dynamics and mitochondrial autophagy is an important mechanism to maintain mitochondrial homeostasis and ensure mitochondrial quality (Wu et al. 2019). Drp1 is a major protein in mitochondrial fission, and microtubule-associated protein 1 light chain 3 (LC3) is a marker molecule of autophagy (Heckmann et al. 2017). LC3 is the first autophagy membrane protein found in higher eukaryotic cells. There are two forms of LC3: cytoplasmic LC3-I and membrane-bound LC3-II. The formation of the LC3 precursor is first processed into the cytoplasmic soluble form LC3-I, which has an exposed glycine residue at the carboxyl end, and the protein is then activated and transported. Finally, LC3-I is modified into the membrane-bound form LC3-II in a process called LC3 transformation. LC3-II, as a marker of autophagy, is detected in preautophagy and autophagy. The total amount of LC3-II or the ratio of LC3-II to LC3-I has been correlated with the number of autophagic vesicles (Tanida et al. 2004; Shirihai et al. 2015). When Drp1 is recruited by mitochondrial outer membrane molecules, it can be transposed to the mitochondrial outer membrane and concentrated in the potential division sites of mitochondria. Previous studies have shown that when multiple Drp-1 molecules surround mitochondria, mitochondria break into many small circular punctate organelles, resulting in increased permeability of the mitochondrial outer membrane and an increased protein ratio of LC3-II/LC3-I. They regulate the process of mitochondrial autophagy by interacting with LC3 connector protein or the LC3 receptor (Shirihai et al. 2015). At the same time, IF results show that the fluorescence intensity of total LC3 protein in the cytoplasm is enhanced, a punctate aggregation phenomenon is presented, and autophagy in cells is enhanced (Tang et al. 2021).
Autophagy is a highly conserved lysosome-dependent cellular degradation process in eukaryotic cells. LC3 can directly recognize and bind proteins on the mitochondrial membrane and then induce mitochondrial autophagy. This process is precisely regulated by more than 30 autophagy-associated genes (ATGs). Atg32 is the only mitochondrial autophagy receptor molecule in yeast cells, and BCL2L13 in mammalian cells is the homolog of Atg32 in yeast cells. Deletion of the Atg32 gene can lead to a low level of mitochondrial autophagy in yeast cells. The exogenous expression of BCL2L13 can compensate for the decrease in mitochondrial autophagy caused by the deletion of Atg32 in yeast cells to a certain extent (Mao et al. 2011). Studies have found that Drp1 can interact with the LC3 receptor BCL2L13 and induce mitochondrial autophagy (Wu et al. 2016). BCL2L13 induces mitochondrial autophagy through a Parkin-independent mechanism. BCL2L13 is located in the outer membrane of mitochondria and directly binds to LC3 through its LIR motif to induce mitochondrial autophagy. The ability of BCL2L13 to induce mitochondrial autophagy is regulated by its phosphorylation modification. The phosphorylation of the BCL2L13 Ser272 site can promote its binding to LC3 and greatly enhance its ability to induce mitochondrial autophagy (Murakawa et al. 2015).
Increased autophagy has now been reported in brain lesions of ischemic stroke (Zhang et al. 2020a), traumatic brain injury (TBI) (Luo and Tao 2020), hemorrhagic stroke (Zhang and Liu 2020), mitochondrial encephalomyopathies (Zhang et al. 2020b) and epilepsy (Lv and Ma 2020), suggesting that autophagy is upregulated in response to various neurological insults and is likely a component of excitotoxicity, which contributes to neural death (Descloux et al. 2018; Davis et al. 2021). The role of autophagy in epilepsy has gained increasing attention. Previous studies reported that cell autophagy in the hippocampus was increased in some drug-induced epileptic models (Attia et al. 2019; Cao et al. 2020). Consistent with these above reports, we found an increase in mitochondrial autophagy levels in the hippocampus following the expression of BCL2L13 and LC3 proteins in the young rat hippocampus after PTZ kindling. This suggested that PTZ kindling induced mitochondrial autophagy in neurons of the hippocampus.
Therefore, we hypothesized that the down regulation of Drp1 played a protective role in brain injury kindling by PTZ following a reduction in mitochondrial autophagy. In the present study, Mdivi-1 reduced the expression of Drp1 protein in the young rat hippocampus. Subsequently, the protein expression levels of BCL2L13, LC3 and caspase 3 were decreased. The above results implied that the BCL2L13/LC3 mitophagy pathway was also inhibited by Mdivi-1. Inhibition of mitochondrial fission reduced mitochondrial autophagy and neuronal necrosis in the hippocampi of young epileptic rats. These results were consistent with those reported by Wu Q et al. (Wu et al. 2018) that mitophagy was enhanced following the accumulation of LC3 at 24 h in the experimental TBI, and then neuron death was reduced by Mdivi-1 through decreasing LC3 expression and the inhibition of autophagy. A similar study found that the expression of Drp1 was down regulated, and the expression of LC3-II/LC3-I was also downregulated, inhibiting abnormal autophagy in mitochondria (Deng et al. 2020). The above results showed that inhibition of mitochondrial fission can protect against epileptic brain injury by inhibiting the BCL2L13/LC3 mitophagy signaling pathway mediated by Drp1.
It is well recognized that the breakdown of mitochondrial functions may facilitate activation of the mitochondria-associated intrinsic apoptosis pathway (Waseem et al. 2021). In agreement with results reported by previous studies (Henshall 2007; Mendez-Armenta et al. 2014), apoptosis signaling pathways in seizure-induced neuronal death showed that the level of cleaved caspase 3 of the hippocampus was markedly increased after seizure attacks induced by PTZ. Inhibition of mitochondrial fission by Mdivi-1 efficiently reduced caspase 3 expression in the young rat hippocampus following PTZ kindling, consistent with the results of Ko AR (Ko et al. 2016) and Kim J (Kim and Kang 2018), which showed that impaired mitochondrial fission played important roles in mitosis, apoptosis and programmed necrosis following status epilepticus (SE). Previous studies on the relationship between mitophagy and apoptosis showed mitophagy-mediated hippocampal neuron apoptosis in animal experiments in different models (Huang et al. 2020; Liu et al. 2021). Therefore, it is well recognized that the breakdown of mitochondrial functions may facilitate activation of the mitochondria-associated intrinsic apoptosis pathway. According to the results in the PTZ-induced epileptic young rat model, Mdivi-1 inhibited the expression of Drp1 protein, resulting in decreases in the expression levels of BCL2L13, LC3 and caspase 3. Although Mdivi-1 cannot directly inhibit the process of mitophagy, it alleviated PTZ-induced mitochondrial fission by inhibiting the GTPase activity of Drp1. Therefore, we speculate that inhibiting mitochondrial fission could reduce mitochondrial autophagy through the BCL2L13/LC3 autophagy signaling pathway and ultimately mitigate necrosis and apoptosis in nerve cells induced by PTZ kindling.
Conclusion
In conclusion, there is a close connection among mitochondrial fission, mitophagy and apoptosis in a rat model of epilepsy. We found that mitochondrial fission, mitochondrial autophagy and neuronal apoptosis increased, which induced neuronal injury and cognitive decline in a PTZ-induced epileptic young rat model. Inhibition of Drp1-mediated mitochondrial fission can reduce recurrent seizures and neuronal damage and improve rat cognition by inhibiting the BCL2L13/LC3 mitophagy signaling pathway through decreasing the protein levels of Drp1, BCL2L13, LC3 and caspase 3 in PTZ-induced epilepsy. The protective effect of inhibiting mitochondrial fission on hippocampal neurons in young epileptic rats may provide a new idea for the treatment of epilepsy.
Data availability
Not applicable.
Code availability
Not applicable.
References
Attia GM, Elmansy RA, Elsaed WM (2019) Neuroprotective effect of nilotinib on pentylenetetrazol-induced epilepsy in adult rat hippocampus: involvement of oxidative stress, autophagy, inflammation, and apoptosis. Folia Neuropathol 57:146–160. https://doi.org/10.5114/fn.2019.84423
Cao J, Tang C, Gao M, Rui Y, Zhang J, Wang L, Wang Y, Xu B, Yan BC (2020) Hyperoside alleviates epilepsy-induced neuronal damage by enhancing antioxidant levels and reducing autophagy. J Ethnopharmacol 257:112884. https://doi.org/10.1016/j.jep.2020.112884
Carter AN, Born HA, Levine AT, Dao AT, Zhao AJ, Lee WL, Anderson AE (2017) Wortmannin attenuates seizure-induced hyperactive PI3K/Akt/mTOR signaling, impaired memory, and spine Dysmorphology in rats. eNeuro 4. https://doi.org/10.1523/ENEURO.0354-16.2017
Costa-Ferro ZS, Souza BS, Leal MM, Kaneto CM, Azevedo CM, Da SI, Soares MB, Ribeiro-dos-Santos R, Dacosta JC (2012) Transplantation of bone marrow mononuclear cells decreases seizure incidence, mitigates neuronal loss and modulates pro-inflammatory cytokine production in epileptic rats. Neurobiol Dis 46:302–313. https://doi.org/10.1016/j.nbd.2011.12.001
Davis SE, Roth JR, Aljabi Q, Hakim AR, Savell KE, Day JJ, Arrant AE (2021) Delivering progranulin to neuronal lysosomes protects against excitotoxicity. J Biol Chem 297:100993. https://doi.org/10.1016/j.jbc.2021.100993
Deng X, Liu J, Liu L, Sun X, Huang J, Dong J (2020) Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int J Biol Sci 16:1403–1416. https://doi.org/10.7150/ijbs.41768
Descloux C, Ginet V, Rummel C, Truttmann AC, Puyal J (2018) Enhanced autophagy contributes to excitotoxic lesions in a rat model of preterm brain injury. Cell Death Dis 9:853. https://doi.org/10.1038/s41419-018-0916-z
Dhir A (2012) Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci,Chapter 9:t9–t37. https://doi.org/10.1002/0471142301.ns0937s58
Dulac M, Leduc-Gaudet JP, Reynaud O, Ayoub MB, Guerin A, Finkelchtein M, Hussain SN, Gouspillou G (2020) Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J Physiol 598:3691–3710. https://doi.org/10.1113/JP279802
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jette N (2017) Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 88:296–303. https://doi.org/10.1212/WNL.0000000000003509
Filiz AK, Gumus E, Karabulut S, Tastemur Y, Taskiran AS (2021) Protective effects of lamotrigine and vitamin B12 on pentylenetetrazole-induced epileptogenesis in rats. Epilepsy Behav 118:107915. https://doi.org/10.1016/j.yebeh.2021.107915
Galluzzi L, Pietrocola F, Levine B, Kroemer G (2014) Metabolic control of autophagy. Cell 159:1263–1276. https://doi.org/10.1016/j.cell.2014.11.006
Gelfuso EA, Reis SL, Pereira A, Aguiar D, Beleboni RO (2020) Neuroprotective effects and improvement of learning and memory elicited by erythravine and 11alpha-hydroxy-erythravine against the pilocarpine model of epilepsy. Life Sci 240:117072. https://doi.org/10.1016/j.lfs.2019.117072
Harris H, Rubinsztein DC (2011) Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 8:108–117. https://doi.org/10.1038/nrneurol.2011.200
Heckmann BL, Boada-Romero E, Cunha LD, Magne J, Green DR (2017) LC3-associated phagocytosis and inflammation. J Mol Biol 429:3561–3576. https://doi.org/10.1016/j.jmb.2017.08.012
Henshall DC (2007) Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans 35:421–423. https://doi.org/10.1042/BST0350421
Hernandez-Ceron M, Martinez-Lazcano JC, Rubio C, Custodio V, Gonzalez-Guevara E, Castillo-Perez C, Paz C (2017) Participation of the dentate-rubral pathway in the kindling model of epilepsy. J Neurosci Res 95:1495–1502. https://doi.org/10.1002/jnr.23974
Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, Mai S, Yang J (2017) Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS One 12:e185129. https://doi.org/10.1371/journal.pone.0185129
Huang Y, Gao X, Zhou X, Xie B, Zhang Y, Zhu J, Zhu S (2020) Mitophagy in the Hippocampus is excessive activated after cardiac arrest and cardiopulmonary resuscitation. Neurochem Res 45:322–330. https://doi.org/10.1007/s11064-019-02916-z
Kaur H, Patro I, Tikoo K, Sandhir R (2015) Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int 89:40–50. https://doi.org/10.1016/j.neuint.2015.07.009
Khurana DS, Valencia I, Goldenthal MJ, Legido A (2013) Mitochondrial dysfunction in epilepsy. Semin Pediatr Neurol 20:176–187. https://doi.org/10.1016/j.spen.2013.10.001
Kim JE, Kang TC (2017) p47Phox/CDK5/DRP1-mediated mitochondrial fission evokes PV cell degeneration in the rat dentate gyrus following status epilepticus. Front Cell Neurosci 11:267. https://doi.org/10.3389/fncel.2017.00267
Kim JE, Kang TC (2018) Differential roles of mitochondrial translocation of active Caspase-3 and HMGB1 in neuronal death induced by status epilepticus. Front Cell Neurosci 12:301. https://doi.org/10.3389/fncel.2018.00301
Ko AR, Hyun HW, Min SJ, Kim JE (2016) The differential DRP1 phosphorylation and mitochondrial dynamics in the regional specific Astroglial death induced by status epilepticus. Front Cell Neurosci 10:124. https://doi.org/10.3389/fncel.2016.00124
Landes T, Martinou JC (2011) Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochim Biophys Acta 1813:540–545. https://doi.org/10.1016/j.bbamcr.2011.01.021
Lee DS, Kim TH, Park H, Kim JE (2022) CDDO-me attenuates Clasmatodendrosis in CA1 astrocyte by inhibiting HSP25-AKT mediated DRP1-S637 phosphorylation in chronic epilepsy rats. Int J Mol Sci 23. https://doi.org/10.3390/ijms23094569
Liu J, Liu L, Han YS, Yi J, Guo C, Zhao HQ, Ling J, Wang YH (2021) The molecular mechanism underlying mitophagy-mediated hippocampal neuron apoptosis in diabetes-related depression. J Cell Mol Med 25:7342–7353. https://doi.org/10.1111/jcmm.16763
Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, Wilcox KS (2016) Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. J Neuropathol Exp Neurol 75:366–378. https://doi.org/10.1093/jnen/nlw008
Luo C, Tao L (2020) The function and mechanisms of autophagy in traumatic brain injury. Adv Exp Med Biol 1207:635–648. https://doi.org/10.1007/978-981-15-4272-5_46
Lv M, Ma Q (2020) Autophagy and epilepsy. Adv Exp Med Biol 1207:163–169. https://doi.org/10.1007/978-981-15-4272-5_10
Mao K, Wang K, Zhao M, Xu T, Klionsky DJ (2011) Two MAPK-signaling pathways are required for mitophagy in Saccharomyces cerevisiae. J Cell Biol 193:755–767. https://doi.org/10.1083/jcb.201102092
Matyas A, Borbely E, Mihaly A (2021) Hippocampal sclerosis in pilocarpine epilepsy: survival of peptide-containing neurons and learning and memory disturbances in the adult NMRI strain mouse. Int J Mol Sci 23. https://doi.org/10.3390/ijms23010204
Mendez-Armenta M, Nava-Ruiz C, Juarez-Rebollar D, Rodriguez-Martinez E, Gomez PY (2014) Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxidative Med Cell Longev 2014:293689. https://doi.org/10.1155/2014/293689
Mukheem MM, Mundlamuri RC, Mariyappa N, Aravind KR, Velmurugan J, Bhargava GK, Suvarna A, Shivashankar N, Raghavendra K, Asranna A, Thennarasu K, Jamuna R, Rose DB, Saini J, Sinha S (2021) P300 in mesial temporal lobe epilepsy and its correlation with cognition - a MEG based prospective case-control study. Epilepsy Behav 114:107619. https://doi.org/10.1016/j.yebeh.2020.107619
Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun 6:7527. https://doi.org/10.1038/ncomms8527
Oettinghaus B, D'Alonzo D, Barbieri E, Restelli LM, Savoia C, Licci M, Tolnay M, Frank S, Scorrano L (2016) DRP1-dependent apoptotic mitochondrial fission occurs independently of BAX, BAK and APAF1 to amplify cell death by BID and oxidative stress. Biochim Biophys Acta 1857:1267–1276. https://doi.org/10.1016/j.bbabio.2016.03.016
Palikaras K, Lionaki E, Tavernarakis N (2016) Mitophagy: in sickness and in health. Mol Cell Oncol 3:e1056332. https://doi.org/10.1080/23723556.2015.1056332
Parone PA, Da CS, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC (2008) Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3:e3257. https://doi.org/10.1371/journal.pone.0003257
Qiu X, Cao L, Yang X, Zhao X, Liu X, Han Y, Xue Y, Jiang H, Chi Z (2013) Role of mitochondrial fission in neuronal injury in pilocarpine-induced epileptic rats. Neuroscience 245:157–165. https://doi.org/10.1016/j.neuroscience.2013.04.019
Rexius-Hall ML, Khalil NN, Andres AM, McCain ML (2020) Mitochondrial division inhibitor 1 (mdivi-1) increases oxidative capacity and contractile stress generated by engineered skeletal muscle. FASEB J 34:11562–11576. https://doi.org/10.1096/fj.201901039RR
Schurr J, Coras R, Rossler K, Pieper T, Kudernatsch M, Holthausen H, Winkler P, Woermann F, Bien CG, Polster T, Schulz R, Kalbhenn T, Urbach H, Becker A, Grunwald T, Huppertz HJ, Gil-Nagel A, Toledano R, Feucht M et al (2017) Mild malformation of cortical development with Oligodendroglial hyperplasia in frontal lobe epilepsy: a new Clinico-pathological entity. Brain Pathol 27:26–35. https://doi.org/10.1111/bpa.12347
Shirihai OS, Song M, Dorn GN (2015) How mitochondrial dynamism orchestrates mitophagy. Circ Res 116:1835–1849. https://doi.org/10.1161/CIRCRESAHA.116.306374
Shlobin NA, Sander JW (2022) Learning from the comorbidities of epilepsy. Curr Opin Neurol 35:175–180. https://doi.org/10.1097/WCO.0000000000001010
Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12:2245–2256. https://doi.org/10.1091/mbc.12.8.2245
Su SH, Wu YF, Lin Q, Wang DP, Hai J (2019) URB597 protects against NLRP3 inflammasome activation by inhibiting autophagy dysfunction in a rat model of chronic cerebral hypoperfusion. J Neuroinflammation 16:260. https://doi.org/10.1186/s12974-019-1668-0
Suchomelova L, Lopez-Meraz ML, Niquet J, Kubova H, Wasterlain CG (2015) Hyperthermia aggravates status epilepticus-induced epileptogenesis and neuronal loss in immature rats. Neuroscience 305:209–224. https://doi.org/10.1016/j.neuroscience.2015.08.006
Tang S, Tang T, Gao G, Wei Q, Sun K, Huang W (2021) Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem 123:151796. https://doi.org/10.1016/j.acthis.2021.151796
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518. https://doi.org/10.1016/j.biocel.2004.05.009
Taskiran AS, Ozdemir E, Gumus E, Ergul M (2020) The effects of salmon calcitonin on epileptic seizures, epileptogenesis, and postseizure hippocampal neuronal damage in pentylenetetrazole-induced epilepsy model in rats. Epilepsy Behav 113:107501. https://doi.org/10.1016/j.yebeh.2020.107501
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107:378–383. https://doi.org/10.1073/pnas.0911187107
Wang J, Chen Y, Wang Q, van Luijtelaar G, Sun M (2019) The effects of lamotrigine and ethosuximide on seizure frequency, neuronal loss, and astrogliosis in a model of temporal-lobe epilepsy. Brain Res 1712:1–6. https://doi.org/10.1016/j.brainres.2019.01.031
Waseem R, Shamsi A, Kazim SN, Islam A (2021) An insight into mitochondrial dysfunction and its implications in neurological diseases. Curr Drug Targets 22:1585–1595. https://doi.org/10.2174/1389450121999201230204050
Wong M (2010) Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia 51:27–36. https://doi.org/10.1111/j.1528-1167.2009.02341.x
Wong M (2013) Cleaning up epilepsy and neurodegeneration: the role of autophagy in epileptogenesis. Epilepsy Curr 13:177–178. https://doi.org/10.5698/1535-7597-13.4.177
Wong M, Crino PB (2012) Tuberous sclerosis and epilepsy: role of astrocytes. Glia 60:1244–1250. https://doi.org/10.1002/glia.22326
Wu W, Lin C, Wu K, Jiang L, Wang X, Li W, Zhuang H, Zhang X, Chen H, Li S, Yang Y, Lu Y, Wang J, Zhu R, Zhang L, Sui S, Tan N, Zhao B, Zhang J et al (2016) FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions. EMBO J 35:1368–1384. https://doi.org/10.15252/embj.201593102
Wu Q, Gao C, Wang H, Zhang X, Li Q, Gu Z, Shi X, Cui Y, Wang T, Chen X, Wang X, Luo C, Tao L (2018) Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int J Biochem Cell Biol 94:44–55. https://doi.org/10.1016/j.biocel.2017.11.007
Wu NN, Zhang Y, Ren J (2019) Mitophagy, mitochondrial dynamics, and homeostasis in cardiovascular aging. Oxidative Med Cell Longev 2019:9825061. https://doi.org/10.1155/2019/9825061
Wu H, Li G, Chen W, Luo W, Yang Z, You Z, Zou Y (2022) Drp1 knockdown represses apoptosis of rat retinal endothelial cells by inhibiting mitophagy. Acta Histochem 124:151837. https://doi.org/10.1016/j.acthis.2021.151837
Xie R, Li T, Qiao X, Mei H, Hu G, Li L, Sun C, Cheng C, Cui Y, Hong N, Liu Y (2021) The protective role of E-64d in hippocampal Excitotoxic neuronal injury induced by glutamate in HT22 hippocampal neuronal cells. Neural Plast 2021:7174287. https://doi.org/10.1155/2021/7174287
Xue Y, Xie N, Cao L, Zhao X, Jiang H, Chi Z (2011) Diazoxide preconditioning against seizure-induced oxidative injury is via the PI3K/Akt pathway in epileptic rat. Neurosci Lett 495:130–134. https://doi.org/10.1016/j.neulet.2011.03.054
Zapata-Munoz J, Villarejo-Zori B, Largo-Barrientos P, Boya P (2021) Towards a better understanding of the neuro-developmental role of autophagy in sickness and in health. Cell. Stress 5:99–118. https://doi.org/10.15698/cst2021.07.253
Zhang Y, Liu C (2020) Autophagy and hemorrhagic stroke. Adv Exp Med Biol 1207:135–147. https://doi.org/10.1007/978-981-15-4272-5_8
Zhang C, Wang R, Liu Z, Bunker E, Lee S, Giuntini M, Chapnick D, Liu X (2019) The plant triterpenoid celastrol blocks PINK1-dependent mitophagy by disrupting PINK1's association with the mitochondrial protein TOM20. J Biol Chem 294:7472–7487. https://doi.org/10.1074/jbc.RA118.006506
Zhang Y, Cao Y, Liu C (2020a) Autophagy and ischemic stroke. Adv Exp Med Biol 1207:111–134. https://doi.org/10.1007/978-981-15-4272-5_7
Zhang X, Zheng Y, Chen Z (2020b) Autophagy and mitochondrial Encephalomyopathies. Adv Exp Med Biol 1207:103–110. https://doi.org/10.1007/978-981-15-4272-5_6
Author contributors
Dr. Qiong Fang participated in the study design, experiment, data analysis and wrote the manuscript; Dr. Shaojuan Zheng participated in the experiment, data analysis and wrote the manuscript; Dr. Qiaobin Chen contributed to study design, data analysis and manuscript preparation; Dr. Lang Chen participated in the manuscript revision and data analysis; Dr. Yating Yang, Ying Wang, Huixia Zhang, and Jiafan Chen participated in the experiment and data analysis.
Funding
The present study was sponsored by Fujian provincial health technology project (Grant No: 2019-ZQN-11), Fujian Provincial Traditional Chinese Medicine Science and Technology Program (Grant No: 2021zyjc03), Natural Science Foundation of Fujian Province by Fujian Science and Technology Department (Grant No: 2022 J01414), College Students’ Innovative Entrepreneurial Training Plan Program (Grant No: 202110392010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Ethical approval was given by the medical ethics committee of Fujian Medical University (2018–014).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that there are no conflicts of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 192 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, Q., Zheng, S., Chen, Q. et al. The protective effect of inhibiting mitochondrial fission on the juvenile rat brain following PTZ kindling through inhibiting the BCL2L13/LC3 mitophagy pathway. Metab Brain Dis 38, 453–466 (2023). https://doi.org/10.1007/s11011-022-01077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01077-3