Abstract
Sporadic Alzheimer’s disease is an age-related neurological and psychiatric disorder characterized by impaired energy metabolism. Oxidative stress and neuroinflammation have been implicated in pathophysiology of sporadic type of dementia. The central streptozotocin administration induces behavioral and biochemical alterations resembling those in sporadic type of Alzheimer’s patients. The present study was designed to investigate the effects of chronic pretreatment with cyclooxygenase-1 or cyclooxygenase-2 or cyclooxygenase-3 selective inhibitors on cognitive dysfunction and oxidative stress markers in intracerebroventricular streptozotocin-treated rats. Chronic treatment with valeryl salicylate (5 and 10 mg/kg, i.p.) and etoricoxib (5 and 10 mg/kg, i.p.) on a daily basis for a period of 21 days, beginning 1 h prior to first intracerebroventricular streptozotocin injection, significantly improved streptozotocin-induced cognitive impairment. However, phenacetin (20 and 40 mg/kg, i.p.) failed to restore the cognitive performances of streptozotocin-treated rats. Besides, improving cognitive dysfunction, chronic administration of highly selective cyclooxygenase-1 and/or cyclooxygenase-2 inhibitors (valeryl salicylate and etoricoxib, respectively), but not cyclooxygenase-3 inhibitor (phenacetin), significantly reduced elevated malondialdehyde, nitrite levels, and restored reduced glutathione and superoxide dismutase levels. Furthermore, cyclooxygenase-1 and/or cyclooxygenase-2 inhibitors significantly increased the survival of pyramidal neurons. In summary, we demonstrate for the first time that both cyclooxygenase-1 and cyclooxygenase-2 isoforms, but not cyclooxygenase-3, are involved in the progression of neuronal damage in intracerebroventricular streptozotocin-treated rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporadic Alzheimer’s disease (SAD) is a progressive, irreversible, age-related neurological disorder associated, biochemically, with impaired brain glucose and energy metabolism, oxidative stress, and impaired cholinergic neurotransmission, morphologically, in neuronal damage and loss in hippocampal volume and allied structures accompanied by astrogliosis and neuroinflammation and finally behavioral abnormalities manifested as progressive learning and memory deficits. The process of aging seems to be the major causative factor of SAD. This may partly be explained because of the multiple changes at the cellular and molecular level (Hoyer 1995). It is interesting to note that the key mechanism of neuronal death in SAD revolves around impaired energy metabolism (Salkovic-Petrisic and Hoyer 2007), mitochondrial dysfunction (Wang et al. 2009), excitotoxicity (Song et al. 2008), neuroinflammation, and oxidative stress (Tahirovic et al. 2007). In spite of all the immense research carried out till date, a complete understanding of etiopathogenesis mechanisms of SAD is still the area of thirst.
Intracerebroventricular (ICV) administration of streptozotocin (STZ) is a well-established, validated, and widely accepted animal model to study SAD. It induces cognitive impairment by inducing degradation of phospholipids (Prasad et al. 1998) and thus increases free fatty acid, the arachidonic acid (Muller et al. 1998). In addition, ICV-STZ induces gliosis in the cortex, hippocampal CA1 region, corpus callosum, and medial and lateral septum (Terwel et al. 1995; Shoham et al. 2007).
Neuroinflammation is one of the mechanisms known to participate in the pathologically affected tissue(s) in several neurodegenerative disorders, including AD (McGeer and McGeer 2003; Rojo et al. 2008). Any injury to the brain results in the generation of high level of glutamate that initiates the inflammatory pathway by activating glial cells, i.e., microglia and astrocytes (Tuppo and Arias 2005) and infiltrating leukocytes (Engelhardt et al. 1993). Cyclooxygenase (COX) is a rate-limiting enzyme in the metabolism of arachidonic acid to prostanoids (O’Banion 1999; Smith et al. 2000), particularly prostaglandins (PGs), which significantly contribute to neuroinflammation (Hein and O’Banion 2009). Different isoforms of COX enzyme, i.e., COX-1 and COX-2, have been identified (Smith et al. 2000). In addition, a COX-1 variant, termed COX-3, has been recently cloned and characterized (Chandrasekharan et al. 2002). Accumulating data indicates that COX activity and PG levels are higher in the brains of AD patients than in control brains (Pasinetti and Aisen 1998; Consilvio et al. 2004). Both COX-1 and COX-2 protein levels are elevated in several non-neuronal cell populations following any brain insult (Schwab et al. 2002; Zhao et al. 2006), and their respective genetic deletion or pharmacological inhibition attenuates the inflammatory responses and brain injury (Candelario-Jalil et al. 2003; Doré et al. 2003; Kelsen et al. 2006; Xiang et al. 2007). However, the role of COX-3 in various neurological disorders is not fully established.
Despite differential role of COX isoforms in various neurological disorders, their role in the pathogenesis of SAD is not known. Nonetheless, the potential of nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of AD is still a matter of debate (Aisen et al. 2003). In a number of studies, the animal models used to study the role of COX isoforms in the pathophysiology of AD are genetic models, pertinent to familial AD. However, the role of COX isoforms in experimental SAD remains to be elucidated. Therefore, the present study was designed to investigate the effects of chronic pretreatment with COX-1 or COX-2 or COX-3 selective inhibitors on cognitive dysfunction and oxidative stress in rat following central STZ administration.
Materials and Methods
Animal
Male Wistar rats bred in the central animal house of the facility of ISF College of Pharmacy, Moga, Punjab, India, and weighing between 210 and 240 g were used at the start of the surgery. Animals were acclimatized to laboratory conditions before experimentation. The animals were kept in groups of three, in plastic cages with soft bedding, under standard conditions of light and dark cycle, and with free access to food and water ad libitum. All the experiments were carried out between 08:00 AM and 16:00 AM. The protocol was approved by the Institutional Animal Ethics Committee and carried out in accordance with the Indian National Science Academy Guidelines for the use and care of animals.
Surgery and ICV-STZ Administration
Rats were acclimatized to the laboratory conditions 2 h before recording locomotor activity. The animals with normal locomotor activity were selected for surgery. Each rat was anesthetized with intraperitoneally (i.p.) injection of ketamine hydrochloride (70 mg/kg, i.p.) immediately followed by diazepam (4 mg/kg, i.p.). The animal was laid on surgery board, and the head was positioned straight, hair were trimmed, and a midline sagittal incision was made in the scalp. Burr holes were drilled in the skull on both the sides over the lateral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.8 mm lateral to sagittal suture, and 3.6 mm beneath the cortical surface of brain. Two heparinized polypropylene tubes of approximately 3 cm length were cannulated 3.6 mm deep into the brain and fitting exactly into the two burr holes made in the skull. A sham surgery was done by giving a cut in skin as before but without burr holes in the skull (Tiwari et al. 2009; Kumar et al. 2006a). The open end of cannula was closed; dental cement was applied, allowed to dry to fix the cannula with skull; the cut skin was sutured; and povidone iodine solution was applied. A single dose of gentamicin (40 mg/kg, i.p.) was injected following surgery, and a daily application of antiseptic powder (Neosporin®) was done to prevent sepsis. Motor activity was assessed again 72 h after surgery, and the animals with similar motor activity count as compared to that of presurgery values and without any blood clot in the ICV cannulas were selected and used in study.
Behavioral Assessment
Assessment of Motor Activity
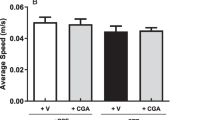
Locomotor activity was assessed before (on day 0), 72 h after surgery before the first dose of STZ administration (on day 1), and on day 21 (after the last dose of COX inhibitor). Each animal was observed over a period of 5 min in a square (30-cm) closed arena equipped with infrared light-sensitive photocells using a digital photoactometer. The apparatus was placed in a darkened light and sound-attenuated and ventilated testing room. The values are expressed as counts per 5 min.
Modified Elevated plus Maze Test
The elevated plus maze consisted of two opposite open arms (50 × 10 cm), crossed with two closed arms of the same dimensions with 40 cm high walls (Sharma and Kulkarni 1991). The arms were connected with central square (10 × 10 cm). Acquisition of memory was assessed on day 20 after the first dose of STZ injection. Rats were placed individually at one end of the open arm facing away from the central square. The time taken to move from the open arm and enter one of the closed arms was recorded as initial transfer latency (ITL). Animals were allowed to explore the maze for 30 s after recording ITL and returned to home cage. One day after ITL, the rat was placed similarly on the open arm, and retention latency (seconds) was noted on day 21.
Spatial Navigation Task
The acquisition and retention of a spatial navigation task was examined using a Morris water maze (Duckworth et al. 1999). Water maze consists of a cylindrical pool (150 cm in diameter, 75 cm in height, filled with non-toxic and non-irritant opaque paint maintained at approximately 25 ± 1°C and measuring 50 cm deep), placed in a darkened room illuminated by a sparse light. Region within 15 cm from border was called as border zone, whereas inner region was called central zone that was further divided into four imaginary quadrants. In the center of the north quadrant was a 16 × 16-cm square transparent platform submerged 2.5 cm beneath the water surface. During testing, the investigator stood at the west edge of the pool wearing a white lab coat. Testing was performed from 9:00 AM to 11:00 AM on the day of observation.
Maze Acquisition Test (Learning)
All the rats underwent training over four consecutive days starting from day 17 following STZ administration consisting of six swimming trials per day. Each trial lasted for 90 s after which the rats that had not found the platform were placed on it and also allowed to rest there for 60 s. Five minutes was maintained during training. Each rat was randomly started in the east, west, or south quadrant and started twice in each of these quadrants during 1 day session. The rat was started by being placed at the edge of the pool in the center of the appropriate quadrant, facing the wall for each trail, and latency to find the platform was recorded. The average of six trials per animal represents initial acquisition latency (IAL).
Maze Retention Test (Testing for Consolidation of the Learned Task)
Following training for 4 days, on day 5 (day 21 after ICV-STZ administration), a retention was conducted in which platform was removed and rats were allowed to swim for 90 s. All the rats were started in the south quadrant and were tested for only one trail during which they were tracked for memory of the platform location by number of entries made into the target quadrant, latency to reach the target quadrant, and the time spent in the target quadrant in which the hidden platform was originally placed during training were used as indices of retention.
Biochemical Assessment
Biochemical tests were carried out 24 h after the last behavioral test on day 21 following ICV-STZ administration, i.e., on day 22.
Tissue Preparation
Animals were sacrificed by decapitation, and the brains were removed and rinsed with ice-cold isotonic saline. Brain tissue samples were then homogenized with ice-cold 0.1-M phosphate buffer (pH 7.4) 10 times (w/v). The homogenate was centrifuged at 2,000×g for 15 min, and aliquots of supernatant were separated and used for biochemical estimation.
Measurement of Lipid Peroxidation
The quantitative measurement of lipid peroxidation in the brain was performed according to the method of Wills (1966). In this, 0.1 ml of supernatant was incubated with 0.5 ml Tris–HCl (0.1 M, pH 7.4) for 2 h. To this, 1 ml of trichloroacetic acid (10%, w/v) was added and centrifuged at 1,000×g for 10 min. To 1 ml supernatant, 1 ml (0.67%, w/v) thiobarbituric acid (TBA) was added and kept in the boiling water bath for 10 min, cooled, and added 1 ml distilled water. The amount of lipid peroxidation products was measured by reaction with TBA at 532 nm using the spectrophotometer (UV-1700, Shimadzu, Japan). The values were calculated using molar extinction coefficient of chromophore (1.56 × 105 M−1 cm−1) and expressed as micromoles per milligram protein.
Estimation of Reduced Glutathione
Reduced glutathione (GSH) in the brain was estimated according to the method described by Ellman (1959). A 1-ml supernatant was precipitated with 1 ml of 4% sulfosalicylic acid and cold-digested at 4°C for 1 h. The samples were centrifuged at 1,200×g for 15 min at 4°C. To 1 ml of this supernatant, 2.7 ml of phosphate buffer (0.1 M, pH 8) and 0.2 ml of 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB) were added. The yellow color developed was read immediately at 412 nm (UV-1700 Spectrophotometer, Shimadzu, Japan). Results were calculated using molar extinction coefficient of chromophore (1.36 × 104 M−1 cm−1) and expressed as nanomoles per milligram protein.
Estimation of Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO), was determined with a colorimetric assay with Greiss reagent (0.1% N-(1-naphthyl)ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% phosphoric acid) as described by Green et al. (1982). Equal volumes of supernatant and Greiss reagent were mixed, the mixture was incubated for 10 min at room temperature in the dark, and the absorbance at 540 nm was determined with UV-1700 Spectrophotometer (Shimadzu, Japan). The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and expressed as micromoles per milligram protein.
Estimation of Superoxide Dismutase
Superoxide dismutase (SOD) activity is measured according to a method described by Misra and Fridovich (1972), by following spectrophotometrically the auto-oxidation of epinephrine at pH 10.4. In this method, supernatant of the tissue was mixed with 0.8 ml of 50 mM glycine buffer, pH 10.4, and the reaction was started by the addition of 0.02 ml (−)-epinephrine. After 5 min, the absorbance was measured at 480 nm (UV-1700 Spectrophotometer, Shimadzu, Japan). The activity of SOD was expressed as percent activity of vehicle-treated control.
Acetylcholinesterase Levels
Acetylcholinesterase (AChE) is a marker of extensive loss of cholinergic system in the forebrain. The quantitative measurement of acetylcholinesterase levels in brain was performed according to the method of Ellman et al. (1961). The assay mixture contained 0.05 ml of supernatant, 3 ml of 0.01 M sodium phosphate buffer (pH 8), 0.1 ml of acetylthiocholine iodide, and 0.1 ml of DTNB (Ellman reagent). The change in absorbance was measured immediately at 412 nm using UV-1700 Spectrophotometer, Shimadzu, Japan. Results were calculated using molar extinction coefficient of chromophore (1.36 × 104 M−1 cm−1) and expressed as percentage of control.
Lactate Dehydrogenase Activity
Lactate dehydrogenase activity (LDH) catalyzes the interconversion of lactate and pyruvate. The amount of this enzyme may be used as a marker of tissue breakdown. LDH in brain was assayed using a kit purchased from Rekon Diagnostics Ltd., Mumbai, India. The absorbance was determined at 340 nm (UV-1700 Spectrophotometer, Shimadzu, Japan), and the activity of LDH is expressed as units per liter.
Protein Estimation
The protein content was measured by the biuret method using bovine serum albumin as standard.
Drugs and Treatment Schedule
All the doses of COX inhibitors were selected on the basis of earlier reports in which significant antioxidant and neuroprotective property was demonstrated at doses 5 and 10 mg/kg for valeryl salicylate, a selective COX-1 inhibitor (Liu et al. 2006); 5 and 10 mg/kg for etoricoxib, a selective COX-2 inhibitor (doses were selected on the basis of their IC50 value for COX-2; Gierse et al. 2005); and 20 and 40 mg/kg for phenacetin, a selective COX-3 inhibitor (Ishida et al. 2007).
In the present study, the animals were divided into 11 groups. In group I, the sham control group, only sham surgery was performed and no treatment was given; group II received ICV-STZ (3 mg/kg) injections on days 1 and 3; groups III and IV received valeryl salicylate at doses 5 and 10 mg/kg (i.p.), respectively, 1 h before ICV-STZ on day 1 and continued once daily for a period of 21 days; group V (per se) received valeryl salicylate (10 mg/kg, i.p.) for 21 days; groups VI and VII received etoricoxib at doses 5 and 10 mg/kg (i.p.), respectively, 1 h before ICV-STZ on day 1 and continued once daily for a period of 21 days; group VIII (per se) received etoricoxib (10 mg/kg, i.p.) for 21 days; groups IX and X received phenacetin at doses 20 and 40 mg kg−1 (i.p.), respectively, 1 h before ICV-STZ on day 1 and continued once daily for a period of 21 days; and group XI (per se) received phenacetin (10 mg/kg, i.p.) for 21 days. The time schedule for administration of ICV-STZ and selective COX-1 or COX-2 or COX-3 inhibitor is explained in Fig. 1.
Time schedule for administration of ICV-STZ, vehicle and selective cyclooxygenase inhibitors in rats. The control group received vehicle only. In ICV-STZ-treated groups, two doses of STZ were administered to rats on days 1 and 3, whereas in drug-treated groups, selective cyclooxygenase inhibitors were administered 1 h before ICV-STZ injection on day 1 and continued once daily for a period of 21 days. In addition, the drug per se group received only cyclooxygenase inhibitors, continuously for 21 days. The behavioral observations were started from day 17 onward, and the animals were sacrificed 24 h after the last behavioral test on day 21 following ICV-STZ infusion, i.e., on day 22, to carry out biochemical estimation
Statistical Analysis
The results are expressed as mean ± SEM of n (number of animals studied) observations. The behavioral data of learning in Morris water maze, modified elevated plus maze, and motor activity were analyzed by repeated measures two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for multiple comparisons. The biochemical data and memory performance in Morris water maze were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. In all tests, p < 0.05 was set to be statistically significant.
Results
Effect of COX Inhibitors on Locomotor Activity in ICV-STZ-Treated Rats
In the present experiment, the mean scores of locomotor activity did not differ significantly between all the groups when assessment is performed on 1 h before (on day 0) and 72 h after surgery (before the first dose of STZ administration, i.e., on day 1) and on day 21. Chronic administration of selective COX-1, COX-2, or COX-3 inhibitors for 21 days had no effect on locomotor activity on day 21 as compared to that observed in respective groups on day 1 (Fig. 2).
Effect of COX Inhibitors on Memory Performance in Modified Elevated plus Maze Test in ICV-STZ-Treated Rats
In the present experiment, the mean ITL on day 20 after ICV-STZ treatment for each rat was relatively stable and showed no significant variation. All the rats entered the closed arm within 90 s. Following training, sham control and selective COX-1, COX-2, or COX-3 inhibitor-treated animals (per se) entered closed arm quickly, and the mean retention transfer latency (RTL) to enter closed arm on day 21 was shorter as compared to ITL on day 20 of each group, respectively. The ICV-STZ-treated rats performed poorly throughout the experiment and increased the mean RTL on day 21 as compared to ITL on day 20 demonstrating that ICV-STZ induced cognitive dysfunction (p < 0.05; F 1, 40 = 297.86). Multiple comparisons two-way ANOVA indicated a significant drug treatment × session interaction. Chronic administration of valeryl salicylate (5 or 10 mg/kg, i.p.) and etoricoxib (5 and 10 mg/kg, i.p.) for 21 days beginning prior to ICV-STZ treatment significantly decreased the mean RTL as compared to ICV-STZ-treated group on day 21 (p < 0.05 vs ICV-STZ-treated group, F 7, 40 = 12.45; Fig. 3) indicating improvement of memory impairment. However, phenacetin (20 or 40 mg/kg, i.p.) treatment in ICV-STZ rats failed to decrease the mean RTL on day 21.
Effect of selective cyclooxygenase inhibitors on transfer latency in the modified elevated plus maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats on day 21. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; c p < 0.05 vs VAL (5); d p < 0.05 vs ETO (5)
Effect of COX Inhibitors on Spatial Navigation Task in ICV-STZ-Treated Rats
Acquisition
The measure of acquisition of spatial navigation task using Morris water maze is shown in Fig. 4a–c. The change in escape latency was observed onto a hidden platform produced by training trials. Although the escape latency to reach the submerged platform decreased gradually in all groups during the entire training period from day 17 to day 20 after ICV-STZ infusion in rats (p < 0.05; F 3, 120 = 209.1), the overall session escape latency of the ICV-STZ-treated group was worse than that of the sham control group (p < 0.05; F 7, 40 = 15.93; Fig 4a) indicating that ICV-STZ caused significant learning impairment as compared to sham control group. Further, sham-operated, selective COX-1, COX-2, or COX-3 inhibitor-treated normal animals (per se) quickly learned to swim directly to the platform in the Morris water maze from day 17 to day 20. Multiple comparisons two-way ANOVA indicated a significant treatment × session interaction. Valeryl salicylate (5 or 10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) treatment significantly decreased the IAL to reach the platform in the pre-trained rats as compared to ICV-STZ administered rats on day 17 onward, following ICV-STZ administration (p < 0.05; F 21, 120 = 3.11). Conversely, phenacetin (20 or 40 mg/kg, i.p.) failed to decrease significantly the IAL following ICV-STZ administration (Fig. 4c).
a Effect of selective cyclooxygenase-1 inhibitor, i.e., valeryl salicylate (VAL) (5 or 10 mg/kg, i.p.) on acquisition of spatial learning in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats. Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ. b Effect of selective cyclooxygenase-2 inhibitor, i.e., etoricoxib (ETO) (5 or 10 mg/kg, i.p.) on acquisition of spatial learning in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats. Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ. c Effect of selective cyclooxygenase-3 inhibitor, i.e., phenacetin (PHE) (20 or 40 mg/kg, i.p.) on acquisition of spatial learning in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats. Values are mean ± SEM. a p < 0.05 vs sham
Retention
In the probe trial, on day 21, following ICV-STZ administration when the platform removed, ICV-STZ-treated group failed to remember the precise location of the platform (Fig. 5), made less number of entries in the target quadrant (Fig. 6), and spent significantly less time in the target quadrant as compared to sham control group indicating cognitive impairment (p < 0.05; Fig. 7). Selective COX-1, COX-2, or COX-3 inhibitor in normal animals (per se) did not alter mean escape latency (Fig. 5), number of entries (Fig. 6), and time spent in the target quadrant (Fig. 7) as compared to sham control group. Valeryl salicylate (10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) starting before ICV-STZ administration caused a significant decline mean latency to enter platform area on day 5 and improved retention of spatial navigation task as compared to ICV-STZ-treated rats (p < 0.05; F 10, 65 = 74.62). On the other hand, valeryl salicylate (5 mg/kg, i.p.) and phenacetin (20 or 40 mg/kg, i.p.) had no effect in ICV-STZ-treated rats.
Effect of selective cyclooxygenase inhibitors on memory performance in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats on day 21. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; d p < 0.05 vs ETO (5)
Effect of selective cyclooxygenase inhibitors on number of entries in the platform area during memory performance in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats on day 21. ETO Etoricoxib (5 or 10 mg/kg, i.p.); PHE Phenacetin (20 or 40 mg/kg, i.p.); VAL Valeryl salicylate (5 or 10 mg/kg, i.p.); Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ, d p < 0.05 vs ETO (5)
Effect of selective cyclooxygenase inhibitors on time spent in the target quadrant during memory performance in the Morris water maze test in intracerebroventricular streptozotocin (ICV-STZ)-treated rats on day 21. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; d p < 0.05 vs ETO (5)
Further, valeryl salicylate (10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) starting before ICV-STZ administration caused a significant higher entries into platform area (p < 0.05; F 10, 65 = 67.89) and time spent in target quadrant (p < 0.05; F 10, 65 = 42.86) on day 21 and improved retention of spatial navigation task. On the contrary, valeryl salicylate (5 mg/kg, i.p.) and phenacetin (20 or 40 mg/kg, i.p.) had no effect on entries into platform area and time spent in the target quadrant.
Effect of COX Inhibitors on Brain Acetylcholinesterase Activity in ICV-STZ-Treated Rats
ICV-STZ administration showed significant increase in brain AChE activity as compared to sham control group (p < 0.05). Valeryl salicylate per se (10 mg/kg, i.p.), etoricoxib per se (10 mg/kg, i.p.), and phenacetin per se (40 mg/kg, i.p.) treatment did not cause any change in the brain AChE activity as compared to sham control group. However, chronic administration of valeryl salicylate (5 or 10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) significantly restored brain AChE activity on day 21 following ICV-STZ administration as compared to ICV-STZ-treated group (p < 0.05). However, there was no significant difference between low and high doses of valeryl salicylate-treated groups. On the other hand, phenacetin (20 or 40 mg/kg, i.p.) was not able to restore brain AChE activity in ICV-STZ administered rats (Fig. 8).
Effect of selective cyclooxygenase inhibitors on acetylcholinesterase activity in the brain of intracerebroventricular streptozotocin (ICV-STZ)-treated rats. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; d p < 0.05 vs ETO (5)
Effect of COX Inhibitors on Brain Oxidative Stress in ICV-STZ-Treated Rats
Effect of COX Inhibitors on Brain Lipid Peroxidation in ICV-STZ-Treated Rats
ICV-STZ-administered rats had significantly increased level of thiobarbituric acid reacting substances (TBARS) in brain in comparison to sham control animals (p < 0.05; Table 1). In this study, treatment with any of the selective COX inhibitors in normal control animals (per se) did not alter brain TBARS level as compared to sham control group. On the contrary, similar administration of valeryl salicylate (5 or 10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) significantly reduced elevated TBARS level in the brains of ICV-STZ-administered rats in comparison to the levels observed in ICV-STZ-treated rats (p < 0.05). However, administration of phenacetin (20 or 40 mg/kg, i.p.) did not alter elevated TBARS level in the brain of ICV-STZ-treated rats (Table 1).
Effect of COX Inhibitors on Brain Reduced Glutathione in ICV-STZ-Treated Rats
ICV-STZ administered rats showed significantly decreased level of GSH in the brain (p < 0.05; Table 1). In the present study, administration of selective COX-1, COX-2, or COX-3 inhibitor had no effect on GSH level in normal animals as compared to sham control group. On the other hand, valeryl salicylate (10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) improved depleted GSH level in brain as compared to ICV-STZ-treated rats (p < 0.05). Conversely, valeryl salicylate (5 mg/kg, i.p.) and phenacetin (20 or 40 mg/kg, i.p.) had no effect on GSH level in brain of ICV-STZ-treated rats (Table 1).
Effect of COX Inhibitors on Brain Superoxide Dismutase Activity
ICV-STZ-administered animals showed significantly decreased activity of SOD in the brain in comparison to sham control animals (p < 0.05; Table 1). In the present study, administration of selective COX-1, COX-2, or COX-3 inhibitor had no effect on SOD activity in normal animals as compared to sham control group. On the other hand, valeryl salicylate (5 or 10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) significantly attenuated the reduction in SOD activity in brain of ICV-STZ-administered rats as compared to ICV-STZ-treated rats. Further, there was no significant difference between low and high doses of valeryl salicylate-treated groups. Conversely, no significant increase in SOD activity was observed in phenacetin (20 or 40 mg/kg, i.p.)-treated rats as compared to ICV-STZ-treated rats.
Effect of COX Inhibitors on Brain Nitrite Level in ICV-STZ-Treated Rats
ICV-STZ-administered rats significantly increased brain nitrite level in comparison to sham control animals (p < 0.05; Fig. 9). Administration of selective COX-1, COX-2, or COX-3 inhibitor had no effect on nitrite level in normal animals as compared to sham control group. Treatment with valeryl salicylate (10 mg/kg, i.p.) and etoricoxib (10 mg/kg, i.p.) in ICV-STZ-administered animals markedly reduced the brain nitrite level as compared to ICV-STZ-administered rats. However, pretreatment of ICV-STZ-administered rats with low dose of COX-1 or COX-2 inhibitor did not show significant decline in brain nitrite level. Conversely, similar administration of phenacetin (20 or 40 mg/kg, i.p.) had no effect on elevated brain nitrite level in ICV-STZ-treated rats (Fig. 9).
Effect of selective cyclooxygenase inhibitors on nitrite level in the brain of intracerebroventricular streptozotocin (ICV-STZ)-treated rats. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; c p < 0.05 vs VAL (5); d p < 0.05 vs ETO (5)
Effect of COX Inhibitors on Brain Lactate Dehydrogenase Activity in ICV-STZ-Treated Rats
The LDH activity was used as cell death marker. ICV-STZ administration showed significant increase in brain LDH when compared with sham control rats (p < 0.05). Administration of selective COX inhibitors per se treatment did not cause any change in the brain LDH activity as compared to sham control rats. However, treatment with valeryl salicylate (10 mg/kg, i.p.) and etoricoxib (5 or 10 mg/kg, i.p.) in ICV-STZ-treated rats significantly reduced the increased LDH activity when compared with ICV-STZ-treated group (p < 0.05). Conversely, treatment with low dose of valeryl salicylate (5 mg/kg, i.p.) and phenacetin (20 or 40 mg/kg, i.p.) failed to reduce increased LDH activity in ICV-STZ rats when compared with ICV-STZ-treated group (Fig. 10).
Effect of selective cyclooxygenase inhibitors on lactate dehydrogenase (LDH) activity in the brain of intracerebroventricular streptozotocin (ICV-STZ)-treated rats. ETO etoricoxib (5 or 10 mg/kg, i.p.), PHE phenacetin (20 or 40 mg/kg, i.p.), VAL valeryl salicylate (5 or 10 mg/kg, i.p.). Values are mean ± SEM. a p < 0.05 vs sham; b p < 0.05 vs ICV-STZ; d p < 0.05 vs ETO (5)
Discussion
The present study examined the effect of cyclooxygenase (COX-1, COX-2, or COX-3) inhibitors to define the role of COX isoforms in the pathogenesis of ICV-STZ induced neurotoxicity, an animal model of SAD. Accumulating data indicates that central administration of STZ causes significant impairment of learning and memory accompanied by marked increase in AChE levels, which further supports the involvement of cholinergic system (Sonkusare et al. 2005; Agrawal et al. 2009). Intriguingly, treatments with COX-1 and/or COX-2, but not COX-3, inhibitors significantly improved the memory performances in Morris water maze and modified elevated plus maze paradigms and also reduced increased AChE activity in ICV-STZ-treated rats. It is well-known that ICV-STZ administration causes IR desensitization that result in alteration in glucose and energy metabolism. Energy failure results in ATP depletion and uncontrolled leakage of ions across the cell membrane that leads to membrane depolarization and release of the neurotransmitters such as glutamate and dopamine. Excessive glutamate release and stimulation of its receptors results in excessive Ca2+ influx that further activates phospholipases (Lipton 1999; Phillis and O’Regan 2003). In a recent study, it has been reported that administration of lercanidipine, L-type Ca2+ channel blocker, improved cognitive dysfunction by reducing oxidative stress and increased AChE activity which suggests that Ca2+ overactivity plays a vital role in ICV-STZ induced SAD (Sonkusare et al. 2005). This is further supported by the previous finding that STZ-induced ATP depletion results in increased intracellular Ca2+ influx that further activates phospholipid hydrolysis and release of AA, which is metabolized by COX into PGs (Moskowitz et al. 1984; Sun et al. 1993; Sheridan et al. 2001).
It is well reported that COX activation and/or induction occurs secondary to N-methyl-d-aspartate (NMDA) receptor activation and increased Ca2+ influx (Miettinen et al. 1997). This increase in COX activity contributes to the neuronal and synaptic loss that is associated with neurodegeneration following increased production of oxidative stress and the neurotoxic actions of PGs (Kukreja et al. 1986; Bezzi et al. 1998). Accumulating data indicates that arachidonic acid cascade is involved in memory acquisition and retention (Yang and Chen 2008). More importantly, it is also evident that the induction of PG formation by IL-1β is involved in loss of working and spatial memory following IL-1β administration (Hein et al. 2007). It can be suggested that the formation of PGs in general by COX enzyme is required for IL-1β effect. However, PGE2 is a likely candidate for influencing memory (Matsumoto et al. 2004). It has been reported that prostaglandins such as PGE1 and PGE2 cause neurotoxicity and stimulate the release of glutamate from astrocytes (Kukreja et al. 1986; Hewett et al. 2000). Moreover, bilateral intrahippocampal injection of PGE2, endotoxin, or IL-1β, the potent inducers of COX-2, significantly impaired memory that resembles Alzheimer’s dementia, which was attenuated by pretreatment with selective COX-2 or nonselective COX (COX-1/2) inhibitors (Nogawa et al. 1997; Jain et al. 2002). These data along with the results of the present study strongly suggest that COX-1 and/or COX-2, but not COX-3, are involved in learning and memory impairment following ICV-STZ injections.
Oxidative stress is also considered to be one of the major determinants of ICV-STZ neurotoxicity (Sharma and Gupta 2002; Tiwari et al. 2009). It is well established that generation of free radicals and subsequent oxidative stress occurs prior to cytopathology and plays a key role in excitotoxic damage as evidenced by the presence of lipid peroxidation products, including isoprostanes, in brain tissues from AD patients (Markesbery 1999; Singh et al. 2003). In transgenic mice model, EP2−/− mice fail to escalate the inflammatory oxidative response seen in EP2+/+ mice following LPS administration, as assayed by levels of lipid peroxidation (Montine et al. 2002). Indeed, free radicals are intermediate products in COX-mediated PG synthesis during the peroxidase activity of COX that results in production of reactive oxygen species (ROS; Pepicelli et al. 2002; Candelario-Jalil et al. 2003) which is the important mediator of neuronal injury (Madrigal et al. 2003). A growing body of evidence indicates that ROS formation and subsequent oxidative stress may cooperate in a series of molecular events that link to memory impairment (Bruce-Keller et al. 1998; Kumar et al. 2006a, b).
Further, NO as a precursor for free radicals reacts with superoxide anions produced as a result of excitotoxicity. Peroxidase activity of COX and oxidative stress after central administration of STZ increase the toxic intermediates like peroxynitrite, nitric dioxide, and nitron ion (Shoham et al. 2007). Indeed, COX inhibition is reported to decrease iNOS activity (Goodwin et al. 1999). Moreover, it has been recently reported that NSAIDs have shown to scavenge hydrogen peroxide and NO-derived free radicals and preventing glutamate-mediated excitotoxicity and subsequent oxidative stress (Fass et al. 2000). These findings implicate that neuroinflammation due to overexpression of COX isoforms in ICV-STZ-treated rat (s) brain may cause increased oxidative stress. However, in most cases, the signal transduction mechanism in increased oxidative stress has not been fully understood. In the present study, treatment with selective COX-1 and/or COX-2 inhibitors significantly attenuated oxidative stress, reduced lipid peroxidation and nitrite level, and improved antioxidant defenses such as SOD and GSH in brain of ICV-STZ-administered rats. Further, no significant modification in oxidative and nitrosative stress was observed in animals treated with selective COX-1, COX-2, or COX-3 inhibitor. Conversely, in the present study, inhibition of COX-3 failed to decrease the oxidative stress markers in the same model which is supported by previous report that COX-3 may play an ancillary role in inflammation in AD (Cui et al. 2004). Thus, it is plausible that ICV-STZ induces activation of COX-1 and/or COX-2 that results in oxidative and nitrosative stress-mediated neurotoxicity, and protective effects of COX-1 and/or COX-2, but not COX-3, inhibitors are attributed partly by reducing oxidative and nitrosative stress in this experimental model of dementia. Based on our finding and previous reports, a possible scheme for signal transduction pathway involved in the neuroprotection by COX-1 and COX-2 inhibitor in ICV-STZ induced sporadic Alzheimer’s disease in rats is shown in Fig. 11.
In the present study, brain LDH activity, a well-known marker of cell death, was also evaluated in order to gain an insight into neuroprotective role of COX inhibitors in experimental dementia. It has been reported that there is an increase in LDH activity after ICV-STZ administration in brain of rats (Hoyer and Lannert 2007). Consistent to this, in the present study, ICV-STZ-treated animals showed a marked increase in LDH which was prevented by pretreatment with COX-1 and/or COX-2, but not COX-3, inhibitor. Recent studies have demonstrated that selective inhibition of the inducible isoform of COX (i.e., COX-2) protected against ischemic neuronal injury in rat (Nogawa et al. 1997). Indeed, naproxen, a nonselective COX-1/2 inhibitor, has shown attenuation of neuronal death mediated by excessive activation of neuronal NMDA receptors in vivo (Silakova et al. 2004). Another study demonstrates a neuroprotective effect of rofecoxib, a selective COX-2 inhibitor, protects against excitotoxic degeneration in rats presumably mediated through the inhibition of p38 MAPK phosphorylation (Scali et al. 2003). In addition, COX-1 and/or COX-2 inhibitors also showed the neuroprotective effect in several studies by ameliorating brain inflammation (Salzberg-Brenhouse et al. 2003; Silakova et al. 2004). These data along with the results of the present study support the neuroprotective effect of COX-1 or COX-2 inhibitors in this experimental model and further suggest that COX-1 and/or COX-2 activity is involved in the biochemical events that lead to neuronal cell death following ICV-STZ administration.
In conclusion, regardless of the exact mechanisms of neuroprotection, the present data suggest that inhibition of COX-1 and/or COX-2, but not COX-3, contributed to the attenuation of oxidative stress, restoration of altered AChE activity, and improvement of neurological and cognitive performance and neuronal cell survival in ICV-STZ-treated demented rats. Nonetheless, our findings do not completely rule out the participation of COX-3 in memory formation. One possibility of failure of COX-3 inhibitor to show neuroprotection is that COX-3 might be sparsely distributed, and/or it is also possible that COX-3 might not be induced in the brain areas that are prone to neuronal damage following central STZ injection. Thus, further studies are needed to confirm the role of COX-3 in pathophysiology of dementia.
References
Agrawal R, Tyagi E, Shukla R, Nath CA (2009) Study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology 56:779–787
Aisen PS, Schafer KA, Grundman M et al (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression—a randomized controlled trial. J Am Med Assoc 289:2819–2826
Bezzi P, Carmignoto G, Pasti L et al (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285
Bruce-Keller AJ, Li YJ, Lovell MA et al (1998) 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J Neuropathol Exp Neurol 57:257–267
Candelario-Jalil E, Alvarez D, Merino N, Leon OS (2003) Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res 47:245–253
Chandrasekharan NV, Dai H, Roos KLT et al (2002) COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A 99:13926–13931
Consilvio C, Vincent AM, Feldman EL (2004) Neuroinflammation, COX-2, and ALS—a dual role? Exp Neurol 187:1–10
Cui JG, Kuroda H, Chandrasekharan NV et al (2004) Cyclooxygenase-3 gene expression in Alzheimer hippocampus and in stressed human neural cells. Neurochem Res 29:1731–1737
Doré S, Otsuka T, Mito T et al (2003) Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol 54:155–162
Duckworth EA, Koutouzis TK, Borlongan CV et al (1999) Rats receiving systemic 3-nitropropionic acid demonstrate impairment of memory in Morris water maze. Psychobiology 27:561–566
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Engelhardt JI, Tajti J, Appel SH (1993) Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol 50:30–36
Fass U, Panickar K, Personett D et al (2000) Differential vulnerability of primary cultured cholinergic neurons to nitric oxide excess. NeuroReport 11:931–936
Gierse JK, Zhang Y, Hood WF et al (2005) Valdecoxib: assessment of cyclooxygenase-2 potency and selectivity. J Pharmacol Exp Ther 312:1206–1212
Goodwin DC, Landino LM, Marnett LJ (1999) Reactions of prostaglandin endoperoxide synthase with nitric oxide and peroxynitrite. Drug Metab Rev 31:273–294
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Ann Biochem 126:131–138
Hein AM, O’Banion MK (2009) Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol 40:15–32
Hein AM, Stutzman DL, Bland ST et al (2007) Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience 150:754–763
Hewett SJ, Uliasz TF, Vidwans AS, Hewett JA (2000) Cyclooxygenase-2 contributes to N-methyl-D-aspartate-mediated neuronal cell death in primary cortical cell culture. J Pharmacol Exp Ther 293:417–425
Hoyer S (1995) Age-related changes in cerebral oxidative metabolism. Implications for drug therapy. Drugs Aging 6:210–218
Hoyer S, Lannert H (2007) Long-term abnormalities in brain glucose/energy metabolism after inhibition of the neuronal insulin receptor: implication of tau-protein. J Neural Transm Suppl 72:195–202
Ishida T, Sato T, Irifune M, Tanaka KI, Nakamura N, Nishikawa T (2007) Effect of acetaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol 21:757–767
Jain NK, Patil CS, Kulkarni SK, Singh A (2002) Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res 133:369–376
Kelsen J, Kjaer K, Chen G et al (2006) Parecoxib is neuroprotective in spontaneously hypertensive rats after transient middle cerebral artery occlusion: a divided treatment response? J Neuroinflammation 3:31
Kukreja RC, Kontos HA, Hess ML, Ellis EF (1986) PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59:612–619
Kumar A, Seghal N, Padi SV, Naidu PS (2006a) Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rats. Eur J Pharmacol 551:58–66
Kumar P, Padi SSV, Naidu PS, Kumar A (2006b) Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol 17:485–492
Lipton SA (1999) Neuronal protection and destruction by NO. Cell Death Differ 6:943–951
Liu XJ, Lee TL, Wong PTH (2006) Cyclooxygenase-1 inhibition shortens the duration of diazepam-induced loss of righting reflex in mice. Anesth Analg 102:135–140
Madrigal JLM, Moro MA, Lizasoain I et al (2003) Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology 28:1579–1588
Markesbery WR (1999) The role of oxidative stress in Alzheimer disease. Arch Neurol 56:1449–1452
Matsumoto Y, Yamaguchi T, Watanabe S, Yamamoto T (2004) Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology 46:1195–1200
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27:741–749
Miettinen S, Fusco FR, Yrjanheikki J et al (1997) Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A(2). Proc Natl Acad Sci U S A 94:6500–6505
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM (2002) Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem 83:463–470
Moskowitz N, Schook W, Puszkin S (1984) Regulation of endogenous calcium-dependent synaptic membrane phospholipase A2. Brain Res 290:273–280
Muller D, Nitsch RM, Wurtman RJ, Hoyer S (1998) Streptozotocin increases free fatty acids and decreases phospholipids in rat brain. J Neural Transm 105:1271–1281
Nogawa S, Zhang FY, Ross ME, Iadecola C (1997) Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci 17:2746–2755
O’Banion MK (1999) COX-2 and Alzheimer’s disease: potential roles in inflammation and neurodegeneration. Expert Opin Investig Drugs 8:1521–1536
Pasinetti GM, Aisen PS (1998) Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 87:319–324
Pepicelli O, Fedele E, Bonanno G et al (2002) In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E-2 extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem 81:1028–1034
Phillis JW, O’Regan MH (2003) The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol 15:61–90
Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR (1998) Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res 23:81–88
Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB (2008) Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res 39:1–16
Salkovic-Petrisic M, Hoyer S (2007) Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm 72:217–233
Salzberg-Brenhouse HC, Chen EY, Emerich DF et al (2003) Inhibitors of cyclooxygenase-2, but not cyclooxygenase-1 provide structural and functional protection against quinolinic acid-induced neurodegeneration. J Pharmacol Exp Ther 306:218–228
Scali C, Giovannini MG, Prosperi C, Bellucci A, Pepeu G, Casamenti F (2003) The selective cyclooxygenase-2 inhibitor rofecoxib suppresses brain inflammation and protects cholinergic neurons from excitotoxic degeneration in vivo. Neuroscience 117:909–919
Schwab JM, Beschorner R, Meyermann R, Gozalan F, Schluesener HJ (2002) Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg 96:892–899
Sharma M, Gupta YK (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489–2498
Sharma AC, Kulkarni SK (1991) Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuropsychopharmacol Biol Psychiatry 16:117–125
Sheridan AM, Sapirstein A, Lemieux N, Martin BD, Kim DK, Bonventre JV (2001) Nuclear translocation of cytosolic phospholipase A(2) is induced by ATP depletion. J Biol Chem 276:29899–29905
Shoham S, Bejar C, Kovalev E, Schorer-Apelbaum D, Weinstock M (2007) Ladostigil prevents gliosis, oxidative–nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology 52:836–843
Silakova JM, Hewett JA, Hewett SJ (2004) Naproxen reduces excitotoxic neurodegeneration in vivo with an extended therapeutic window. J Pharmacol Exp Ther 309:1060–1066
Singh A, Naidu PS, Kulkarni SK (2003) Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res 37:1245–1252
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Song MS, Rauw G, Baker GB, Kar S (2008) Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci 28:1989–2002
Sonkusare S, Srinivasan K, Kaul C, Ramarao P (2005) Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 77:1–14
Sun FF, Fleming WE, Taylor BM (1993) Degradation of membrane phospholipids in the cultured human astroglial cell line UC-11MG during ATP depletion. Biochem Pharmacol 45:1149–1155
Tahirovic I, Sofic E, Sapcanin A et al (2007) Reduced brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. Neurochem Res 32:1709–1717
Terwel D, Prickaerts J, Meng FP, Jolles J (1995) Brain enzyme activities after intracerebroventricular injection of streptozotocin in rats receiving acetyl-L-carnitine. Eur J Pharmacol 287:65–71
Tiwari V, Kuhad A, Bishnoi M, Chopra K (2009) Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol Biochem Behav 93:183–189
Tuppo EE, Arias HR (2005) The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol 37:289–305
Wang XL, Su B, Zheng L, Perry G, Smith MA, Zhu XW (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem 109:153–159
Wills ED (1966) Mechanism of lipid peroxide formation in animal. Biochems J 99:667–676
Xiang Z, Thomas S, Pasinetti G (2007) Increased neuronal injury in transgenic mice with neuronal overexpression of human cyclooxygenase-2 is reversed by hypothermia and rofecoxib treatment. Curr Neurovasc Res 4:274–279
Yang HW, Chen C (2008) Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des 14:1443–1451
Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM (2006) Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res 84:1–14
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhull, D.K., Jindal, A., Dhull, R.K. et al. Neuroprotective Effect of Cyclooxygenase Inhibitors in ICV-STZ Induced Sporadic Alzheimer’s Disease in Rats. J Mol Neurosci 46, 223–235 (2012). https://doi.org/10.1007/s12031-011-9583-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9583-6