Abstract
Intracerebroventricular (ICV) injection of streptozotocin (STZ) is a well established procedure to induce neuroinflammation leading to dementia in experimental animals. However, the optimal dose of STZ has not been determined. In the present study, rats were ICV injected with 1.5, 3 and 6 mg of STZ per kg of body weight. After 21 days, neuroinflammatory markers i.e. TNF-α, IL-1β, ROS and nitrite were quantified in the hippocampus. Memory function was assessed by the radial arm maze test after 9, 12, 15, 18, 21 days following STZ injection. STZ treatment significantly increased neuroinflammatory markers and decreased memory functions in a dose dependent manner showing optimum effects at the dose of 3 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rodent models have notably contributed to elucidate the underlying mechanisms of Alzheimer’s disease (AD), the leading causes of dementia in the world (Neha et al. 2014). The pathogenesis of AD is complex and the mechanism of neurodegeneration is disparate in different AD patients (Chetelat 2013). In comparison to the familial AD type (2–5% of total AD patients), sporadic AD (sAD) constitutes over 90% of total AD population (Blennow et al. 2006). Very little is known about the mechanism of neurodegeneration in sAD (Kamat et al. 2016). Although the “Amyloid cascade” hypothesis supports the neurodegeneration process in familial AD but it fails to explain the pathophysiological mechanisms in the sporadic type (Chetelat 2013). The sporadic AD has been explained by different investigators on the basis of oxidative-nitrosative stress (Reeta et al. 2017), neuroinflammation (Nazem et al. 2015) and impaired energy metabolism (Grieb 2016). The decreased ability of brain tissue to metabolize glucose has been considered as a cause of dementia (Vlassenko et al. 2010) and it was reported that the people with type-2 diabetes are at a higher risk of AD (Chatterjee and Mudher 2018).
Several chemically induced models of AD, such as intracerebroventricular (ICV) injection of colchicines (Sil et al. 2015), okadaic acid (Costa et al. 2012), amyloid beta peptide (Gupta et al. 2018) in rat/mouse have been widely used for studying sAD like pathology. ICV injection of streptozotocin (STZ), a glucosamine-nitrosourea compound present in bacteria Streptomyces achromogenes (Lewis and Barbiers 1959), induces a rat/mouse model of dementia (Grieb 2016). ICV STZ injected experimental rat (STZ-ER) develops glucose hypometabolism(so called type-3 diabetes), oxidative stress, neuroinflammation resulting in dementia as commonly found in some human sAD patients (Rajasekar et al. 2014; Salkovic-Petrisic et al. 2013).
STZ-ER or ICV STZ injected mice are prepared using different doses of STZ at different duration of studies (Correia et al. 2011; Halawany et al. 2017; Ishrat et al. 2009; Nakhate et al. 2018). Though the STZ injection at the dose of 0.5 mg/kg body wt. failed to exhibit memory changes after one week of injection, the memory and learning were impaired in ICV STZ injected rats at the dose of 1 mg/kg body wt. (Salkovic-Petrisic et al. 2006) and 1.25 mg/kg body wt. (Prickaerts et al. 2000) after 3–4 weeks. The learning and memory were also impaired at the dose of 1.5 mg/kg body wt. after 10–21 days (Sonkusare et al. 2005). Disruptions of learning and memory were noted with 3 mg/kg body wt. (Dhull et al. 2012) and with 6 mg/kg body wt. where 3 mg/kg body wt. was injected twice at about 3–4 weeks of ICV STZ injection (Rai et al. 2013). It appears from these studies that the memory parameters were significantly impaired at 3 weeks of ICV STZ injection. Though different doses of ICV STZ were used to assess memory functions, the optimum dose required for memory induction remains to be deciphered. These memory impairments after ICV STZ injection in rats are probably linked to increased oxidative stress (Reeta et al. 2017; Singh and Kumar 2016) and decreased energy metabolism (Grieb 2016; Prickaerts et al. 2000).
The neuroinflammation was detectable from the immunohistochemical studies in rats at the dose of 1 mg/kg body wt. of ICV STZ injection but pronounced effects were noted at the dose of 3 mg/kg body wt. after 3 weeks (Kraska et al. 2012). Though pro-inflammatory markers were not investigated in the rodent brains at lower ICV STZ doses (1/1.5 mg/kg body wt.), the level of TNFα was increased in rat brain at the dose of 3 mg/kg body wt at different time points of 2 weeks (Ahmed et al. 2013), 3 weeks (Singh and Kumar 2016) and 4 weeks (Reeta et al. 2017). TNFα and IL 1 β, reactive oxygen species (ROS) and nitrite levels were also found to be increased in rats at the dose of 6 mg/kg body wt (injected twice at 3 mg/kg body wt. in 2 days) after 3 weeks of ICV STZ injection (Rai et al. 2013). All these studies indicate that STZ induced optimum neuroinflammation occurred around 3 weeks at different doses.
Therefore, the memory impairments and increased neuroinflammatory markers were reported in STZ-ER at different doses of STZ at different time points but the dose related responses of ICV STZ injection on these parameters at a specific time point in rats are not available in the literature. However, pronounced memory impairments and neuroinflammatory changes at a specific time point following ICV STZ injection in rats with different STZ doses might help to identify the optimum dose of STZ required to prepare a suitable STZ-ER. Therefore, this study has been designed to investigate the changes of hippocampal neuroinflammatory markers along with the alteration of different memory parameters (working memory error, reference memory error and latency for food retrieval in the first and four baited arms in radial arm maze) in rats after 3 weeks of bilateral ICV injection of STZ at one time at different doses (1.5 mg/kg body wt., 3 mg/kg body wt. and 6 mg/kg body wt.) in an attempt to identify the optimum dose of STZ for neuroinflammation and memory impairments in STZ-ER .
Methodology
Animals
Male albino Charles-Foster rats with an average initial body weight between 180 and 210 g were used in the study. The rats were kept in standard laboratory conditions (constant room temperature 26–28 º C and 12:12 h light /dark cycle) in accordance to the institutional Animals Ethics Commitee protocols.
Design of experiments
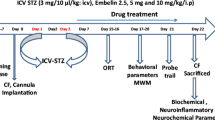
30 rats were randomly distributed into 5 groups having 6 rats in each as follows: control, sham operated (artificial CSF, ICV injection) and STZ-ER1 (STZ at 1.5 mg/kg body wt. ICV), STZ-ER2 (STZ at 3 mg/kg body wt. ICV) and STZ-ER3 (STZ at 6 mg/kg body wt. ICV). All the rats were subjected to habituation (10 days) and training (15 days) prior to the assessment of memory parameters (working memory error, reference memory error, latency for food retrieval in first and four baited arms) in a radial 8 arm maze. Thereafter behavioural studies were carried out for 27 days in the following protocol: Testing-1 to 5 days as pre operative control, STZ/artificial CSF ICV injection on 6th day, post operative memory assessment- 15th, 18th, 21st, 24th and 27th day(i.e. 9, 12, 15, 18, 21 days after STZ injection). All the rats were sacrificed under ether anaesthesia followed by decapitation on 28th day of study (23rd day after STZ injection). The brain was carefully dissected out from the skull and was dipped into ice-cold phosphate buffered saline for a while. Thereafter hippocampii from both sides of the brain were isolated out using microforceps keeping it on an aluminium foil covered ice cold platform for the measurement of TNF-α, IL-1β, ROS and nitrite.
Preparation of experimental rat by ICV streptozotocin injection
The rats were anaesthetised with thiopentone (40 mg/kg body weight i.p.) and head was fixed on the stereotaxic apparatus following the procedure described by Sil et al. (2015). For approaching lateral ventricles on both sides, two burr holes were made on the skull surface at AP: − 0.6 mm from bregma, L: ± 1.5 mm following the atlas of Paxinos and Watson (1986). A steel microcannula (0.45 mm diameter) connected to a microsyringe( Hamilton, Germany) was inserted stereotaxically into the lateral ventricles at 2.8 mm below the cortical surface. 5 µl of artificial CSF containing different doses of STZ (1.5 mg/kg body wt. / 3 mg/kg body wt. / 6 mg/kg body wt.) were microinfused slowly (1 µl/min) into each side of the lateral ventricle to prepare different STZ-ER groups of rats. Sham-operated rats similarly received the same volume of vehicle (artificial CSF) during the surgery. The microcannula was kept in place after microinfusion for 2–3 min to prevent back flow of the injected fluid. The holes on the skull were then covered with sterile bone wax and the muscles and skin were then sutured separately. Finally, neosporin powder was sprayed over the sutured area to prevent any sort of infection.
Radial arm maze testing for memory
A modified protocol of Mizuno et al. (2000) was followed to conduct memory testing in rats in a radial 8-arm maze (RAM) apparatus. Recordings were monitored independently by 2 observers to avoid biased data. 4 parameters were tested following habituation and training for preoperative (1–5 days) and post operative (15, 18, 21, 24 and 27 days) memory assessment (Sil and Ghosh 2016; Gupta et al. 2018). The following memory parameters were calculated as follows:
-
Working memory error: Repeated entry into a particular baited arm was counted as one memory error.
-
Reference memory error: Entry into any non baited arm was accounted as a reference memory error.
Latency for food retrieval in the first baited arm: Time taken by a rat to obtain food pellet in the first baited arm of a trial was defined as latency for food retrieval in the first baited arm. When the rat was unable to finish the memory task within the stipulated time of 5 min/ trial, the latency was noted as 5 min.
Latency for food retrieval in all four baited arms: Time taken to retrieve food pellet in all four baited arms of a trial was defined as latency for food retrieval in the first baited arm. When a rat was unable to execute the job within the stipulated time of 5 min/ trial, the latency was noted as 5 min.
These parameters were calculated for each rat in 3 trial sessions of a day. The mean value/day of a memory parameter of a rat was calculated from the data of 3 trials. Following this procedure, the preoperative test values of a memory parameter for different rat groups were calculated. Similarly, calculations for all the memory parameters were also obtained on 15th, 18th, 21st, 24th and 27th day of the behavioural studies (Post-operative memory assessment).
ROS estimation
A modified protocol of Socci et al. (1999) was followed for the estimation of hippocampal ROS levels. Isolated hippocampii were homogenised in 500 µl of ice-cold (40 m M) Tris-HCl buffer (pH 7.4). The homogenates obtained were thereafter centrifuged at 15000 g (for 20 min at 4oC) and the supernatant (sample) was collected. 10 µl of each sample was mixed with 5 µM DCF-DA (Loba Chemie, India) and incubated in a water bath at 37 ° C for 30 min. The fluorescence emitted from the fluorescent product DCF was estimated by spectrofluorometry (JASCO FP 6200) at an excitation wavelength and emission wavelength of 495 and 529 nm, respectively. Values for ROS have been expressed as percent of control.
Nitrite estimation
Nitrite levels of hippocampus were estimated following a modified method of Green et al. (1982). Hippocampal homogenate was obtained following the same protocol as outlined above for ROS estimation. Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulphanilamide and 2.5% phosphoric acid] was added to 10 µl of the hippocampal homogenate. The resultant mixture was thereafter incubated for 10 min at 37 ° C. Then the absorbance was measured in a spectrofluorometer at wavelength of 550 nm. Results have been expressed as percent of control.
Protein estimation
The method of Lowry et al. (1951) was followed to estimate protein amounts in samples (brain homogenates). For protein sample estimations, a standard curve was drawn from standard protein values prior to the experiment using BSA (1 mg/ml) [Rai et al. 2013].
Cytokine stimation
TNF-α
Protocol of Csolle and Sperlagh (2010) was followed for preparing the samples. Homogenization was performed in 500 µl of 10 mM Tris-HCl buffer containing 1 mM EGTA, 1 mM EDTA, 0.2 mM PMSF and 4M urea per 0.1 g tissue. The hippocampal homogenates were centrifuged at 15000 x g (for 20 min at 4oC) and the resultant supernatant was then collected. A solution containing 10 mM Tris-HCl buffer, 1% BSA and 0.2% Tween-20 was added to the supernatant. Measurement of TNF-α level was done using 50 µl of this solution (sample). Rat TNF-Flex Set and BD Cytometric Bead Array rat soluble protein master buffer kit (BD Biosciences, USA) was used for estimation of hippocampal TNF-α level. TNF-α level in the samples were expressed as picograms of TNF- α /100 mg protein. Values was analysed in BD FACS verse instrument using FCAP Array software.
IL-1β
Commercial rat IL-1β ELISA Kit (Ray Bio, Norcross, GA) was used for estimation of hippocampal IL-1β levels. Assay samples were prepared by the same protocol as described for TNF- α assay and 100 µl of the sample solution was used for determination of IL-1β levels according to protocol prescribed in the kit(Ray Bio). Reading of absorbance was taken at 450 nm/well in a 96 well plate reader indicated the IL-1β content of the samples. IL-1β level in the sample was expressed as picograms of IL-1β/mg protein in the sample.
Statistical analysis
Data are expressed as means ± SEM (Standard error of mean). One-way ANOVA was employed to compare the data of the control, sham-operated and experimental STZ -ER groups followed by the Tukey-Kramer multiple comparison test with the statistical package for social science software (SPSS; 19.0.0, USA).
Results
Immunological parameters
TNF- α, IL-1β, ROS and Nitrite levels of hippocampus
There was an increase in hippocampal TNF-α/ IL-1β/ ROS and nitrite levels of STZ-ER1, STZ-ER2 rats and STZ-ER3 rats compared to control/sham operated rats (p < 0.001). TNF-α/ IL-1β/ ROS and nitrite levels in STZ-ER2 rats were higher compared to STZ-ER1 group (p < 0.001) but no change was found in the cytokine and oxidative-nitrosative stress marker levels between STZ-ER2 and STZ-ER3 (Fig. 1).
The hippocampal TNF-α (a), IL-1β (b), ROS (c), nitrite (d) levels in different experimental groups of rats. Significant increase in hippocampal neuroinflammatory parameters of STZ-ER1 (a p < 0.001), STZ-ER2 (b p < 0.001) and STZ-ER3 (c p < 0.001) rats compared to control and sham-operated rats. Significant increase in STZ-ER2 compared to STZ-ER1 rats (d p < 0.001) and significant increase of these parameters in STZ-ER3 rats compared to STZ-ER1 rats (e p < 0.001). Abbreviations: STZ-ER1 = intracerebroventricular streptozotocin injected experimental rats at the dose of 1.5 mg/kg body wt. group; STZ-ER2 = intracerebroventricular streptozotocin injected experimental rats at the dose of 3 mg/kg body wt. group; STZ-ER3 = intracerebroventricular streptozotocin injected experimental rats at the dose of 6 mg/kg body wt. group. Sham = Artificial CSF injected sham operated group. Values are expressed in mean ± SEM (n = 06)
Memory parameters
Working memory error (WME) and Reference memory error (RME)
WME and RME were gradually increased in a time dependent manner after 15th day of testing (9 days after streptozotocin injection) in ICV STZ rats. Memory impairments in the STZ-ER1 was indicated by the increase in WME/RME (p < 0.001) from 15th to 27th day of testing compared to control/sham operated rats. There was also an increase (p < 0.001) in the WME (at 18th, 21st and 27th day of testing) and RME (at 18th, 24th and 27th day of testing) in STZ-ER1 rats compared to that of the previous assessment in STZ-ER1 rats. WME/RME were again increased(p < 0.001) in STZ-ER2/ STZ-ER3 rats at all post operative testing time-points compared to that of the previous assessment. The WME/RME of STZ-ER2/ STZ-ER3 rats were increased at all post operative testing time points (p < 0.001) compared to corresponding WME /RME of STZ-ER1 rats. No significant difference was observed between STZ-ER2 and STZ-ER3 in WME/RME at any time-points (Fig. 2a, 2b).
(a) Working memory errors(WME); (b) Reference memory error(RME); (c) Latency for food retrieval in the first baited arm (L1B); (d) Latency for food retrieval in all four baited arms (L4B) of different groups of rats. aSignificant between the corresponding day of STZ-ER1 and control/ sham-operated rats( p < 0.001) in all the four memory parameters; b Significant between a time point and the previous time point of STZ-ER1 in WME,RME and L1B at p < 0.01 whereas in L4B at p < 0.001; cSignificant between the corresponding day of STZ-ER2/ STZ-ER3 and control/ sham-operated rats at p < 0.001 in all the 4 memory parameters; dSignificant between a time point and the previous time point of STZ-ER2/ STZ-ER3 at p < 0.001 in all the four parameters; eSignificant between a time point of STZ-ER2/STZ-ER3 and the corresponding time point of STZ-ER1 at p < 0.001 in all the four memory parameters. Abbreviations for experimental groups of rats are same as Fig. 1. Values are expressed in mean ± SEM (n = 06)
Latency for food retrieval in the first baited arm and Latency for food retrievalin all four baited arms
Time taken to retrieve food in both the first baited arm and all four baited arms gradually increased in a time dependent manner after 15th day of testing (9 days after streptozotocin injection). There was an increase (p < 0.001) in both the latency for food retrieval in the first baited arm (at 21st, 24th and 27th day of testing) and latency for food retrieval in all four baited arms (at 24th and 27th day of testing) of STZ-ER1 compared to that of control/sham operated rats. There was also a significant increase in the time taken for both the parameters in STZ-ER1 (at 24th day of testing for latency for food retrieval in the first baited arm and at 21st and 24th day of testing for latency for food retrieval in all four baited arms) as compared to that of previous assessment. Increased latencies of these two parameters(p < 0.001) were observed in STZ-ER2/ STZ-ER3 rats at all post operative testing time-points compared to that of the previous assessment. The time taken for food retrieval in the above mentioned parameters of STZ-ER2/ STZ-ER3 rats were also increased (p < 0.001) from 15th -27th day of testing compared to the values of STZ-ER1 at the corresponding time-points. No significant difference was observed between STZ-ER2 and STZ-ER3 in these memory parameters at any time-points (Fig. 2c, 2d).
Dose related responses of STZ
The effects of STZ in different doses on the observed parameters (4 neuroinflammatory markers and 4 memory parameters) showed that the responses were significantly increased at the dose of 3 mg/kg body wt. from that of 1.5 mg/kg body wt. However, the responses of STZ were not further increased at the dose of 6 mg/kg body wt compared to that of 3 mg/kg body wt. (Figs. 1 and 2). Therefore, a plateau like effect was found in the dose response curve of STZ in all the observed parameters and these plateau are shown in TNFα and working memory error (Fig. 3a) and nitrite and reference memory error (Fig. 3b).
Discussion
The mechanism of memory impairments in STZ-ER rat/mouse model has been reviewed by several researchers (Grieb 2016; Kamat et al. 2016). STZ can bind with glucose transporter 2 (GLUT 2) and may cross cellular membrane (Lenzen 2008). Brain expresses GLUT 2 receptors like many other glucose sensing cells such as beta cells of pancreas and liver cells. GLUT2 is particularly found in hypothalamic areas and brain stem and they are localized in neurons, astrocytes, tanycytes and epithelial cells lining cerebral ventricles (Thorens 2011). The glucose entry into the cell through GLUT2 may be influenced by this interaction of STZ and GLUT2. Moreover, it was reported that insulin/IGF signalling related genes expression were principally impaired in hippocampus and frontal cortex in STZ injected monkey (Lee et al. 2014). The brain insulin resistance could be induced by STZ through these mechanisms which may again result in the type 2 diabetic state in brain (Grieb 2016). The hypometabolism of glucose in brain may cause proamyloidogenic effect (Vlassenko et al. 2010) and/or oxidative stress (Rai et al. 2013). The free radicals generated in oxidative stress in turn initiate DNA damage (DNA methylation), protein carbonylation, mitochondrial abnormalities (impaired ATP synthesis) and other changes that lead to dementia (Kamat et al. 2016).
In the STZ-ER of the present study, the production of ROS and NO were enhanced probably due to glucose hypometabolism and oxidative stress (Rai et al. 2013). The neuroinflammatory state in STZ-ER was also indicated from several studies of different laboratories (Nazem et al. 2015; Rai et al. 2013). There was an increase in pro-inflammatory markers such as TNF-α, IL-1β in brain regions of cerebral cortex and hippocampus in STZ-ER (Rai et al. 2013) and this neuroinflammation was blocked by COX inhibitors (Dhull et al. 2012). Neuroinflammation is a multifaceted process involving both neurons and glia (Rai et al. 2013), and STZ can affect both of the cells (Thorens 2011). STZ induced activation of glial cells evoke the production of TNF-α, IL-1β in addition to ROS and NO ( Nazem et al. 2015). These inflammatory mediators may up-regulate inducible COX-2 which produces prostaglandins (PGs), and PGE2 may increase inflammatory mediators by acting on microglia (Aloisi 2001; Shingo et al. 2012). In the present study the STZ induced neuroinflammation is evident from the elevated levels of hippocampal TNF-α, IL-1β, ROS and nitrite. Moreover, the present study shows that the inflammatory mediators were significantly increased at the dose of 3 mg/kg body wt. of STZ than that of 1.5 mg/ kg body wt. Probably the cellular and molecular events involved in the process of neuroinflammation by STZ were affected more at the higher dose of STZ. However, it was also noted that further increase of the dose of STZ (i.e. 6 mg/ kg body wt.) failed to produce any significant change in the observed parameters than that of previous dose of 3 mg/ kg body wt. The results on neuroinflammatory parameters indicate that the dose related response of STZ is not a straight line and a plateau like effect was evident between 3 mg/kg body wt and 6 mg/kg body wt of STZ.
In the present study, different memory parameters were assessed in the STZ-ER using a radial 8 arm maze. There was a decline in memory (as indicated by increased error in working and reference memory, higher latencies to retrieve the food in the first baited arm and in all 4 baited arms) in all the STZ-ER groups compared to that of control or sham operated rats. The memory impairments in the STZ-ER have also been previously investigated using different tests like Morris water maze tasks and Y maze tasks by different investigators (Halawany et al. 2017; Dhull et al. 2012). The Morris water maze task was performed to investigate the reference memory (spatial) in ICV STZ injected (3 mg/kg body wt.) mice where the training was prolonged in the STZ treated groups as they required more time and traversed a longer path to locate the submerged platform (Halawany et al. 2017). Similar results were reported from another study where escape latency and distance travelled were higher while the time spent in the target quadrant was significantly decreased in a Morris water maze task after STZ injection (3 mg/kg body wt. ) in the lateral ventricles of rats (Ahmed et al. 2013). The increased time spent in Morris water maze task as reported by other investigators may be corroborated with increased latencies for food retrieval in the present study and thus a similar memory error with respect to spatial reference is demonstrated. Several other investigators also showed that STZ injection in the lateral ventricles of rats caused memory impairment in a Morris water maze ( Kamat et al. 2016; Dhull et al. 2012). Memory impairments were also reported in ICV colchicine injected rats in radial arm maze (Sil and Ghosh 2016). The present study showed a significant increase of memory errors in rats injected with 3 mg/ kg body wt. of STZ than that of 1.5 mg/kg body wt. of STZ injected rats. It may be mentioned here that the memory errors and latencies were not significantly increased in STZ-ER injected with 6 mg/ kg body wt. compared to that of STZ-ER receiving 3 mg/kg body wt. of STZ. These results on memory parameters probably indicate that the neurotoxicity induced by STZ has reached an optimum level at the dose of 3 mg/kg body wt.
The cause-effect relation between neuroinflammatory markers and memory impairments cannot be identified from this study. The changes of inflammatory markers and memory impairments in STZ-ER in short duration study might throw light in this area. The relation between inflammatory markers and memory impairments should further be examined with blocking of inflammatory cascades (IL2 blocker, cox 2 blocker etc.) at different points after ICV STZ injection. The dose response effect of STZ on inflammatory markers/ memory parameters at 3rd week has been determined in this study but the dynamics of STZ effect on brain in time dependent manner remains to be investigated. The dose response effect of STZ on inflammatory markers/ memory parameters in short duration experiment (e.g., 1,2,3,7 days) and long duration experiment (e.g., 8, 12, 16, 20 weeks) might provide information about the status of dose response curve in STZ-ER in a time dependent manner, and thus the time course of STZ effect in different doses on brain functions may be understood.
The dose related response of STZ on inflammation (i.e., TNF-α, IL-1β, ROS and nitrite) and memory parameters (i.e., working and reference memory errors, latency to retrieve the food in the first baited arm and in all 4 baited arms) in STZ-ER are similar in the present study. It appears from the results of the present study that the single ICV injection of STZ bilaterally at the dose of 3 mg/kg body wt. produces the optimum effects on memory parameters and neuroinflammatory markers after 3rd week of STZ injection and this optimum dose of STZ may be used for the preparation of ICV STZ injected animals for drug development and other experimental purposes.
Abbreviations
- AD:
-
Alzheimer’s Disease
- sAD:
-
sporadic Alzheimer’s Disease
- ICV:
-
intracerebroventricular
- STZ:
-
streptozotocin
- STZ-ER:
-
intracerebroventricular streptozotocin injected experimental rats
- TNF – α:
-
Tumor Necrosis Factor – α
- IL-1β:
-
Interleukin − 1β
- ROS:
-
Reactive oxygen species
- WME:
-
Working Memory Error
- RME:
-
Referrence Memory Error
References
Ahmed ME, Khan MM, Javed H, Vaibhav K, Khan A, Tabassum R, Ashafaq M, Islam F, Safhi MM, Islam F (2013) Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem Int 62:492–501
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Blennow K, Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403
Chatterjee S, Mudher A (2018) Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front Neurosci 12:383
Chételat G (2013) Alzheimer disease: Aβ-independent processes-rethinking preclinical AD. Nat Rev Neurol 9(3):123–124
Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA (2011) Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res Rev 10:264–273
Costa AP, Tramontina AC, Biasibetti R, Batassini C, Lopes MW, Wartchow KM (2012) Neuroglial alterations in rats submitted to the okadaic acid-induced model of dementia. Behav Brain Res 226(2):420–427
Csolle C, Sperlagh B (2010) Peripheral origin of IL-1β production in the rodent hippocampus under in vivo systemic bacterial lipoplysaccharide (LPS) challenge and it’s regulation by P2 × 7 receptors. J Neuroimmunol 219:38–46
Dhull DK, Jindal A, Dhull RK, Aggarwal S, Bhateja D, Padi SSV (2012) Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J Mol Neurosci 46:223–235
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Grieb P (2016) Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol 53:1741–1752
Gupta P, Sil S, Ghosh R, Ghosh A, Ghosh T (2018) Intracerebroventricular Aβ-induced neuroinflammation alters peripheral immune responses in rats. J Mol Neurosci 66: 572-586. https://doi.org/10.1007/s12031-018-1189-9
Halawany AME, Sayed NSE, Abdallah HM, Dine RSE (2017) Protective effects of gingerol on streptozotocin-induced sporadic Alzheimer’s disease: emphasis on inhibition of β-amyloid, COX-2, alpha-, beta-secretases and APH1a. Sci Rep 7:2902
Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, Ahmad A, Shrivastav P, Islam F (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Research 1281:117–127
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad AS (2016) Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s Disease (sAD)-like pathology. Mol Neurobiol 53(7):4548–4562
Kraska A, Santin MD, Dorieux O, Joseph-Mathurin N, Bourrin E (2012) In vivo cross-sectional characterization of cerebral alterations induced by intracerebroventricular administration of streptozotocin. PLoS One 7(9):e46196
Lee Y, Kim YH, Park SJ, Huh JW, Kim SH, Kim SU, Kim JS, Jeong KJ (2014) Insulin/IGF signaling-related gene expression in the brain of a sporadic Alzheimer’s disease monkey model induced by intracerebroventricular injection of streptozotocin. J Alzheimer’s Dis 38:251–267
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin induced diabetes. Diabetologia 51:216–226
Lewis C, Barbiers AR (1959) “Streptozotocin, a new antibiotic” In vitro and in vivo evaluation. Antibiot Annu 7:247–254
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T (2000) Involvement of brainderived Neurotrophic factor in spatialmemory formation and maintenance in a radial arm maze test in rats. J Neurosci 20:7116–7121
Nakhate KT, Bharne AP, Verma VS, Aru DN, Kokare DM (2018) Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β- secretase. Biomed Pharmacother 101:379–390
Nazem A, Sankowski R, Bacher M, Al-Abed Y (2015) Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflammation 12:2–15
Neha, Sodhi RK, Jaggi AS, Singh N (2014) Animal models of dementia and cognitive dysfunction. Life Sci 109:73–86
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, San Diego
Prickaerts J, De Vente J, Honig W, Steinbusch H, Ittersum MMV, Blokland A, Steinbusch HW (2000) Nitric oxide synthase does not mediate neurotoxicity after an i.c.v. injection of streptozotocin in the rat. J Neural Transm 107:745–766
Rai S, Kamat PK, Nath C, Shukla R (2013) A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. J Neuroimmunol 254:1–9
Rajasekar N, Dwivedi S, Nath C (2014) Protection of streptozotocin induced insulin receptor dysfunction, neuroinflammation and amyloidogenesis in astrocytes by insulin. Neuropharmacology 86:337–352
Reeta KH, Singh D, Gupta YK (2017) Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress cholinergic functions neuroinflammation. Neurochem Int 108:146–156
Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P (2006) Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem 96:1005–1015
Salkovic-Petrisic M, Knezovic A, Hoyer S, Riederer P (2013) What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J Neural Transm 120:233–252
Shingo AS, Kanabayashi T, Murase T, Kito S (2012) Cognitive decline in STZ-3V rats is largely due to dysfunctional insulin signalling through the dentate gyrus. Behav Brain Res 229:378–383
Sil S, Ghosh T (2016) Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s disease. J Neuroimmunol 291:115–124
Sil S, Ghosh R, Sanyal M, Guha D, Ghosh TK (2015) A comparison of neurodegeneration linked with neuroinflammation in brain areas of rats after intracerebroventricular colchicine injection. J Immunotoxicol 13:182–190
Singh A, Kumar A (2016) Comparative analysis of intrahippocampal amyloid beta (1–42) and it is intracerebroventricular streptozotocin models of Alzheimer’s Disease: Possible behavioral, biochemical,mitochondrial,cellular and histopathological evidences. J Alzheimers Dis Parkinsonism 6:208
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155:109–117
Sonkusare S, Srinivasan K, Kaul C, Ramarao P (2005) Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 77:1–14
Thorens B (2011) Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab (Suppl 1):82–88
Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA (2010) Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A 107(41):17763–17767
Acknowledgements
This research work was funded by the Department of Science and Technology, Govt. of India, INSPIRE Fellowship Scheme (DST/INSPIRE Fellowship/2013/938 dt. 26.11.2014). Dr. Debajit Bhowmick [Centre for Research in Nanoscience and Nanotechnology, University of Calcutta], Dr. Arijit Ghosh( Research Associate, DBT) and Sudeshna Das ( M.Sc student, 2015) are acknowledged for their assistances in parts of the work.
Funding
The study was funded by INSPIRE Fellowship Scheme (DST/INSPIRE Fellowship/2013/938 dt. 26.11.2014), Department of Science and Technology, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
The institutional animal ethical committee rules and regulations were strictly followed during the experimental procedures (IAEC/IV/Proposal/TKG-04/2015 dated 19.01.2015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 106 kb)
Rights and permissions
About this article
Cite this article
Ghosh, R., Sil, S., Gupta, P. et al. Optimization of intracerebroventricular streptozotocin dose for the induction of neuroinflammation and memory impairments in rats. Metab Brain Dis 35, 1279–1286 (2020). https://doi.org/10.1007/s11011-020-00588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00588-1