Abstract
Vascular endothelial growth factor (VEGF) regulates angio/neurogenesis and also tightly links to the pathogenesis of Alzheimer’s disease (AD). Although exercise has a beneficial effect on neurovascular function and cognitive function, the direct effect of exercise on VEGF-related signaling and cognitive deficit in AD is incompletely understood. Therefore, the purpose of this study was to investigate the protective effect of exercise on angiostatin/VEGF cascade and cognitive function in AD model rats. Wistar male rats were randomly divided into five groups: control (CON), injection of DMSO (Sham-CON), CON-exercise (sham-EX), intrahippocampal injection of Aβ (Aβ), and Aβ-exercise (Aβ-EX). Rats in EX groups underwent treadmill exercise for 4 weeks, then the cognitive function was measured by the Morris Water Maze (MWM) test. mRNA levels of hypoxia-induced factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 2 (VEGFR2), and angiostatin were determined in hippocampus by RT-PCR. We found that spatial learning and memory were impaired in Aβ-injected rats, but exercise training improved it. Moreover, exercise training increased the reduced mRNA expression level of VEGF signaling, including HIF1α, VEGF, and VEGFR2 in the hippocampus from Aβ-injected rats. Also, the mRNA expression level of angiostatin was elevated in the hippocampus from Aβ-injected rats, and exercise training abrogated its expression. Our findings suggest that exercise training improves cognitive function in Aβ-injected rats, possibly through enhancing VEGF signaling and reducing angiostatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease that is responsible for cognitive dysfunction. The memory impairment is emanated from the accumulation of amyloid beta (Aβ) in the brain, especially in the hippocampus (Wang et al. 2011), and Aβ plaque forming in the vascular walls, called cerebral amyloid angiopathy (CAA). The co-existing of neuritic plaque and CAA in AD brain leads to neuronal impairment and cerebrovascular dysfunction (Hong et al. 2020; Zlokovic 2011), which induces tissue injury and neuronal death, and then finally result in cognitive decline in AD (Bell and Zlokovic 2009).
Vascular endothelial growth factor (VEGF) plays a pivotal role in angiogenesis and neurogenesis (Muche et al. 2015). VEGF expression is regulated by hypoxia-induced factor-1α (HIF-1α), then binds to its high- affinity receptor vascular endothelial growth factor receptor 2 (VEGFR2) to exert angiogenesis and neurogenesis (Waltenberger et al. 1994; Zhang et al. 2009). The expression of HIF-1α (Liu et al. 2008; Soucek et al. 2003) and VEGF was downregulated in AD mice brain (Echeverria et al. 2017; Guo et al. 2019; Provias and Jeynes 2014), and overexpression of VEGF ameliorated the Aβ deposition and cognitive decline in an AD rodent model (Cao et al. 2004; Wang et al. 2011). However, some studies reported that the level of VEGF was higher in plasma and brain tissue from patients with AD than in healthy subjects (Chiappelli et al. 2006; Cho et al. 2017; Mahoney et al. 2019). Therefore, the contribution of VEGF signaling to the pathogenesis of AD and cognitive dysfunction remains controversial. Angiostatin is known to angiogenesis inhibitor (Eriksson et al. 2003), by blocking the formation of VEGF through the inhibition of P42/44 MAP kinase activity, and it finally leads to angiogenesis inhibition (Liang et al. 2016). Arteries in patients with diabetes, the VEGF expression was negatively correlated the angiostatin expression (Chung et al. 2006). It indicates that angiostatin might be a key regulator in VEGF signaling. However, the role of angiostatin and its expression in AD is still unclear.
Exercise has numerous beneficial effects on brain health and cerebrovascular function (Hong et al. 2020) and pathogenesis in AD (Azimi et al. 2018). Exercise training increased cerebral blood flow with reduction of Aβ plaque formation and improving cognitive decline (Alfini et al. 2019). It also induces hippocampal neurogenesis and angiogenesis (Ballard 2017) with increases in the expression of HIF-1α (Dornbos et al. 2013; Smeyne et al. 2015) and VEGF/VEGFR2 in the brain of rodent models (Ding et al. 2006; Lou et al. 2008) and in plasma in a patient with AD (Pedrinolla et al. 2020). Furthermore, exercise training reduced the mRNA level of angiostatin in hindleg muscles from myocardial infarction induced rats (Ranjbar et al. 2016). Although beneficial effects of exercise training on VEGF signaling are well reported, it is unclear whether exercise can positively regulate angiostatin-associated VEGF signaling cascade in the hippocampus of AD with an improvement of cognitive dysfunction.
Therefore, our study aimed to investigate the effect of exercise on cognitive function and angiostatin/VEGF signaling cascade in the hippocampus of Aβ-injected rats. To answer these questions, we first determined whether the spatial learning and memory are impaired in Aβ-injected, and then we determined whether exercise training could improve cognitive deficits by modulating angiostatin/VEGF signaling cascade in Aβ-injected rats. Hence, we hypothesize that exercise training would improve cognitive decline in Aβ-injected rats by positively regulating the mRNA level of angiostatin/VEGF signaling cascade in the hippocampus from Aβ-injected rats.
Material and methods
Animals and experimental design
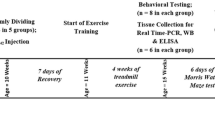
Seventy Wistar male rats (8 weeks age) were purchased from the Pasteur Institute of Iran. Rats were housed in a temperature-controlled (25 ± 2ºC) animal facility with 12 h light–dark cycles and allowed free access to water and chow. All rats were randomly divided into five experimental groups (n = 14 per group): control (CON), injection of DMSO (Sham-CON), CON-exercise (Sham-EX), intrahippocampal injection of Aβ (Aβ), and Aβ-exercise (Aβ-EX). At 10 weeks of age, DMSO and Aβ1-42 were injected for 7 consecutive days, and then the exercise groups performed a 4-week treadmill aerobic exercise training. 24 h after the last treadmill session animals from each group were randomly allocated to be either sacrificed (n = 6) or subjected to the Morris Water Maze test (n = 8) for 5 consecutive days (Fig. 1a). These animal experiments were carried out in accordance with the National Institutes of Health (NIH) Guideline for the Care and Use of Laboratory Animals and were approved by the Ethics Committee on the use of animals at Tarbiat Modares University.
Aβ1-42 preparation and intrahippocampal injection
The detail Aβ1-42 preparation was described previously (Mohammadpour et al. 2015). Briefly, Aβ1-42 peptides were dissolved in 3% dimethyl sulfoxide (DMSO) at a concentration of 5 μg/μl. Aβ solution was incubated at 37 °C for seven days to form neurotoxic fibrils (Azimi et al. 2018).
Rats were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (25 mg/kg, i.p.) and placed in a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). Holes were drilled in the skull above the hippocampus using the following coordinates: 3.8 mm posterior to bregma; + 2.2 mm lateral to the sagittal suture (Yang et al. 2005). The needle of a 1 µl Hamilton micro-syringe was inserted 2.7 mm below the surface of the skull. Thereafter, a total 1 µl of the Aβ solution (5 µg/µl) was injected into each side of the hippocampus. The infusion duration was 5 min, and the needles were left in place for an additional 60 s to allow diffusion of the solution away from the needle tip. Sham-CON and Sham-EX rats were injected with DMSO using the same surgical procedures. To make sure that the injection sites were in the Cornu Ammonis (CAl) area, 100 µm thick brain sections of two additional rats were taken, and the accuracy of the injection site was verified using an optical microscope.
Exercise training protocol
At 11 weeks of age, Sham-EX and Aβ-EX rats were subjected to five consecutive days of exercise training for 4 weeks on a rodent treadmill apparatus. Preceding the exercise training, rats were acclimated to running on a treadmill for 1 week. The exercise protocol consisted of running on a treadmill at 10-15 m/min, 5 days/week for 4 weeks, and then the intensity and duration of exercise gradually increased by the following week. The treadmill running sessions were conducted between 8:00 am and 2 pm. During the first to the second week of the training session, animals ran on the treadmill at 10 m/min for 30 min; each session included 2 × 15 min intervals and 5 min breaks between intervals. In the third week, the rats ran on a treadmill at 15 m/min for 45 min that each session included 3 × 15 min intervals and 5 min breaks between the session. In the fourth week, the rats ran 60 min on the treadmill that each session included 4 × 15 min intervals and 5 min breaks between intervals at a speed of 15 m/min (Fig. 1b) (Dao et al. 2013).
Morris water maze (MWM) test
Spatial learning was evaluated by the MWM test. A dark circular stainless steel pool (136 cm diameter, 60 cm height) was filled with water (23–25 °C). The pool contained various prominent visual cues. The pool was divided into four equal quadrants (Northeast (NE), Northwest (NW), Southeast (SE), and Southwest (SW)). A transparent circular-shaped platform was submerged 1 cm beneath the water surface in the Northwest quadrant (target quadrant) of the pool. The platform's location was constant during all trials. A video camera connected to a tracking system (EthoVision XT7, Netherland) was mounted over the pool to record the swimming trace for later analysis. The acquisition test was conducted in four training trials each day for four consecutive days. In each trial, an animal was placed in the wall of the pool at one of the four starting points of a quadrant in random order (north, east, south, west) and was allowed to detect the hidden platform within a period of 90 s. Rats detecting the platform within this period were allowed to stay on the platform for 20 s, while rats not reaching the platform within 90 s were gently guided to the platform and allowed to rest for 20 s. Several parameters, including escape latency, traveled distance, and swimming speed, were recorded in each trial to assess spatial learning ability (Azimi et al. 2018). The probe test was performed on experimental day 5. The platform was deleted from the water, and then each rat was placed in the water in opposite of the target quadrant and was allowed to swim freely for the 60 s. The percentage of time spent in the target quadrant was measured as spatial memory retention (Narenji et al. 2015). After the last probe test, the platform was elevated above the water surface and was covered with a piece of aluminum foil and then placed in the opposite of the target quadrant (southeast quadrant). This phase assessed the Visual-Motor coordination of rats (Narenji et al. 2015).
RNA extraction and real-time PCR
24 h after the final exercise session, rats were euthanized by intraperitoneal injection of ketamine and xylazine. Then brain was removed from the skull bone, and the hippocampi were carefully separated and rapidly frozen in liquid nitrogen and then stored at -80 °C. Right hippocampus samples of rats were powdered with a cold mortar and pestle. Total RNA was isolated using Isol RNA-Lysis reagent (5PRIME, QIAGEN Group), and total RNA was extracted according to the procedures described in the manufacturer's instructions. Total RNA was assayed using the Nanodrop spectrophotometer (Thermo Scientific, Wilmington, USA) to assess purity and concentration. First-strand cDNA was synthesized from total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems). Primer sequences (listed in Table 1) were designed using the NCBI primer design tool. All primers were purchased from Applied Biosystems, USA. A 20 µl reaction mixture containing 10 µl SYBR Green Mastermix (Amplicon) and the appropriate concentrations of gene-specific primers plus 1000 ng/µl of cDNA template was loaded in each well of a 96-well plate. All PCR reactions were performed in duplicate. PCR was performed with thermal conditions as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. GAPDH primers were used as the endogenous control, and the 2–ΔΔCt method was used to analyze the value of relative mRNA expression (Yuan et al. 2006).
Statistical analysis
All the data were described as mean ± SEM. Statistical analyses were performed by SPSS 18 software (SPSS, Inc., Chicago, IL, USA). Shapiro–Wilk test was used to determine the normality of the distribution. Comparisons between groups were performed by one-way ANOVA, followed by Tukey’s honestly significant difference (HSD) post hoc test. Statistical significance was set at p < 0.05.
Results
Exercise ameliorates cognitive dysfunction in AD pathology-induced rat model
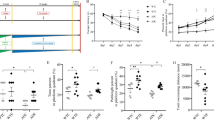
The acquisition phase data obtained from CON and Sham-CON indicates that no significant difference was found in escape latency (p > 0.05), traveled distance (p > 0.05) between the groups (Fig. 2a, b). However, the escape latency and traveled distance were significantly increased in Aβ group compared to CON group (p < 0.001) in the acquisition phase (Fig. 2a, b). Furthermore, exercise training significantly reduced the escape latency and traveled distance in Aβ-EX group compared to Aβ group (p < 0.001; Fig. 2a, b). However, there were no significant differences in mean swimming speed between groups (p > 0.05) (data not shown).
Effect of exercise training on spatial learning in Aβ-injected rat model by the Morris Water Maze test. CON, control; Sham-CON, DMSO injected; sham-EX, control with exercise training (EX); Aβ, Aβ-injected; AD-EX, Aβ-injected with EX; ns, not significant. a-b: effect of exercise training on mean scape latency (sec) and mean traveled distance (cm) in the acquisition phase. c-d: effect of exercise training on the percentage of the time spent in the target quadrant in the probe trial and mean escape latency to the visible platform. Bar graph values are presented as mean ± SEM. n = 8. *P < 0.05 vs. CON; #P < 0.05 vs. AD
In the probe phase, a significant decrease in the percentage of time spent in the target quadrant in Aβ group compared to CON group (p < 0.001), while exercise significantly increased the percent time spent in the target quadrant in Aβ-EX group compared to Aβ group (p < 0.001; Fig. 2c). Also, the percent time spent in the target quadrant was increased in sham-EX group compared to CON group (p < 0.001; Fig. 2c). However, there was no significant difference on the visible platform day for escape latency among the groups (Fig. 2d).
The effect of exercise on mRNA levels of HIF1, VEGF, VEGFR2, and Angiostatin in hippocampus from AD rat
As shown in Fig. 3a, the mRNA expression level of HIF-1α was significantly reduced in the hippocampus from Aβ-injected brain compared with CON brain (p < 0.05), but its expression was significantly elevated by exercise training in the hippocampus from Aβ-EX brain compared to Aβ-injected brain (p < 0.05).
The effect of exercise on angiostatin/VEGF signaling in the hippocampus of Aβ-injected rat model. CON, control; Sham-CON, DMSO injected; sham-EX, control with exercise training (EX); Aβ, Aβ-injected; AD-EX, Aβ-injected with EX. a-d: effect of exercise training on mRNA level of HIF1, VEGF, VEGFR2, and angiostatin. Bar graph values are presented as mean ± SEM. n = 6. *P < 0.05 vs. CON; #P < 0.05 vs. AD
We also found that the mRNA expression levels of VEGF and VEGFR2 were significantly diminished in the hippocampus from Aβ-injected brain compared to CON brain, but these expressions were significantly increased in the hippocampus from Aβ-EX brain compared to Aβ-injected brain (p < 0.05; Fig. 3b, c). Moreover, the mRNA expression levels of VEGF and VEGFR2 increased in the hippocampus from Sham-EX brain compared with CON brain. However, there was no significant difference in VEGF and VEGFR2 in the hippocampus between Aβ-EX brain compared to CON brain (p > 0.05; Fig. 3b, c).
We found that the mRNA expression level of angiostatin was significantly higher in the hippocampus from Aβ-injected brain compared to CON brain, but its expression was not significantly diminished in the hippocampus from Aβ-EX brain compared to Aβ-injected brain (Fig. 3d).
Discussion
The present findings demonstrate the first evidence that AD pathology and exercise training contribute to the mRNA level of angiostatin in the Aβ-injected brain. Also, our current study provides evidence that exercise training ameliorates spatial learning and memory deficits in Aβ-injected rats, possibly through VEGF signaling. Specifically, our results demonstrate that 1) the impaired spatial learning and memory is ameliorated by exercise training in Aβ-injected rat; 2) exercise training increased the reduced mRNA expression level of VEGF signaling including HIF1α, VEGF, and VEGFR2 in hippocampus from Aβ-injected rat, 3) mRNA expression level of angiostatin was elevated in hippocampus from Aβ-injected rat, and exercise training positively regulated its expression.
Memory and cognitive deficits are the most primary clinical manifestation in patients with AD, are implicated with the deposition of Aβ plaques in the hippocampal area and CAA in the brain (Rosa and Fahnestock 2014). Previous studies have shown that spatial learning impairment and memory deficits were developed after Aβ microinjection in the hippocampus of rodent models (Sharma et al. 2016; Wu et al. 2017). Our data aligned with the previous finding that the escape latency and traveled distance were significantly increased in the Aβ group compared to the CON group in the acquisition phase (Fig. 2a, b), as well as a significant decrease in the percentage of time spent in the target quadrant in Aβ group compared to CON group in probe phase (Fig. 2c). Our findings suggest that Aβ injection directly impairs cognitive function in the rat model. The beneficial effect of exercise training on cognitive decline in patients with AD (Gomes-Osman et al. 2017; Morris et al. 2017) and animal models (Azimi et al. 2018; Kim et al. 2014; Wang and Wang 2016) are well demonstrated. Exercise training elevates low density lipoprotein receptor-related protein 1(LRP-1) expression in the AD hippocampus, in turn, increase Aβ clearance, finally improving cognitive function and preventing the progression of AD pathology (Khodadadi et al. 2018). Our results also revealed that exercise training ameliorated spatial learning and memory impairment in the Aβ-EX group compared to the Aβ group (Fig. 2a-c). Collectively, current findings provide evidence that exercise training has protective effects on spatial learning and memory, possibly through the prevention of Aβ pathology. However, our study has a limitation that the Aβ accumulation in the hippocampus from the brain was not directly measured. This limitation should be addressed in future studies.
HIF-1α is tightly interacting with VEGF to initiate angiogenesis and its expression is regulated by AMP-activated protein kinase (AMPK) in AD (Ogunshola and Antoniou 2009; Zhang et al. 2009). HIF-1α expression was lower in AD brains compared with age-matched controls (Liu et al. 2008), and overexpression of HIF-1α protected neurons against Aβ-induced neurotoxicity and reduced Aβ accumulation in AD mice model (Liu et al. 2008; Mechlovich et al. 2014; Soucek et al. 2003; Zheng et al. 2015). Furthermore, VEGF is one of the main angiogenesis factors regulating neurogenesis and is also involved in AD pathogenesis as well as cognitive impairment in AD (Guo et al. 2019; Provias and Jeynes 2014; Wang et al. 2011). Previous studies reported that the mRNA and protein expression of HIF-1 (Liu et al. 2008; Schubert et al. 2009), VEGF, VEGFR2 are downregulated in the hippocampus and cortex of the AD brain (Guo et al. 2019; Provias and Jeynes 2014; Wang et al. 2011). These findings align with our results, in part, the mRNA levels of HIF-1, VEFG, and VEGFR2 were significantly reduced in the hippocampus of the Aβ group compared to CON groups (Fig. 3a-c). Moreover, VEGF treatment ameliorates cognitive deficits in AD by reducing Aβ accumulation in the AD brain (Cao et al. 2004; Guo et al. 2019; Wang et al. 2011) and cerebral vessels with reduction of Aβ-induced vascular regression and apoptosis (Religa et al. 2013). The expression of VEGF is decreased with an elevation of Aβ, while VEGF treatment improves the cognitive impairment, concurrently decreased the BACE1 (β-site APP cleaving enzyme) and increased ADAM10 (α-secretase cleaving APP) expression in the TG2576 mice brain (Guo et al. 2019). Furthermore, Aβ acts as an anti-angiogenic factor inhibiting the migration and permeability of VEGF in endothelial cells, in turn, blocks VEGF signaling by direct interaction with VEGFR2 in Aβ-treated human umbilical vein endothelial cells (HUVECs) and human brain microvascular endothelial cells (HBMECs) (Patel et al. 2010). Elevated Aβ plaque formation in brain tissue and CAA might impair Aβ clearance process possibly through a decrease of VEGF signaling, and it exacerbates AD pathology and cognitive decline in the AD. These previous findings support our data, in part, reduced mRNA level of VEGF signaling induced by Aβ injection might accelerate spatial learning and memory deficits in Aβ group (Fig. 3a-c). Collectively, current data suggest that the reduced the mRNA expression of VEGF signaling might induce spatial learning and memory deficits, possibly through exacerbating amyloidogenic pathway in Aβ-injected rat brain.
Several clinical investigations report that exercise training delay or prevent progression of AD pathogenesis and cognitive impairment (Kim et al. 2014; Morris et al. 2017) via a decrease in Aβ accumulation, which was accompanied by increased mRNA levels of HIF-1, VEGF and VEGFR2 in the hippocampus of rats (Ding et al. 2006; Dornbos et al. 2013; Lou et al. 2008), as well as patient with AD (Pedrinolla et al. 2020). Exercise training increases HIF-1α expression in neurons and astrocytes from the ischemic rat brain (Otsuka et al. 2019), pressure overloaded in mouse heart (Tian et al. 2020), as well as in patients with hypertension (Muangritdech et al. 2020). However, no study has reported the beneficial effect of exercise on the regulation of HIF-1α in AD, especially in the Aβ-injected rat model. Our study result reported for the first time that exercise training elevates the mRNA level of HIF-1α in the hippocampus from Aβ-injected rats. Aforementioned, VEGF signaling improves cognitive function by modulating the amyloidogenic pathway (Bürger et al. 2009; Guo et al. 2019; Wang et al. 2011). VEGF administration increased ADAM10 and reduced BACE1 expressions in the brain of Tg2576 mice, which led to the improvement in cognitive function (Guo et al. 2019). Furthermore, Wang et al. also reported that VEGF treatment in PDGF-hAPP transgenic mice protects memory impairment and decreased Aβ deposition in the brain (Wang et al. 2011). These findings suggested that exercise-induced improvement of VEGF signaling might increase in both central and peripheral Aβ clearance in the AD brain, eventually ameliorating learning and memory deficits. Our findings also showed that exercise training elevates the mRNA levels of HIF-1, VEGF, and VEGFR2 in the hippocampus of the Aβ-EX group compared to Aβ groups (Fig. 3a-c) with improved spatial learning and memory (Fig. 2a-c). These findings collectively imply that elevated VEGF signaling by exercise training might ameliorate the impairment of spatial learning and memory, possibly through converting amyloidogenic to a non-amyloidogenic pathway in Aβ-injected rat brain.
Angiostatin is identified as an endogenous angiogenic inhibitor that inhibiting both angiogenesis and vascular permeability (Eriksson et al. 2003). It has been indicated that angiostatin attaches to integrins and inhibits p42/p44 MAP kinase pathway that playing a role in regulating VEGF and controlling its downstream pathways (Sima et al. 2004). Chung et al. have reported that angiostatin production is inversely associated with VEGF expression, but its expression is positively correlated with matrix metalloproteinase-2 and-9 (MMP-2 & 9) expressions in human diabetic arterial vasculature (Chung et al. 2006). This study results explained that elevated angiostatin could be attributed to the pathogenesis of impaired angiogenesis in diabetes mellitus. Furthermore, we firstly show that the mRNA level of angiostatin was elevated in the hippocampus from the Aβ group with reduced VEGF mRNA level (Fig. 3d) while 10 weeks of aerobic training reduced the mRNA level of angiostatin in cardiac and hindlimb muscles from rats with myocardial infarction (Ranjbar et al. 2016). Our study demonstrates that exercise training reduced the mRNA level of angiostatin in the hippocampus of Aβ-injected rats (Fig. 3d, 12% lower vs. Aβ) although it was not statistically significant. It suggests that angiostatin may partially contribute to the beneficial effect of exercise on cognitive function in Aβ-injected rats. Along with angiostatin, more importantly, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and HIF-1α have been suggested to enhance cognitive function in the brain by exercise training. They are upstream pathways of VEGF signaling that both are regulated by AMPK (Ohno et al. 2012) and exercise training ameliorates cognitive impairment through the increase of AMPK activity, in turn, leads to upregulation of PGC-1α in the hippocampus of Aβ-injected rats model (Azimi et al. 2018). It suggested that the elevation of AMPK/PGC-1α signaling might be a possible potential mechanism for improvement of spatial learning and memory deficits although angiostatin is not significantly regulated by exercise training in the Aβ-injected rat model.
In conclusion, the current study highlights the importance of exercise training as an effective approach to reduce/prevent the deterioration of spatial learning and memory in Aβ-injected rats, possibly through enhancing VEGF signaling, but not in angiostatin. Further investigation is required to elucidate the direct causality between VEGF signaling and angiostatin on cognitive deficits in Aβ-injected rats. These findings will provide the missing clues for developing a therapeutic strategy to protect and/or prevent cognitive decline in AD.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC (2019) Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis 67(2):671–684
Azimi M, Gharakhanlou R, Naghdi N, Khodadadi D, Heysieattalab S (2018) Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/FNDC5/BDNF pathway. Peptides 102:78–88
Ballard HJ (2017) Exercise makes your brain bigger: Skeletal muscle VEGF and hippocampal neurogenesis. J Physiol 595(17):5721
Bell RD, Zlokovic BV (2009) Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118(1):103–113
Bürger S, Noack M, Kirazov LP, Kirazov EP, Naydenov CL, Kouznetsova E, Schliebs R (2009) Vascular endothelial growth factor (VEGF) affects processing of amyloid precursor protein and β-amyloidogenesis in brain slice cultures derived from transgenic Tg2576 mouse brain. Int J Dev Neurosci 27(6):517–523
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36(8):827
Chiappelli M, Borroni B, Archetti S, Calabrese E, Corsi MM, Franceschi M, Licastro F (2006) VEGF gene and phenotype relation with Alzheimer’s disease and mild cognitive impairment. Rejuvenation Res 9(4):485–493
Cho S-J, Park MH, Han C, Yoon K, Koh YH (2017) VEGFR2 alteration in Alzheimer’s disease. Sci Rep 7(1):1–11
Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB (2006) Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and-9 in human type 2 diabetic arterial vasculature. Circ Res 99(2):140–148
Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA (2013) Treadmill exercise prevents learning and memory impairment in Alzheimer’s disease-like pathology. Curr Alzheimer Res 10(5):507–515
Ding Y-H, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y (2006) Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res 3(1):15–23
Dornbos D III, Zwagerman N, Guo M, Ding JY, Peng C, Esmail F, Ding Y (2013) Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res 91(6):818–827
Echeverria V, Barreto EG, Ávila-Rodriguezc MV, Tarasov V, Aliev G (2017) Is VEGF a key target of cotinine and other potential therapies against Alzheimer disease? Curr Alzheimer Res 14(11):1155–1163
Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ (2003) Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett 536(1–3):19–24
Gomes-Osman J, Cabral DF, Hinchman C, Jannati A, Morris TP, Pascual-Leone A (2017) The effects of exercise on cognitive function and brain plasticity–a feasibility trial. Restor Neurol Neurosci 35(5):547–556
Guo H, Xia D, Liao S, Niu B, Tang J, Hu H, Cao B (2019) Vascular endothelial growth factor improves the cognitive decline of Alzheimer’s disease via concurrently inducing the expression of ADAM10 and reducing the expression of β-site APP cleaving enzyme 1 in Tg2576 mice. Neurosci Res 142:49–57
Hong J, Hong S-G, Lee J, Park J-Y, Eriksen JL, Rooney BV, Park Y (2020) Exercise training ameliorates cerebrovascular dysfunction in a murine model of Alzheimer’s disease: role of the P2Y2 receptor and endoplasmic reticulum stress. Am J Physiol-Heart Circ Physiol 318(6):H1559–H1569
Khodadadi D, Gharakhanlou R, Naghdi N, Salimi M, Azimi SM, Shahed A (2018) The effect of 4 weeks of exercise preconditioning on soluble amyloid beta level and memory impairment in rats with Alzheimer’s disease induced by Aβ1-42 injection. Razi J Med Sci 24(165):74–84
Kim B-K, Shin M-S, Kim C-J, Baek S-B, Ko Y-C, Kim Y-P (2014) Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehab 10(1):2
Liang Y-Z, Zeng Z-L, Hua L-L, Li J-F, Wang Y-L, Bi X-Z (2016) Expression and significance of angiostatin, vascular endothelial growth factor and matrix metalloproteinase-9 in brain tissue of diabetic rats with ischemia reperfusion. Asian Pac J Trop Med 9(6):587–591
Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong C-X (2008) Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 582(2):359–364
Lou S-J, Liu J-Y, Chang H, Chen P-J (2008) Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res 1210:48–55
Mahoney ER, Dumitrescu L, Moore AM, Cambronero FE, De Jager PL, Koran MEI, Schneider JA (2019) Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer’s disease. Mol Psychiatry 1–9
Mechlovich D, Amit T, Bar-Am O, Mandel SBH, Youdim M, Weinreb O (2014) The novel multi-target iron chelator, M30 modulates HIF-1α-related glycolytic genes and insulin signaling pathway in the frontal cortex of APP/PS1 Alzheimer’s disease mice. Curr Alzheimer Res 11(2):119–127
Mohammadpour JD, Hosseinmardi N, Janahmadi M, Fathollahi Y, Motamedi F, Rohampour K (2015) Non-selective NSAIDs improve the amyloid-β-mediated suppression of memory and synaptic plasticity. Pharmacol Biochem Behav 132:33–41
Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, Swerdlow RH (2017) Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS One 12(2):e0170547
Muangritdech N, Hamlin MJ, Sawanyawisuth K, Prajumwongs P, Saengjan W, Wonnabussapawich P, Manimmanakorn A (2020) Hypoxic training improves blood pressure, nitric oxide and hypoxia-inducible factor-1 alpha in hypertensive patients. Eur J Appl Physiol 120(8):1815–1826
Muche A, Bigl M, Arendt T, Schliebs R (2015) Expression of vascular endothelial growth factor (VEGF) mRNA, VEGF receptor 2 (Flk-1) mRNA, and of VEGF co-receptor neuropilin (Nrp)-1 mRNA in brain tissue of aging Tg2576 mice by in situ hybridization. Int J Dev Neurosci 43:25–34
Narenji SA, Naghdi N, Azadmanesh K, Edalat R (2015) 3α-diol administration decreases hippocampal PKA (II) mRNA expression and impairs Morris water maze performance in adult male rats. Behav Brain Res 280:149–159
Ogunshola O, Antoniou X (2009) Contribution of hypoxia to Alzheimer’s disease: is HIF-1α a mediator of neurodegeneration? Cell Mol Life Sci 66(22):3555–3563
Ohno H, Shirato K, Sakurai T, Ogasawara J, Sumitani Y, Sato S, Kizaki T (2012) Effect of exercise on HIF-1 and VEGF signaling. J Phys Fitness Sports Med 1(1):5–16
Otsuka S, Sakakima H, Terashi T, Takada S, Nakanishi K, Kikuchi K (2019) Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3γ after focal brain ischemia in rats. Brain Struct Funct 224(2):727–738
Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Paris D (2010) Alzheimer’s β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem 112(1):66–76
Pedrinolla A, Venturelli M, Fonte C, Tamburin S, Di Baldassarre A, Naro F, Muti E (2020) Exercise training improves vascular function in patients with Alzheimer’s disease. Eur J Appl Physiol 1–13
Provias J, Jeynes B (2014) Reduction in vascular endothelial growth factor expression in the superior temporal, hippocampal, and brainstem regions in Alzheimer’s disease. Curr Neurovasc Res 11(3):202–209
Ranjbar K, Rahmani-Nia F, Shahabpour E (2016) Aerobic training and l-arginine supplementation promotes rat heart and hindleg muscles arteriogenesis after myocardial infarction. J Physiol Biochem 72(3):393–404
Religa P, Cao R, Religa D, Xue Y, Bogdanovic N, Westaway D, Cao Y (2013) VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci Rep 3:2053
Rosa E, Fahnestock M (2014) Amyloid-Beta, BDNF, and the mechanism of neurodegeneration in Alzheimer’s disease handbook of neurotoxicity. Springer, pp 1597–1620
Schubert D, Soucek T, Blouw B (2009) The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci 29(7):1323–1334
Sharma S, Verma S, Kapoor M, Saini A, Nehru B (2016) Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol Res 38(9):838–850
Sima J, Zhang SX, Shao C, Fant J, Ma J-X (2004) The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett 564(1–2):19–23
Smeyne M, Sladen P, Jiao Y, Dragatsis I, Smeyne RJ (2015) HIF1α is necessary for exercise-induced neuroprotection while HIF2α is needed for dopaminergic neuron survival in the substantia nigra pars compacta. Neuroscience 295:23–38
Soucek T, Cumming R, Dargusch R, Maher P, Schubert D (2003) The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 39(1):43–56
Tian X, Zhou N, Yuan J, Lu L, Zhang Q, Wei M, Yuan L (2020) Heat shock transcription factor 1 regulates exercise-induced myocardial angiogenesis after pressure overload via HIF-1α/VEGF pathway. J Cell Mol Med 24(3):2178–2188
Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin C-H (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269(43):26988–26995
Wang P, Xie Z-H, Guo Y-J, Zhao C-P, Jiang H, Song Y, Bi J-Z (2011) VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 411(3):620–626
Wang X-Q, Wang G-W (2016) Effects of treadmill exercise intensity on spatial working memory and long-term memory in rats. Life Sci 149:96–103
Wu L, Feng X, Li T, Sun B, Khan MZ, He L (2017) Risperidone ameliorated Aβ1-42-induced cognitive and hippocampal synaptic impairments in mice. Behav Brain Res 322:145–156
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Frautschy SA (2005) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280(7):5892–5901
Yuan JS, Reed A, Chen F, Stewart CN (2006) Statistical analysis of real-time PCR data. BMC Bioinf 7(1):85
Zhang L, Qu Y, Yang C, Tang J, Zhang X, Mao M, Ferriero D (2009) Signaling pathway involved in hypoxia-inducible factor-1α regulation in hypoxic-ischemic cortical neurons in vitro. Neurosci Lett 461(1):1–6
Zheng H, Fridkin M, Youdim M (2015) New approaches to treating Alzheimer's disease. Perspect Med Chem 7:PMC. S13210
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723
Acknowledgments
We express our gratitude to the Kharazmi University of Tehran for financial support. The authors would also like to thank the Trabiat Modarres University for the use of experimental equipment.
Author information
Authors and Affiliations
Contributions
AZ, JH, YP, and HR designed the study, collected and analyzed data. RG and NN involved behavioral study. AZ and MA performed animal experiment. AZ and JH wrote the manuscript. YP, JL, and HR edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The animal experiments were carried out in accordance with the National Institutes of Health (NIH) Guideline for the Care and Use of Laboratory Animals and were approved by the Ethics Committee on the use of animals at Tarbiat Modares University, Tehran, Iran.
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aliasghar Zarezadehmehrizi and Junyoung Hong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zarezadehmehrizi, A., Hong, J., Lee, J. et al. Exercise training ameliorates cognitive dysfunction in amyloid beta-injected rat model: possible mechanisms of Angiostatin/VEGF signaling. Metab Brain Dis 36, 2263–2271 (2021). https://doi.org/10.1007/s11011-021-00751-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00751-2