Abstract
Hypothyroidism has been reported to be associated with cognitive decline. Considering the role of folic acid (FA) in cognitive performance, the present study was designed to investigate the effects of FA on hypothyroidism-induced cognitive impairment, oxidative damage, and alterations in acetylcholinesterase (AChE) activity in rat model of propylthiouracil (PTU)-induced hypothyroidism. In this study, PTU (0.05% in drinking water) and FA (5, 10, and 15 mg/kg, oral gavage) were administered for the rats during 7 weeks. Then, behavioral performance was tested using Morris water maze (MWM) and passive avoidance (PA) tasks. Finally, oxidative stress indicators and AChE activity were assayed in the brain tissues. The impairing effect of hypothyroidism on cognitive performance was markedly alleviated by FA especially at higher doses. In the MWM test, FA reduced escape latency and travelled distance, compared to the non-treated hypothyroid group. In the PA test, latency to enter dark chamber was significantly enhanced by FA compared to the non-treated hypothyroid group (p < 0.05–p < 0.001). Besides, FA attenuated AChE activity and malondialdehyde level but it increased activity of superoxide dismutase enzyme and total thiol content (p < 0.05–p < 0.001). In conclusion, our findings revealed that FA could improve learning and memory ability in hypothyroid rats. The observed protective effects may have been mediated through regulation of oxidative stress and AChE activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid hormones (THs) including thyroxine (T4) and triiodothyronine (T3) are of great importance for neuronal developmental stages and play a key role in maturation, migration, differentiation, and signaling of neurons. THs-related disorders during development and adulthood can lead to serious clinical problems including cognitive dysfunction (Bernal 2007). Since, concentrations of THs are decreased with the increase in age, then hypothyroidism commonly occurs among elderlies and can lead to symptoms of severe cognitive impairment including deterioration of learning and memory, perception, reasoning, problem-solving, decision-making, and language by affecting energy consumption processes, such as glucose metabolism, which is necessary for neurotransmission (Bégin et al. 2008; Annerbo and Lökk 2013). THs҆ receptors are abundantly found in the hippocampus. Therefore, deficiency of THs can lead to structural and physiological changes as well as a decrease in growth and number of hippocampal cells (Cooke et al. 2014). Besides, their deficiency may lead to an imbalance between oxidants and antioxidants, which can result in an increased level of reactive oxygen species (ROS) (Masullo et al. 2018). In addition to the increased production of free radicals, hypothyroidism may lead to a decrease in capacity of antioxidant defense system (Chakrabarti et al. 2016). Studies have shown a strong association between activity of THs and function of neurotransmitter systems especially cholinergic system (Fu et al. 2014; Wang et al. 2015). Clinical and experimental reports have suggested that THs replacement therapy can partially reverse negative effects of hypothyroidism on learning and memory, but a full recovery of T3 content in target tissues, especially in the brain, is far from reach and therefore, more appropriate treatments are required (Chaalal et al. 2019). It has been reported that antioxidant agents are able to decrease the side effects of hypothyroidism on learning and memory (Asiaei et al. 2017; Beheshti et al. 2017; Baghcheghi et al. 2018a, 2020; Khordad et al. 2018). In addition, some of vitamins have been shown to have beneficial effects on hypothyroidism-related cognitive dysfunctions (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2020).
Folic acid (FA) is a member of vitamin B family that plays an important role in cognitive activity by increasing level of vitamins B12 and B6. Deficiency in folate and vitamin B12 may increase the risk of dementia and memory impairment (Ma et al. 2016). Clinical evidence shows that approximately two-thirds of patients with anemia or folate and vitamin B12 deficiency have cognitive impairment (Reynolds 2002). Homocysteine is a sulfur amino acid and its blood level is controlled by FA, B12, and B6 vitamins (Modaghegh et al. 2016). However, there is a direct relationship between the increased homocysteine levels and cognitive impairment (Garcia and Zanibbi 2004). FA deficiency increases homocysteine levels, resulting in DNA damage and apoptosis in the hippocampal neurons. Therefore, administration of FA can improve cognitive function by controlling homocysteine levels and acting as an effective antioxidant (Singh et al. 2011). Moreover, studies have indicated that FA improves both short- and long-term memory (Shooshtari et al. 2012). Administration of FA in hypothyroid rats has been reported to improve oxidative stress and hypothalamic monoamines (Ibrahim et al. 2012). In addition, folate is metabolically bound to choline and is involved in synthesis and release of acetylcholine (Crivello et al. 2010). Folate deprivation genetically or in the diet reduces acetylcholine levels and can influence cognitive activity (Chan et al. 2008).
Given that hypothyroidism is one of the most common diseases leading to cognitive impairment and since, folate deficiency is one of the most important dietary health problems worldwide, this study was done to evaluate the effect of FA on learning and memory, oxidative stress indicators and acetylcholine esterase (AChE) activity in hypothyroid rats.
Materials and methods
Chemicals
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT), acetylthiocholine iodide, and propylthiouracil (PTU) were purchased from Sigma Co.(St. Louis, USA).
5,5´-Dithiobis-2-nitrobenzoic acid (DTNB), 2-thiobarbituric acid (TBA), hydrochloric acid (HCl), trichloroacetic acid (TCA), ethylenediaminetetraacetic acid disodium salt (Na2EDTA), tris (hydroxymethyl) aminomethane (Trizma base), phosphate-buffered saline (PBS), and dimethyl sulfoxide (DMSO) were purchased from Merck Co.(Darmstadt, Germany).
Animals and experimental design
In the present study, 50 male juvenile (21–22 days old) Wistar rats weighing 50–55 g were used. Animals were kept and treated under standard conditions (with 12 h light: dark cycle at 24 ± 2 °C) and they had free access to food and water. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Ethics Committee on Animal Research of Mashhad University of Medical Sciences (Approval No. IR.MUMS.MEDICAL.REC.1399.639).
The animals were randomly divided into 5 groups including (1) Control group, in which the rats received normal drinking water and vehicle instead of FA, (2) Hypothyroid group, in which PTU was added to drinking water and the rats received vehicle instead of FA. Groups 3, 4, and 5 included Hypothyroid—FA 5, Hypothyroid—FA 10, and Hypothyroid—FA 15 groups, in which rats received PTU in their drinking water (0.05%) (Asiaei et al. 2017; Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b, 2020) and were treated with 5, 10, or 15 mg/kg of FA, respectively through oral gavage (Singh et al. 2011; Shooshtari et al. 2012). PTU was added to drinking water daily and treatment by different doses of FA was carried out orally once a day for 7 weeks. Then, behavioral tests were done and finally, blood samples were collected and animals' brains were removed under deep anesthesia using ketamine and xylazine. Serum samples separated from the blood, and hippocampus and cortex samples separated from the brains were kept at − 80° C until further biochemical tests.
Morris water maze (MWM) test
A MWM test was used to assess spatial learning and memory in rats. In this test, a circular black pool (136 cm in diameter, height of 60 cm, and depth of 30 cm) filled with water (22–24 °C) was used that had an escape platform (10 cm in diameter and height of 28 cm). The pool was divided into the following four zones/quadrants: north, south, east, and west. The platform was located in center of southwestern quadrant. For helping the animals in navigation, visual cues were placed around the apparatus. The experiments were performed on 5 consecutive days so that, 4 trials were done in each day. In each trial, the animal was randomly located into the water tank and allowed to find the platform. After finding the platform, it was allowed to stay on the platform for 15 s. If the animal was not able to find the platform during 60 s, it was located on the platform by the experimenter and allowed to stay on it for 15 s. The animals rested outside the apparatus for 20 s between each trail. The time latency to find the platform and length of the swimming path were recorded by a video tracking system. On the 6th day, the probe test was performed in such a way that the platform was removed and the rat was allowed to swim for 60 s. The time spent and distance traveled in the target quadrant were recorded (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b).
Passive avoidance test
The passive avoidance (PA) test helps to study non-spatial memory. In this test, an apparatus containing 2 chambers (including dark and light chambers), separated by a small guillotine door was used. Floor of the dark chamber was covered with steel bars at a distance of 1 cm. An electric shock was applied to these bars by a stimulator. The experiment was performed in three phases: (1) habituation phase where the animals were placed in the apparatus for 2 consecutive days (each day for 5 min) and allowed to move freely between 2 chambers; (2) training phase where the animals were placed in the light chamber and the guillotine door was opened 20 s later. As soon as the animal entered the dark room, the door was closed and an electric shock (2 mA for 2 s) was applied to the animal's feet; and (3) retention phase, done 3, 24, 48, and 72 h after the training phase, where the animals were placed in the light chamber, the guillotine door was opened, and the time latency in entering the dark compartment, the time spent in the light and dark compartments, and frequency of entering into the dark compartment were recorded (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b). In retention trails, shock punishment was not applied.
Biochemical tests
Blood samples were centrifuged at 500 g for 10 min and the obtained serums were used for measuring levels of thyroxin. Serum level of thyroxin was measured using a radioimmunoassay method in the Navid Medical laboratory (Mashhad City, Razavi Khorasan Province, Iran). Homogenates of cerebral cortex and hippocampus (10% w/v) were prepared in ice-cold PBS (0.1 M, pH 7.4). Then, the homogenates were centrifuged at 4 °C, at 10,000 × g to separate supernatants for estimation of malondialdehyde (MDA) and total thiol concentrations as well as superoxide dismutase (SOD) and AChE activities.
Measurement of MDA and total thiol concentrations
As a marker of lipid peroxidation, MDA was measured in the hippocampus and cortex. Briefly, one ml of each sample was added to 2 ml of TBA/TCA/HCl reagent and the reaction mixtures were incubated in a boiling bath for 45 min. After cooling, the whole solutions were centrifuged at 1,000 g for 10 min. Finally, the supernatants were collected and absorbance of pink chromogen was measured at 535 nm using a spectrophotometer (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b). MDA concentration was calculated by the following equation:
For measuring thiol content, DTNB was used. In this assay, 50 μl of the homogenates was added to 1 ml of tris–EDTA buffer (pH 8.6) and the first absorbance (A1) was recorded at 412 nm using a spectrophotometer. Afterwards, 20 μl of DTNB solution (10 mM in methanol) was added to each sample and the second absorbance (A2) was recorded at the same wavelength (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b). Total thiol concentration (mM) was calculated by the following equation:
Estimation of SOD activity
SOD activity in the cerebral cortex and hippocampus was assessed based on the ability of the enzyme to inhibit autoxidation of pyrogallol (Madesh and Balasubramanian 1998). According to the method proposed by Madesh et al., each sample (10 μl of supernatant from homogenate) was mixed with MTT and pyrogallol solution and then, it was incubated at room temperature. After 5 min, DMSO was added to solubilize the resultant color. Optical absorbance was measured at 570 nm and activity of SOD was expressed as unit per gram of tissue (Madesh and Balasubramanian 1998).
Estimation of AChE activity
The AChE activity in the supernatants was determined by the Ellmans҆ method using acetylthiocholine iodide as a substrate. Briefly, each sample (50 μl) was added to a solution containing PBS (pH 8), 0.1 ml of DTNB (10 mM), and 0.02 ml of acetylthiocholine (75 mM). Changes in absorbance of the samples were spectrophotometrically recorded at 412 nm within 10 min and AChE activity was estimated as μmol/g tissue/min (Ellman et al. 1961).
Statistical analysis
Statistical analysis of the data was performed using the SPSS software version 11.5 and normal distribution of the data was checked by the Kolmogorov–Smirnov test. Data about learning phase in MWM test were analyzed by the repeated -measures analysis of variance (ANOVA) followed by Tukey’s post -hoc test. Other data were analyzed by one-way ANOVA followed by Tukey’s post -hoc test. All data were expressed as means ± SEM and a p-value of < 0.05 was considered as statistically significant.
Results
Morris water maze
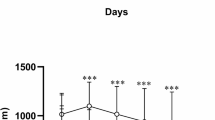
The time latency and distance traveled to reach the platform during 5-day training in the MWM test were significantly increased in hypothyroid group compared to the control group (P < 0.001 for latency and P < 0.05–P < 0.001 for the traveled distance). The time latency was significantly reduced in all FA-treated groups compared to the hypothyroid group (P < 0.05 to P < 0.001) (Fig. 1a). Moreover, the distance traveled to reach the platform was lower in hypothyroid rats treated with different doses of FA than those in the hypothyroid group (P < 0.05 to P < 0.01) (Fig. 1b).
The results of traveling time (a) and distance (b) during 5 days learning in Morris water maze. The data were expressed as mean ± SEM (n = 10). *P < 0.05, ***P < 0.001 compared to the control group. +P < 0.05, ++P < 0.01, +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid groups. FA: Folic acid
The animals in hypothyroid group showed significant decreases in the time spent and distance traveled in target quadrant on the probe day compared to the control group (P < 0.001 for both variables). The hypothyroid animals treated with FA (5, 10, or 15 mg) better remembered location of the platform and spent longer time and traveled longer distance in target area of MWM test than the hypothyroid group (P < 0.001 for both variables). The animals of the hypothyroid-FA 15 group spent longer time and traveled longer distance in the target area of MWM test than the hypothyroid-FA 10 group (P < 0.05 and P < 0.01, respectively) and traveled a longer distance in the target area than the hypothyroid-FA 5 group (P < 0.05). There was no significant difference in traveling time in the target area of MWM test between FA-treated hypothyroid rats and the control group. The distance traveled in the target area was shorter in the hypothyroid-FA 5 and hypothyroid-FA 10 groups than the control group (P < 0.01 for both variables) but there was no significant difference between hypothyroid-FA 15 and control groups in this regard (Fig. 2).
The results of traveling time (a) and distance (b) in the target quadrant during probe test in Morris water maze. The data were expressed as mean ± SEM (n = 10). **P < 0.01 and ***P < 0.001 compared to the control group. +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid group. #P < 0.05 and ##P < 0.01 comparison between Hypothyroid FA 5 and 10 and Hypothyroid FA 15 group. FA: Folic acid
Passive avoidance
Based on the results presented in Figs. 3 and 4, in the hypothyroid group, the latency to enter and the time spent in the light chamber were decreased while the time spent and frequency of entry to the dark chamber were significantly increased compared to the control group, 3, 24, 48, and 72 h after receiving electric shock (P < 0.01 to P < 0.001). The latency to enter the dark chamber was higher in all FA-treated hypothyroid groups than the control group, 3 h after delivery of electric shock (P < 0.01, P < 0.001 and P < 0.001 for FA doses of 5, 10, and 15, respectively). The latency to enter the dark chamber was higher in the hypothyroid-FA 10 and hypothyroid-FA 15 groups than the hypothyroid group 24, 48, and 72 h after delivery of the shock (P < 0.05 to P < 0.001) but there was no significant difference between the hypothyroid-FA 5 and hypothyroid groups at these time-points (P > 0.05). The latency to enter the dark chamber was significantly higher in the hypothyroid-FA 15 group compared to the hypothyroid-FA 5 group, 3, 24, 48, and 72 h post-shock (P < 0.01, P < 0.001, P < 0.001, and P < 0.05, respectively). As shown in Fig. 3a, the latency in the hypothyroid-FA 15 group was higher compared to the hypothyroid-FA 10 group, 24 h post- shock (P < 0.05).
The results of delay time for entering the dark (a) and the total time spent in the dark (b) in passive avoidance test. The data were expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the control group. +P < 0.05, ++P < 0.01 and +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid group. #P < 0.05, ##P < 0.01, and ##P < 0.01 comparison between Hypothyroid FA 5 and 10 and Hypothyroid FA 15 group. FA: Folic acid
The results of total time spent in the light (a) and the number of entering into the dark (b) in passive avoidance test. The data were expressed as mean ± SEM (n = 10). **P < 0.01 and ***P < 0.001 compared to the control group. +P < 0.05, ++P < 0.01, and +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid group. #P < 0.05, ##P < 0.01, and ###P < 0.001 comparison between Hypothyroid FA 5 and 10 and Hypothyroid FA 15 group. FA: Folic acid
All FA-treated hypothyroid rats spent shorter times in the dark chamber than the hypothyroid group, 3 h post- shock (P < 0.001 for all the three groups) but there was no significant difference in this parameter between the three FA-treated groups. Rats of the hypothyroid-FA 10 and hypothyroid-FA 15 groups also spent shorter times in the dark chamber than the hypothyroid group, 24, 48, and 72 h post-shock (P < 0.01 to P < 0.001) but there was no significant difference between the hypothyroid-FA 5 and hypothyroid groups at these time-points (Fig. 3b). Rats of the hypothyroid-FA 15 group spent shorter time in the dark chamber than the hypothyroid-FA 5 group, 24, 48, and 72 h post-shock (P < 0.05 to P < 0.001). In addition, rats of the hypothyroid-FA 10 group spent shorter time in the dark chamber than the hypothyroid-FA 5 group, 24 and 72 h post- shock (P < 0.05 for both times).
The results also showed that the FA-treated hypothyroid rats spent longer times in the light chamber than the hypothyroid group 3 h post-shock (P < 0.001 for all groups) but there was no significant difference between FA-treated hypothyroid groups in this regard. Moreover, rats of the hypothyroid-FA 10 and hypothyroid-FA 15 groups spent longer times in the light chamber than the hypothyroid group, 24, 48, and 72 h post-shock (P < 0.01 to P < 0.001) but there was no significant difference between hypothyroid-FA 5 and hypothyroid groups (Fig. 4a). The time spent in the light chamber was longer in the hypothyroid-FA 10 and hypothyroid-FA 15 groups than the hypothyroid-FA 5 group, 24, 48, and 72 h post-shock (P < 0.05 to P < 0.001).
The results of entries to the dark chamber (Fig. 4b) showed that frequency of entries was higher in the hypothyroid-FA 5 group than the control group (P < 0.01 to P < 0.001) but there was no significant difference between the hypothyroid-FA 5 and control groups. Rats of the hypothyroid-FA 10 and hypothyroid-FA 15 groups had lower number of entries into the dark chamber than the hypothyroid group at all time-points post- shock (P < 0.05 to P < 0.001). Number of entries was lower in the hypothyroid-FA 10 and hypothyroid-FA 15 groups than the hypothyroid-FA group (P < 0.05 to P < 0.001).
MDA and thiol concentrations in cortical and hippocampal tissues
Hypothyroidism induced by PTU increased MDA level but decreased total thiol level in both cortex and hippocampus (P < 0.001 for all groups) (Figs. 5, 6). FA reduced MDA concentration at all doses while it increased thiol content in the hippocampus (P < 0.05 to P < 0.001). Also, FA decreased MDA level at concentrations of 10 and 15 mg/kg while it increased thiol content in the cortex of hypothyroid rats compared to the hypothyroid group (P < 0.05 to P < 0.001) but there was no significant difference between hypothyroid-FA 5 and hypothyroid groups in terms of cortical levels of MDA and thiol. There was no significant difference between the three FA-treated groups in terms of hippocampal levels of MDA and thiol but in the cortex of the hypothyroid-FA 15 group, MDA concentration was lower (P < 0.01) while thiol content was higher (P < 0.05) than the hypothyroid-FA 5 group (Figs. 5, 6).
The results of malondialdehyde (MDA) in the hippocampus (a) and cortex (b). The data were expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control group. ++P < 0.01 and +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid group. ##P < 0.01 comparison between Hypothyroid FA 5 and Hypothyroid FA 15 group. FA: Folic acid
The results of thiol in the hippocampus (a) and cortex (b). The data were expressed as mean ± SEM (n = 10). ***P < 0.001 compared to the control group. +P < 0.05 and ++P < 0.01 comparison between Hypothyroid FA 10 and 15 and Hypothyroid group; #P < 0.05 comparison between Hypothyroid FA 5 and Hypothyroid FA 15 group. FA: Folic acid
SOD activity in hippocampal and cortical tissues
As demonstrated in Fig. 7, hippocampal and cortical SOD activity was significantly decreased following hypothyroidism induced by PTU administration (P < 0.001 for both tissues). SOD activity was significantly increased in hypothyroid-FA 10 and hypothyroid-FA 15 groups compared to the hypothyroid group (P < 0.01 and P < 0.001) and it was higher in the hypothyroid-FA 15 group than the hypothyroid-FA 5 group (P < 0.001 in the hippocampus and P < 0.01 in the cortex). There was no significant difference between the hypothyroid-FA 5 and hypothyroid groups in terms of both hippocampal and cortical SOD activity (Fig. 7). Both hippocampal and cortical SOD activity were still lower in all FA-treated hypothyroid groups than the control group (P < 0.05 to P < 0.001).
The results of SOD activity in the hippocampus (a) and cortex (b). The data were expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control group. ++P < 0.01 and +++P < 0.001 comparison between Hypothyroid FA 10 and 15 and Hypothyroid group, ##P < 0.01 and ###P < 0.001 comparison between Hypothyroid FA 5 and Hypothyroid FA 15 group. FA: Folic acid
AChE activity in hippocampal and cortical tissues
As indicated in Fig. 8, hippocampal and cortical AChE activity was significantly increased following hypothyroidism induced by PTU administration (P < 0.001 for both cortical and hippocampal tissues). Administration of 10 and 15 mg/kg of FA reduced AChE activity in the hippocampus and cortex of the rats compared to the hypothyroid group (P < 0.05 to P < 0.001) but there was no significant difference between the hypothyroid-FA 5 and hypothyroid groups. AChE activity in the cortex of both hypothyroid-FA 10 and hypothyroid-FA 15 groups was lower than that of the hypothyroid-FA 5 group (P < 0.01 for both groups) but there was no significant difference in the hippocampal AChE activity between FA-treated hypothyroid groups (Fig. 8). The results also showed that AChE activity was higher in the hippocampus of all FA-treated hypothyroid groups and in the cortex of hypothyroid-FA 5 group than the control group (P < 0.05 to P < 0.001).
The results of acetylcholinesterase (AChE) activity in the hippocampus (a) and cortex (b). *P < 0.05 and ***P < 0.001 compared to the control group. The data were expressed as mean ± SEM (n = 10). +P < 0.05, ++P < 0.01, and +++P < 0.001 comparison between Hypothyroid FA 5, 10, and 15 and Hypothyroid group. ##P < 0.01 comparison between Hypothyroid FA 5 and 10 and Hypothyroid FA 15 group. FA: Folic acid
Thyroxin level in the serum
The results indicated that serum level of thyroxin was significantly lower in the hypothyroid group than the control group (P < 0.001). The results also showed that FA was not able to reverse serum level of thyroxin as there was no significant difference between FA-treated hypothyroid and hypothyroid groups. Serum level of thyroxin was lower in all FA-treated hypothyroid groups than the control group (P < 0.001 for all cases) (Fig. 9).
Discussion
In the present study, PTU administration induced a hypothyroidism state as reflected by a low serum level of thyroxin in the hypothyroid group compared to the control group. PTU, as a well-known drug used for treatment of hyperthyroidism, has been frequently applied to produce hypothyroidism in rodents (Asiaei et al. 2017; Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b, 2020). Hypothyroidism, especially during developmental and growth periods has been shown to negatively influence central nervous system (Asiaei et al. 2017; Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b, 2020). Studies in humans and animals have demonstrated that hypothyroidism during developmental period impairs cognitive functions, such as attention, learning, and memory (Hosseini et al. 2010; Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b).
The results of the current study showed that PTU-induced hypothyroidism was accompanied with learning and memory impairment as confirmed by both MWM and PA tests. Also, it was found that rats of the hypothyroid group spent longer time to reach the platform during 5-day learning period in the MWM test. Rats of the hypothyroid group also traveled longer distance to reach the hidden platform than the control group. Interestingly, rats of the hypothyroid group could not remember location of the platform and spent less time and traveled shorter distance in the target area of the probe trial for the MWM test. The results of PA test also showed that rats of the hypothyroid group had a shorter delay but a higher frequency in entering the dark compartment, and spent longer time there, than the control group. These results are consistent with the previous studies showing that hypothyroidism during lactation, infancy, or developmental periods causes cognitive impairments, such as learning and memory dysfunction (Hosseini et al. 2010; Beydoun et al. 2013; Farrokhi et al. 2014). THs are known to be important for non-hippocampal and hippocampal-related learning and memory, synaptic flexibility, and neurogenesis (Cooke et al. 2014; Asiaei et al. 2017; Baghcheghi et al. 2020).
The exact mechanism(s) responsible for adverse effects of hypothyroidism on learning and memory have not been well elucidated. It is suggested that hypothyroidism causes cerebral atrophy and neuro-inflammation as it is also accompanied with production of amyloid beta (Aβ), tau hyperphosphorylation, and impairment of signaling pathways responsible for hippocampal-dependent spatial memory (Beydoun et al. 2013; Chaalal et al. 2019). An imbalance between production of peroxidants and antioxidants and generation of high levels of ROS and RNS (reactive nitrogen species) is also suggested to negatively influence on learning and memory (Venditti and Di 2006).
The results of the current study also showed that hypothyroidism-induced impairment of learning and memory was accompanied with a decrease in thiol content and SOD activity but it caused an increase in MDA level in both hippocampal and cortical tissues. These findings confirmed occurrence of an oxidative stress state in the brain of hypothyroid rats, which may have a role in impairing effects of hypothyroidism on learning and memory as observed in the present study. The results of previous research have shown that MDA levels are reduced in patients with controlled hypothyroidism due to antioxidant mechanisms mediated by THs (Villanueva et al. 2013). Also, it has been previously reported that hypothyroidism is accompanied with a decrease in thiol content and SOD and catalase (CAT) activities in the brain. In this context, Baghcheghi et al. (2017) demonstrated a remarkable decrease in thiol content, SOD, and CAT activities along with the increased MDA concentration in both hippocampal and cortical sections of rats҆ brain.
Interestingly, AChE activity was significantly decreased in the hippocampus and cortex of hypothyroid rats compared to the controls. Acetylcholine is one of the major neurotransmitters involved in cognitive function. It has been previously found that hypothyroidism is associated with dysfunction of cholinergic system (Smith et al. 2002). Also, it has been shown that thyroxin improved choline acetyltransferase activity and acetylcholine level in the brain and consequently, improved learning and memory (Fu et al. 2014). Considering the results of the present study and the mentioned evidence, at least, negative effects of hypothyroidism may be partly due to its effects on the cholinergic system.
Supplementation of THs is widely done to treat hypothyroidism. Levothyroxine has been reported to reduce hippocampal cognitive impairment in hypothyroid mice (Smith et al. 2002; Fu et al. 2014). Recently, vitamins C and E and antioxidant natural products have been suggested to reduce the adverse effects of hypothyroidism on brain functions including learning and memory (Beheshti et al. 2017; Baghcheghi et al. 2018a, 2018b, 2020). Herein, treatment by three doses of FA (i.e., 5, 10, and 15 mg/kg) improved learning and memory in the hypothyroid rats. The results showed that the hypothyroid rats treated with different doses of FA spent less time and traveled shorter distances to reach the platform during 5-day learning period in the MWM test. They also better remembered location of the platform and spent more time and traveled longer distances in the target area of the probe trial for the MWM test. The results of PA test also demonstrated that treatment by different doses of FA prolonged the latency to enter the dark compartment and increased the total time spent in the light compartment while it decreased the time spent in the dark chamber and reduced the number of entries to the dark compartment. To the best of our knowledge, there is no previous study reported about the effects of FA on hypothyroidism-induced learning and memory impairment. Previous studies have shown that administration of vitamin B improves cognitive function in people with low FA levels by decreasing homocysteine (Shooshtari et al. 2012). FA deficiency has been reported to be followed by increases in homocysteine and cognition and learning and memory impairments (Dam et al. 2017).
Our results also showed that FA attenuated MDA level while it increased thiol level and SOD activity in both hippocampal and cortical tissues. It has been previously reported that FA improves level of glutathione (GSH), but causes a significant reduction in brains҆ MDA levels, indicating suppression of lipid peroxidation (S). FA administration in patients with Alzheimer's disease could improve cognition and reduce inflammatory factors (Chen et al. 2016; Calderón Guzmán et al. 2020). FA deficiency increases risk of other neurological disorders including stroke through induction of oxidative DNA damage associated with morphological damage and the increased cell autophagy function (Zhao et al. 2016). Short-term administration of FA for 7 weeks in patients with metformin-treated type 2 diabetes was able to significantly decrease serum levels of MDA (Aghamohammadi et al. 2011). Moreover, FA administration with or without vitamin B12 for 30 days could prevent mitochondrial dysfunction and DNA damage caused by short-term exposure to arsenic trioxide in rats (Majumdar et al. 2009). FA treatment in stressed rats was able to reduce depressive-like behaviors and brains҆ oxidative damage, and ameliorate antioxidant imbalance in the hippocampus (Budni et al. 2012; Réus et al. 2018; Menegas et al. 2020).
For better understanding the underlying mechanism(s), AChE activity was also evaluated in the brain. The results showed that FA decreased AChE activity in the hippocampus and cortex. Considering these results, it seems that at least, improving effects of FA on learning and memory observed in the present study are partly due to its attenuating effect on AChE activity. Also, it has been reported that FA deficiency is associated with dysfunction of cholinergic system and the increased AChE activity (Crivello et al. 2010).
Nevertheless, FA was not able to restore thyroxin concentration to normal levels. Thus, it seems that FA was not able to protect the thyroid grain from damaging effects of PTU. For better understanding the effects of FA on serum level of thyroxin, further investigations are needed using other animal models including rat model of thyroidectomy.
Conclusion
Our findings revealed that FA could improve learning and memory ability in hypothyroid rats. The observed protective effects may have been achieved by suppression of oxidative stress and regulation of AChE activity.
References
Aghamohammadi V, Gargari BP, Aliasgharzadeh A (2011) Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr 30(3):210–215. https://doi.org/10.1080/07315724.2011.10719962
Annerbo S, Lökk J (2013) A clinical review of the association of thyroid stimulating hormone and cognitive impairment. ISRN Endocrinol 2013:856017. https://doi.org/10.1155/2013/856017
Asiaei F, Fazel A, Rajabzadeh AA, Hosseini M, Beheshti F, Seghatoleslam M (2017) Neuroprotective effects of Nigella sativa extract upon the hippocampus in PTU-induced hypothyroidism juvenile rats: a stereological study. Metab Brain Dis 32(5):1755–1765. https://doi.org/10.1007/s11011-017-0025-1
Baghcheghi Y, Beheshti F, Shafei MN, Salmani H, Sadeghnia HR, Soukhtanloo M, Anaeigoudari A, Hosseini M (2018a) The effects of vitamin E on brain derived neurotrophic factor, tissues oxidative damage and learning and memory of juvenile hypothyroid rats. Metab Brain Dis 33(3):713–724. https://doi.org/10.1007/s11011-017-0176-0
Baghcheghi Y, Hosseini M, Beheshti F, Salmani H, Anaeigoudari A (2018b) Thymoquinone reverses learning and memory impairments and brain tissue oxidative damage in hypothyroid juvenile rats. Arq Neuropsiquiatr 76(1):32–40. https://doi.org/10.1590/0004-282X20170182
Baghcheghi Y, Mansouri S, Beheshti F, Shafei MN, Salmani H, Reisi P, Anaeigoudari A, Bideskan AE, Hosseini M (2020) Neuroprotective and long term potentiation improving effects of vitamin E in juvenile hypothyroid rats. Int J Vitam Nutr Res 90(1–2):156–168. https://doi.org/10.1024/0300-9831/a000533
Bégin ME, Langlois MF, Lorrain D, Cunnane SC (2008) Thyroid function and cognition during aging. Curr Gerontol Geriatr Res 2008:474868. https://doi.org/10.1155/2008/474868
Beheshti F, Hosseini M, Shafei MN, Soukhtanloo M, Ghasemi S, Vafaee F, Zarepoor L (2017) The effects of Nigella sativa extract on hypothyroidism-associated learning and memory impairment during neonatal and juvenile growth in rats. Nutr Neurosci 20(1):49–59. https://doi.org/10.1179/1476830514Y.0000000144
Bernal J (2007) Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3(3):249–259. https://doi.org/10.1038/ncpendmet0424
Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman JS, Evans MK, Zonderman AB (2013) Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. J Clin Endocrinol Metab 98(8):3470–3481. https://doi.org/10.1210/jc.2013-1813
Budni J, Zomkowski AD, Engel D, Santos DB, dos Santos AA, Moretti M, Valvassori SS, Ornell F, Quevedo J, Farina M, Rodrigues AL (2012) Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Exp Neurol 240:112–121. https://doi.org/10.1016/j.expneurol.2012.10.024
Calderón Guzmán D, Osnaya Brizuela N, Ortiz Herrera M, Juárez Olguín H, Valenzuela Peraza A, Hernández García E, Barragán Mejía G (2020) Folic acid increases levels of GHS in brain of rats with oxidative stress induced with 3-nitropropionic acid. Arch Physiol Biochem 126(1):1–6. https://doi.org/10.1080/13813455.2018.1484771
Chaalal A, Poirier R, Blum D, Laroche S, Enderlin V (2019) Thyroid hormone supplementation restores spatial memory, hippocampal markers of neuroinflammation, plasticity-related signaling molecules, and β-amyloid peptide load in hypothyroid rats. Mol Neurobiol 56(1):722–735. https://doi.org/10.1007/s12035-018-1111-z
Chakrabarti SK, Ghosh S, Banerjee S, Mukherjee S, Chowdhury S (2016) Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab 20(5):674–678. https://doi.org/10.4103/2230-8210.190555
Chan A, Tchantchou F, Graves V, Rozen R, Shea TB (2008) Dietary and genetic compromise in folate availability reduces acetylcholine, cognitive performance and increases aggression: critical role of S-adenosyl methionine. J Nutr Health Aging 12(4):252–261. https://doi.org/10.1007/BF02982630
Chen H, Liu S, Ji L, Wu T, Ji Y, Zhou Y, Zheng M, Zhang M, Xu W, Huang G (2016) Folic Acid supplementation mitigates alzheimer’s disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm 2016:5912146. https://doi.org/10.1155/2016/5912146
Cooke GE, Mullally S, Correia N, O’Mara SM, Gibney J (2014) Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 24(3):433–440. https://doi.org/10.1089/thy.2013.0058
Crivello NA, Blusztajn JK, Joseph JA, Shukitt-Hale B, Smith DE (2010) Short-term nutritional folate deficiency in rats has a greater effect on choline and acetylcholine metabolism in the peripheral nervous system than in the brain, and this effect escalates with age. Nutr Res 30(10):722–730. https://doi.org/10.1016/j.nutres.2010.09.008
Dam K, Füchtemeier M, Farr TD, Boehm-Sturm P, Foddis M, Dirnagl U, Malysheva O, Caudill MA, Jadavji NM (2017) Increased homocysteine levels impair reference memory and reduce cortical levels of acetylcholine in a mouse model of vascular cognitive impairment. Behav Brain Res 15(321):201–208. https://doi.org/10.1016/j.bbr.2016.12.041
Ellman GL, Courtney KD, Andres VJr, Feather-Stone RM, (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Farrokhi E, Hosseini M, Beheshti F, Vafaee F, Hadjzadeh MA, Dastgheib SS (2014) Brain tissues oxidative damage as a possible mechanism of deleterious effects of propylthiouracil- induced hypothyroidism on learning and memory in neonatal and juvenile growth in rats. Basic Clin Neurosci 5(4):285–294
Fu A, Zhou R, Xu X (2014) The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice. Neural Regen Res 9(8):864–871. https://doi.org/10.4103/1673-5374.131602
Garcia A, Zanibbi K (2004) Homocysteine and cognitive function in elderly people. CMAJ 171(8):897–904. https://doi.org/10.1503/cmaj.1031586
Hosseini M, Dastghaib SS, Rafatpanah H, Hadjzadeh MA, Nahrevanian H, Farrokhi I (2010) Nitric oxide contributes to learning and memory deficits observed in hypothyroid rats during neonatal and juvenile growth. Clinics (sao Paulo) 65(11):1175–1181. https://doi.org/10.1590/s1807-59322010001100021
Ibrahim W, Tousson E, El-Masry T, Arafa N, Akela M (2012) The effect of folic acid as an antioxidant on the hypothalamic monoamines in experimentally induced hypothyroid rat. Toxicol Ind Health 28(3):253–261. https://doi.org/10.1177/0748233711410913
Khordad E, Alipour F, Beheshti F, Hosseini M, Rajabzadeh AA, Asiaei F, Seghatoleslam M (2018) Vitamin C prevents hypothyroidism associated neuronal damage in the hippocampus of neonatal and juvenile rats: a stereological study. J Chem Neuroanat 93:48–56. https://doi.org/10.1016/j.jchemneu.2017.11.011
Ma F, Wu T, Zhao J, Song A, Liu H, Xu W, Huang G (2016) Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci Rep 6:37486. https://doi.org/10.1038/srep37486
Madesh M, Balasubramanian KA (1998) Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 35(3):184–188
Majumdar S, Mukherjee S, Maiti A, Karmakar S, Das AS, Mukherjee M, Nanda A, Mitra C (2009) Folic acid or combination of folic acid and vitamin B(12) prevents short-term arsenic trioxide-induced systemic and mitochondrial dysfunction and DNA damage. Environ Toxicol 24(4):377–387. https://doi.org/10.1002/tox.20442
Masullo LF, Magalhães RA, Lemes RPG, de Almeida Filho TP, de Castro MF, Maia Filho PA, Cunha TOV, Quidute ARP, Fontenele EGP, Sun G, Martins MRA (2018) Levothyroxine replacement Improves oxidative status in primary hypothyroidism. Front Endocrinol (Lausanne) 9:655. https://doi.org/10.3389/fendo.2018.00655
Menegas S, Dal-Pont GC, Cararo JH, Varela RB, Aguiar-Geraldo JM, Possamai-Della T, Andersen ML, Quevedo J, Valvassori SS (2020) Efficacy of folic acid as an adjunct to lithium therapy on manic-like behaviors, oxidative stress and inflammatory parameters in an animal model of mania. Metab Brain Dis 35(2):413–425. https://doi.org/10.1007/s11011-019-00503-3.
Modaghegh MH, Ravari H, Haghighi MZ, Rajabnejad A (2016) Effect of folic acid therapy on homocysteine level in patients with atherosclerosis or Buerger's disease and in healthy individuals: A clinical trial. Electron Physician 8(10):3138-3143. .https://doi.org/10.19082/3138.
Réus GZ, Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Dos Santos TR, Darabas AC, Parzianello M, Matos D, Abatti M, Vieira AC, Fucillini V, Michels M, Dal-Pizzol F, Quevedo J (2018) ω-3 and folic acid act against depressive-like behavior and oxidative damage in the brain of rats subjected to early- or late-life stress. Nutrition 53:120–133. https://doi.org/10.1016/j.nut.2018.03.006
Reynolds EH (2002) Folic acid, ageing, depression, and dementia. BMJ 324(7352):1512–1515. https://doi.org/10.1136/bmj.324.7352.1512
Shooshtari MK, Moazedi AA, Parham GA (2012) Memory and motor coordination improvement by folic acid supplementation in healthy adult male rats. Iran J Basic Med Sci 15(6):1173–1179
Singh R, Kanwar SS, Sood PK, Nehru B (2011) Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cell Mol Neurobiol 31(1):83–91. https://doi.org/10.1007/s10571-010-9557-1
Smith JW, Evans AT, Costall B, Smythe JW (2002) Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev 26(1):45–60. https://doi.org/10.1016/s0149-7634(01)00037-9
Venditti P, Di Meo S (2006) Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 63(4):414–434. https://doi.org/10.1007/s00018-005-5457-9
Villanueva I, Alva-Sánchez C, Pacheco-Rosado J (2013) The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid Med Cell Longev 2013:218145. https://doi.org/10.1155/2013/218145
Wang F, Zeng X, Zhu Y, Ning D, Liu J, Liu C, Jia X, Zhu D (2015) Effects of thyroxine and donepezil on hippocampal acetylcholine content, acetylcholinesterase activity, synaptotagmin-1 and SNAP-25 expression in hypothyroid adult rats. Mol Med Rep 11(2):775–782. https://doi.org/10.3892/mmr.2014.2825
Zhao Y, Huang G, Chen S, Gou Y, Dong Z, Zhang X (2016) Folic acid deficiency increases brain cell injury via autophagy enhancement after focal cerebral ischemia. J Nutr Biochem 38:41–49. https://doi.org/10.1016/j.jnutbio.2016.08.009
Acknowledgements
The authors appreciate the Vice Chancellor for Research and Technology, Mashhad University of Medical Sciences for financial support (NO: 991073).
Funding
This study was funded by Vice Chancellor for Research and Technology, Mashhad University of Medical Sciences with the following grant number: 991073.
Author information
Authors and Affiliations
Contributions
Mahmoud Hosseini conducted conception and design of the project, Sabiheh Amirahmadi, Somaieh Ahmadabady, Mahsa Akbarain, Kataneh Abrari, Arezoo Rajbian, and Farzaneh Vafaee performed the experiments. Mahmoud Hosseini, Arezoo Rajbian, and Farzaneh Vafaee prepared a draft of the manuscript. Mahmoud Hosseini, Arezoo Rajbian, Farzaneh Vafaee performed statistical analysis. Mahmoud Hosseini, Farzaneh Vafaee, and Arezoo Rajbian provided final revision of the manuscript. All authors contributed to manuscript preparation and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in this studies including animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Ethical Committee of the Animal Research of Mashhad University of Medical Sciences (Ethical code: IR.MUMS.MEDICAL.REC.1399.639).
Data availability statement
The authors confirm that all data generated or analyzed during this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amirahmadi, S., Hosseini, M., Ahmadabady, S. et al. Folic acid attenuated learning and memory impairment via inhibition of oxidative damage and acetylcholinesterase activity in hypothyroid rats. Metab Brain Dis 36, 2393–2403 (2021). https://doi.org/10.1007/s11011-021-00815-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00815-3