Abstract
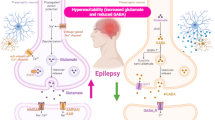
Epilepsy is a long-term neurological disease characterized by convulsions that can be recurrent. It is mainly caused by an imbalance between excitation and inhibition in the central nervous system. Currently, the pathogenesis is still unclear, although it may be related to changes in ion channels, neurotransmitters and glial cells. In recent years, increasing attention has been paid to the role of autophagy in the development of epilepsy. This chapter focuses on the role of the mTOR pathway in epileptogenesis and the relationship between autophagy, glycogen metabolism and Lafora disease and discusses the potential role of autophagy as a target for the treatment of epilepsy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Epilepsy is a long-term neurological disorder characterized by recurrent convulsions. It is caused by the abnormal discharge of nerve cells in the cerebral cortex due to an imbalance between excitatory and inhibitory neurotransmission in the central nervous system. Epilepsy has a high incidence and mortality and causes serious damage to the patient’s physical and mental health and quality of life.
Although epilepsy cannot be cured, 70% of seizures can be controlled by drugs. Traditional antiepileptic drugs, including phenytoin and phenobarbital, have certain clinical effects. However, there are many side effects, such as a high teratogenic rate and hyperactivity and inattention, which are difficult for patients to tolerate. New antiepileptic drugs (such as lamotrigine, levetiracetam, topiramate and oxcarbazepine) have the advantages of good efficacy and few side effects and are easily tolerated by patients (Stafstrom and Carmant 2015). For drug-refractory epilepsy, neurosurgery, nerve stimulation therapy or diet change can be used to relieve epileptic symptoms without drugs (Stafstrom and Carmant 2015).
The etiology of epilepsy is complicated, and the underlying mechanisms have not yet been fully elucidated. Various factors are associated with epilepsy. In younger patients, the most common causes are hereditary, congenital and developmental disorders. In older patients, brain tumors, stroke, cranial trauma, metabolic abnormalities and neurodegenerative diseases are the main influencing factors. The pathogenesis may include changes in ion channels, neurotransmitters and glial cells. Recent studies have emphasized the essential role of autophagy in epileptogenesis.
1 The Role of Autophagy in Epilepsy
Autophagy is a way for cells to self-regulate and maintain normal physiological functions. Cells can eliminate certain toxins, pathogens and misfolded proteins or damaged organelles through autophagy, thus protecting the cell from further damage. It also facilitates the recycling of substances and energy in cells. However, insufficient or excessive autophagy can cause damage to the cells. Gradually, autophagy has been understood as an important method of cellular quality control, and attention has been paid to its role in the process of epileptogenesis and development.
1.1 mTOR Pathway and Epilepsy
The abnormal activation of the mTOR pathway has been shown to cause a variety of epilepsy syndromes, including hereditary epilepsy and various acquired epilepsies. Tuberous sclerosis complex (TSC) is a common autosomal hereditary disease characterized by lesions of multiple organs, including the brain, skin, kidneys, eyes, and lungs (Curatolo et al. 2008). Up to 96% of cases of TSC are associated with epileptic symptoms (Jozwiak et al. 2000). TSC is caused by mutations of the tumor suppressor genes TSC1 and TSC2 (Curatolo et al. 2008). TSC1-deficient mice and TSC2-deficient mice exhibit severe epileptic symptoms, concomitant with the excessive activation of mTOR and impaired autophagy. Hyperexcitability has been detected in the brains of TSC patients and in neurons differentiated from induced pluripotent stem cells derived from TSC patients (Nadadhur et al. 2019; Wang et al. 2007). Reduced inhibitory synapse function has been identified to contribute to the hyperactivity of TSC1-deficient neurons (Bateup et al. 2013). However, it is worth noting that mTOR is actively involved in most key steps of neural development, such as the establishment of neuronal architecture, the maintenance of synaptic strength, and the generation of excitatory pyramidal neurons and inhibitory GABAergic neurons (see Chap. 11). The hyperexcitability observed in TSC patients may be a consequence of excessive mTOR activation in the developing brain. This idea is consistent with the clinical fact that the vast majority of TSC patients first develop epilepsy as infants (Chu-Shore et al. 2010). In this context, it is worth noting that a small fraction of patients do not show seizures until adolescence (Chu-Shore et al. 2010), which may be because the pathogenic effect of TSC inactivation is cell type-specific. The deletion of TSC1 in either neurons or astrocytes results in seizures (Meikle et al. 2008; Zeng et al. 2008). Although TSC1-deficient neurons exhibit reduced inhibitory synaptic transmission (Bateup et al. 2013), the deletion of TSC1 in GABAergic interneurons does not result in spontaneous seizures (Fu et al. 2012; Wang et al. 2007).
Epilepsy caused by cortical malformation, such as focal cortical dysplasia type IIb (FCD type IIb), is a refractory epilepsy with a histopathology similar to that of TSC that is mainly characterized by neurons with abnormal morphology, specifically dysmorphic neurons (DNs) and balloon cells (BCs). Specifically, BCs in the brains of FCD patients exhibit an accumulation of lysosomes and autophagy-related proteins, including Beclin 1, LC3, ATG5, and ATG12, as well as the autophagy regulator DOR and the autophagy receptor P62, indicating that autophagy is impaired in FCD. This deficiency in autophagy can be reversed in vitro by inhibiting mTOR, suggesting that the aberrant activation of mTOR may directly lead to defects in autophagy in FCD type IIb (Yasin et al. 2013). In fact, mutations in either PTEN or DEPDC5 (which encodes GATOR1) have also been reported in patients with FCD type IIb (Schick et al. 2006; Tsai et al. 2017). Both PTEN and GATOR1 are suppressors of mTOR. PTEN is one of the tumor suppressor genes encoding plasma membrane lipid phosphatase, which antagonizes PI3K-Akt signaling upstream factors of mTOR. GATOR1 activates the GTPase RagA/B, thus inhibiting mTORC1 activity under static and low amino acid conditions (Bar-Peled et al. 2013). These lines of evidence suggest that excessive mTOR activation, which results in deficient autophagy, is associated with the FCD type IIb. Consistent with these lines of evidence, PTEN deficiency results in severe epilepsy in mice, concomitant with impaired autophagy (McMahon et al. 2012). These findings strongly suggest that mutations in mTOR or the molecules associated with its pathway may be involved in the pathogenesis of FCD type IIb and FCD type IIb-associated epilepsy.
In addition, in animal models of acquired epilepsy, such as models of kainic acid-induced epilepsy (Shacka et al. 2007) and a model of traumatic brain injury-induced epilepsy (Chen et al. 2007), the mTOR signaling pathway is also overactivated. Therefore, the abnormal hyperactivation of mTOR is generally considered to be one of the main causes of epileptogenesis. Consistent with this idea, clinical trials have shown that treatment with rapamycin (a powerful autophagy inducer) can reduce the frequency of seizures in patients with TSC (Franz et al. 2006). In contrast to traditional antiepileptic drugs, rapamycin has no direct effect on neuronal excitability (Ruegg et al. 2007) but exerts its antiepileptic effects by inhibiting the mTOR signaling pathway. Intervention with the mTOR inhibitor rapamycin not only reduces the number of epileptic seizures but also prevents or reverses the histopathological changes, thereby achieving antiepileptic effects (Wong 2010; Zeng et al. 2009).
Despite these findings, most data claiming that autophagy is involved in seizures are still indirect. As a highly conserved serine/threonine protein kinase, mTOR, in addition to suppressing autophagy, is also a critical regulator of transcription and translation. In mice with a specific deletion of PTEN in a subset of neurons, the levels of the potassium channel subunit Kv1.1, but not of Kv1.2, Kv1 or Kvβ2, in the hippocampus increased, and Kv1.1 distribution was also altered. These observations suggest that mTOR signaling may regulate neuronal excitability by modulating voltage-gated ion channel expression (Nguyen and Anderson 2018). Direct evidence that links autophagy and epilepsy comes from observations of ATG7 conditional knockout mice. The deletion of ATG7 in mature neurons in the CaMKII-cre mouse line results in spontaneous recurrent seizures (McMahon et al. 2012).
1.2 Autophagy, the Metabolism of Glycogen and Lafora Disease
Lafora disease (LD) is an autosomal-derived lethal disease characterized by seizures, progressive myoclonus, cognitive impairment, and typical glycogen-like inclusion bodies, termed Lafora bodies (Ganesh et al. 2006). LD is the most common form of progressive myoclonic epilepsy. Patients generally die between the ages of 20 and 30. The disease is caused by mutations in the epilepsy-associated proteins laforin (encoded by the PME2A gene) or malin (encoded by the PME2B gene). Laforin is a glycogen 6 phosphatase that degrades the glycogen chain. Malin is an ubiquitin E3 ligase. Mutations in laforin and malin cause the abnormal accumulation of basophilic inclusion bodies (Lafora bodies), which are formed by the abnormal accumulation of glycogen, in the cerebral cortex, substantia nigra, globus pallidus and dentate nucleus. Knocking out the laforin gene, the malin gene, or both gene in mice recapitulates most of the symptoms of LD. Normal laforin inhibits the mTOR complex, allowing the autophagy machinery to function properly and maintain its function. In contrast, when laforin is mutated, the autophagy pathway is strongly inhibited by excessive mTOR activation, causing a dysfunction in glycogen clearance. Mutations in malin leads to failures in autophagosome formation. Unlike laforin-regulated autophagy, malin-regulated autophagy is mTOR-independent (Criado et al. 2012). Therefore, mutations in the laforin gene and the malin gene cause impaired autophagy and the accumulation of Lafora bodies in neurons. However, the mechanism by which Lafora bodies result in seizures is unclear.
2 Autophagy as a Target for the Treatment of Epilepsy
In summary, the overactivation of mTOR and insufficient autophagy may promote the development of epilepsy. Therefore, drugs targeting autophagy or mTOR signaling are expected to play a therapeutic role in epilepsy. As an inhibitor of mTOR, rapamycin plays an important role in the treatment of epilepsy. Firstly, the inhibition of the mTOR pathway by rapamycin can prevent seizures and improve the pathology of PTEN or TSC mutated mouse models caused by mTOR signaling disruption. For instance, data from the use of rapamycin in an animal model of TSC suggest that it can delay or inhibit the process of epilepsy by inducing autophagy, reduce the frequency of spontaneous seizures, and improve survival rate in mice (Zeng et al. 2011). Secondly, the addition of rapamycin to cortical sections of patients with cortical dysplasia reduced rhythm oscillations induced by the proconvulsant agent 4-aminopyridine, which blocks type A K+ currents and enhances neurotransmitter release (Cepeda et al. 2010). Furthermore, high-dose rapamycin administration reduces cortical hyperactivation of the mTOR pathway, inhibits sputum, and partially improves cognitive deficits in infantile spasms epileptogenesis (Raffo et al. 2011). Finally, rapamycin may improve the development of epilepsy-related pathology and reduce the incidence of spontaneous epilepsy in a model of temporal lobe epilepsy (Zeng et al. 2009; Huang and Yang 2010). Although the effect of rapamycin depends on the time and duration of administration, and may depend on the model used, mTOR dysregulation is closely related to several hereditary and acquired epilepsy, and some of these epileptogenic processes can be reversed using mTOR inhibitors.
Some antiepileptic drugs, such as carbamazepine, also acts as an enhancer of autophagy, have also been reported to play a role in the metabolic disease alpha-antitrypsin deficiency. Treatment of carbamazepine reduces alpha-antitrypsin liver load and fibrosis. These results provide data for clinical trials of carbamazepine in patients with alpha 1-antitrypsin deficiency (Hidvegi et al. 2010). In addition, carbamazepine can improve cognitive impairment in Alzheimer’s disease (AD) model mice by enhancing autophagic flow (Li et al. 2013). Alzheimer’s disease patients have a higher frequency of seizures. The antiepileptic drug lamotrigine suppresses hyperexcitability in AD model mice (Zhang et al. 2014). Treatment with lamotrigine reduces the occurrence of epileptic spikes and attenuates cognitive deficits in AD model mice, possibly through the induction of autophagy via both mTOR-dependent and mTOR-independent manners (Wu et al. 2015; Zhang et al. 2014). In amyotrophic lateral sclerosis, the widely used antiepileptic drug valproic acid reduces key molecule TDP-25 induced neuronal toxicity by inhibiting endoplasmic reticulum stress-mediated apoptosis and enhancing autophagy (Wang et al. 2015).
One of the most striking features of autophagy is its ability to be induced under different conditions, such as nutritional deficiencies. Based on this feature, autophagy induced by nutritional restriction is considered a potential treatment for epilepsy. In this context, autophagy stimulation and caloric restriction under physiological conditions improves cognitive deficits in normal brain aging (Mattson 2010). Thus, many metabolic interventions for the treatment of epilepsy, including ketogenic diets, intermittent fasting, calorie restriction or specific diets, have been proposed (Hartman and Stafstrom 2013).
3 Conclusions
In this chapter, we discussed the relationship between autophagy and epileptogenesis and how autophagy-related pathways, especially impaired mTOR signaling pathways, lead to epilepsy. We also discussed the role of autophagy as a target of antiepileptic treatment. These studies suggest that the use of different drugs that activate autophagy, including potent mTOR inhibitors such as rapamycin, and mTOR-independent autophagy modulators, such as drugs or dietary ingredients, can be used in the early treatment of epilepsy. They can prevent seizure recurrence and drug tolerance. Through the in-depth study of autophagy, the mTOR signaling pathway and the mechanism underlying epilepsy, new antiepileptic drug targets are expected to be found. Whether these targets act on the mTOR signaling pathway itself or on the other pathway of autophagy they will have a profound impact on the treatment strategy for epilepsy. Although autophagy is an important pathogenesis in epilepsy, the role of autophagy activation in epilepsy treatment remains to be explored.
References
Bar-Peled L, Chantranupong L, Cherniack AD et al (2013) A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340(6136):1100–1106
Bateup HS, Johnson CA, Denefrio CL et al (2013) Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron 78(3):510–522
Cepeda C, André VM, Hauptman JS, Rao SP et al (2010) Differential sensitivity of cortical neurons to 4-aminopyridine and rapamycin in diverse forms of pediatric epilepsy. Society for Neuroscience Annual Meeting. Society for Neuroscience, San Diego, CA
Chen S, Atkins CM, Liu CL et al (2007) Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab 27(5):939–949
Chu-Shore CJ, Major P, Camposano S et al (2010) The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 51(7):1236–1241
Criado O, Aguado C, Gayarre J et al (2012) Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum Mol Genet 21(7):1521–1533
Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372(9639):657–668
Franz DN, Leonard J, Tudor C et al (2006) Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59(3):490–498
Fu C, Cawthon B, Clinkscales W et al (2012) GABAergic interneuron development and function is modulated by the Tsc1 gene. Cereb Cortex 22(9):2111–2119
Ganesh S, Puri R, Singh S et al (2006) Recent advances in the molecular basis of Lafora’s progressive myoclonus epilepsy. J Hum Genet 51(1):1–8
Hartman AL, Stafstrom CE (2013) Harnessing the power of metabolism for seizure prevention: focus on dietary treatments. Epilepsy Behav 26(3):266–272
Hidvegi T, Ewing M, Hale P et al (2010) An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 329(5988):229–232
Huang X, Zhang H, Yang J et al. (2010) Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis 40(1):193–199
Jozwiak S, Schwartz RA, Janniger CK et al (2000) Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol 15(10):652–659
Li L, Zhang S, Zhang X et al (2013) Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-pathology in a mouse model of Alzheimer’s disease. Curr Alzheimer Res 10(4):433–441
Mattson MP (2010) The impact of dietary energy intake on cognitive aging. Front Aging Neurosci 2:5
McMahon J, Huang X, Yang J et al (2012) Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci 32(45):15704–15714
Meikle L, Pollizzi K, Egnor A et al (2008) Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci 28(21):5422–5432
Nadadhur AG, Alsaqati M, Gasparotto L et al (2019) Neuron-glia interactions increase neuronal phenotypes in tuberous sclerosis complex patient iPSC-derived models. Stem Cell Reports 12(1):42–56
Nguyen LH, Anderson AE (2018) mTOR-dependent alterations of Kv1.1 subunit expression in the neuronal subset-specific Pten knockout mouse model of cortical dysplasia with epilepsy. Sci Rep 8(1):3568
Raffo E, Coppola A, Ono T et al (2011) A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis 43(2):322–329
Ruegg S, Baybis M, Juul H et al (2007) Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res 77(2–3):85–92
Schick V, Majores M, Engels G et al (2006) Activation of Akt independent of PTEN and CTMP tumor-suppressor gene mutations in epilepsy-associated Taylor-type focal cortical dysplasias. Acta Neuropathol 112(6):715–725
Shacka JJ, Lu J, Xie ZL et al (2007) Kainic acid induces early and transient autophagic stress in mouse hippocampus. Neurosci Lett 414(1):57–60
Stafstrom CE, Carmant L (2015) Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med 5(6)
Tsai MH, Chan CK, Chang YC et al (2017) DEPDC5 mutations in familial and sporadic focal epilepsy. Clin Genet 92(4):397–404
Wang Y, Greenwood JS, Calcagnotto ME et al (2007) Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol 61(2):139–152
Wang X, Ma M, Teng J et al (2015) Valproate attenuates 25-kDa C-terminal fragment of TDP-43-induced neuronal toxicity via suppressing endoplasmic reticulum stress and activating autophagy. Int J Biol Sci 11(7):752–761
Wong M (2010) Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia 51(1):27–36
Wu H, Lu MH, Wang W et al (2015) Lamotrigine reduces beta-site AbetaPP-Cleaving Enzyme 1 protein levels through induction of autophagy. J Alzheimers Dis 46(4):863–876
Yasin SA, Ali AM, Tata M et al (2013) mTOR-dependent abnormalities in autophagy characterize human malformations of cortical development: evidence from focal cortical dysplasia and tuberous sclerosis. Acta Neuropathol 126(2):207–218
Zeng LH, Xu L, Gutmann DH et al (2008) Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63(4):444–453
Zeng LH, Rensing NR, Wong M (2009) Developing antiepileptogenic drugs for acquired epilepsy: targeting the mammalian target of rapamycin (mTOR) pathway. Mol Cell Pharmacol 1(3):124–129
Zeng LH, Rensing NR, Zhang B et al (2011) Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet 20(3):445–454
Zhang MY, Zheng CY, Zou MM et al (2014) Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice. Neurobiol Aging 35(12):2713–2725
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lv, M., Ma, Q. (2020). Autophagy and Epilepsy. In: Le, W. (eds) Autophagy: Biology and Diseases. Advances in Experimental Medicine and Biology, vol 1207. Springer, Singapore. https://doi.org/10.1007/978-981-15-4272-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-4272-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4271-8
Online ISBN: 978-981-15-4272-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)