Abstract
Due to the high ability of cadmium to cross the blood–brain barrier, cadmium (Cd) causes severe neurological damages. Hence, the purpose of this study was to investigate the possible protective effect of Mangifera indica leaf extract (MLE) against Cd-induced neurotoxicity. Rats were divided into eight groups. Group 1 served as vehicle control group, groups 2, 3 and 4 received MLE (100, 200, 300 mg /kg b.wt, respectively). Group 5 was treated with CdCl2 (5 mg/kg b.wt). Groups 6, 7 and 8 were co-treated with MLE and CdCl2 using the same doses. All treatments were orally administered for 28 days. Cortical oxidative stress biomarkers [Malondialdehyde (MDA), nitric oxide (NO), glutathione content (GSH), oxidized form of glutathione (GSSG), 8-hydroxy-2-deoxyguanosine (8-OHdG), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)], inflammatory cytokines [tumor necrosis factor (TNF-α) and interlukin-1β (IL-1β)], biogenic amines [norepinephrine (NE), dopamine (DA) and serotonin (5-HT)], some biogenic metabolites [3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA)], acetylcholine esterase activity (AChE) and purinergic compound [adenosine triphosphate (ATP)] were determined in frontal cortex of rats. Results indicated that Cd increased levels of the oxidative biomarkers (MDA, NO, GSSG and 8-OHdG) and the inflammatory mediators (TNF-α and IL-1β), while lowered GSH, SOD, CAT, GPx and ATP levels. Also, Cd significantly decreased the AChE activity and the tested biogenic amines while elevated the tested metabolites in the frontal cortex. Levels of all disrupted cortical parameters were alleviated by MLE co-administration. The MLE induced apparent protective effect on Cd-induced neurotoxicity in concern with its medium and higher doses which may be due to its antioxidant and anti-inflammatory activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals are chemical elements that are widely distributed in the earth’s crust, which are very toxic at low and high doses (Viswanadh et al. 2010). Cadmium (Cd) is one of the most harmful and ubiquitous heavy metals and environmental pollutants causing deleterious problems to the human and animal health (Thevenod and Lee 2013). Cd is not degradable and has a long biological half-life time in soft tissues due to its low rate of excretion from the body (Jarup et al. 2000; Wang and Du 2013). Humans mainly exposed to cadmium from the contaminated diet and water. Other sources also include tobacco smoking and industrial emissions (Nair et al. 2013). Once inhaled or ingested, Cd accumulate mainly in the kidney, liver and lungs causing disturbance in the biological systems (Bernard 2008). Due to its high ability to cross the blood–brain barrier (BBB), cadmium was found to cause severe neurological damages (Li et al. 2016). The cerebral cortex has been reported to participate and play a key role in memory, cognition, sensory perception, motor planning, decision making, language and control. Cd is targeting the cortical neurons causing a disturbance in the higher level functions (Yuan et al. 2013). Afifi and Embaby (2016) observed sever degenerative alterations in the cortical structure following the oral administration of Cd for two months. Some neurological disorders has been noticed after Cd exposure such as learning disabilities, memory deficits (Ashok et al. 2015; Li et al. 2012) and Parkinson like symptoms (Okuda et al. 1997). Magnifying evidence has revealed that reactive oxygen species (ROS) are playing a key role in Cd toxicology. The over production of ROS such as hydroxyl radicals, superoxide ions and hydrogen peroxide might be due to the depletion of glutathione pool and protein-bound sulfhydryl groups, in turn the produced ROS overwhelm the antioxidant defenses producing a state of oxidative stress (Liu et al. 2009). Cd has been shown to generate oxidative stress in the brain tissue leading to lipid perxidation, DNA damage and neurochemical alterations (Shagirtha et al. 2011 and Abdel Moneim et al. 2014). Furthermore, it has been reported that oxidative stress was found to be associated with the overproduction of the inflammatory cytokines (Liu et al. 2011). However, the precise mechanism of Cd-induced neurotoxicity is still not clear.
As a result of its ability to scavenge ROS, antioxidants might be used to protect against Cd toxicity. Medicinal plants have been widely used to prevent different degenerative diseases due to their rich polyphenolic content which has strong antioxidant properties (Sreeramulu et al. 2013). Mangifera indica is a tropical tree, also known as mango, belongs to the Anacardiaceae family. The photochemical analysis of Mangifera indica showed that it contains polyphenols, steroids, fatty acids, terpenoids and microelements. The ployphenols includes gallic acid, 3,4-dihydroxy benzoic acid, gallic acid methyl ester, gallic acid propyl ester, mangiferin, catechin, epicatechin, benzoic acid and benzoic acid propyl ester (Núñez Sellés et al. 2002). The pharmacological activities of Mangifera indica include antioxidant, antidiabetic, anti-tumor, antiviral, immunomodulatory, anti-diarrheal, anti-inflammatory, antibacterial and hepatoprotective (Bhowmik et al. 2009; Garrido et al. 2004; Guha et al. 1996; Prasad et al. 2007; Rocha Ribeiro et al. 2007; Sairam et al. 2003; Sarkar et al. 2004).
It has been documented that the methanolic and the aqueous extracts of Mangifera indica L. were found to protect the neuroblastoma cells from oxidative damage (Kawpoomhae et al. 2010). Mangifera indica L. extract also protected the cerebral cortex neurons from the excitotoxicity mediated by glutamate (Lemus-Molina et al. 2009). In the same context, Mangifera indica L. extract prevented the damage of hippocampal CA1 neurons after ischaemia-reperfusion (Martinez Sanchez et al. 2001). In addition, Tau hyperphosphorylation and the atrophy of cortical and hippocampal neurons were decreased after the treatment with Mangifera indica extract indicating its ability to alleviate the memory deficit and learning abilities in diabetic dyslipidemia model (Infante-Garcia et al. 2016).
However, the possible protective effect of M. indica leaf extract (MLE) on Cd-induced neurotoxicity has not been investigated. Therefore, the current study is designed to investigate the potential neuroprotective role of MLE in Cd-induced neuronal damage through estimating the levels of oxidative stress biomarkers, inflammatory cytokines, biogenic amines, some biogenic metabolites, acetylcholine esterase activity and purinergic compound in frontal cortex of male albino rats.
Materials and methods
Animal model
Wistar strain male albino rats (150–170 g) obtained from the NODCAR Animal House, NODCAR, Giza, Egypt, were used for the study. The rats were housed in wire mesh cages under standard conditions (temperature 25–29 °C, 12 h light and 12 h darkness cycles). Animals were fed with pelleted standard rat diet and water ad libitum. All procedures performed in the study were in accordance with the ethical standards of the CMHI bioethical committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Extract preparation
Approximately 2000 g of powdered material was placed in a clean, flat-bottomed glass container and soaked in ten volume of ethanol 70%. The container with its contents was sealed and kept for 5 days. Then extraction was carried out using ultrasonic sound bath accompanied by sonication (40 min). The content filtrated by a piece of clean, white cotton material. The extract then was filtered through Whatman filter paper (Bibby RE200, Sterilin Ltd., UK) and dried by electric oven at 45 °C temperature and continued up to obtain ethanolic (277 g) extract. The gummy extract was stored in an air tight container.
Experimental design
Forty eight rats were divided into 8 groups of six animals each. Group 1 served as normal control. Groups 2, 3 and 4 were treated for 28 days with 100, 200 and 300 mg/kg b.wt of MLE, respectively. Groups 5 treated with CdCl2 in saline (5 mg/kg b.wt) for 28 days. Groups 6, 7 and 8 were treated with MLE (100, 200 and 300 mg/kg b.wt) followed by the administration of CdCl2 (5 mg/kg b.wt) for 28 days. MLE and Cd administrations were done orally. MLE was orally administered at doses of 100, 200 and 300 mg/kg according to a preliminary study in which this dose yielded no signs of toxicity, while CdCl2 was orally administered at 5 mg/kg according to Shagirtha et al. (2011) and Afifi and Embaby (2016).
Sample collection
Rats were sacrificed by decapitation 24 h after the last administration. Brain cortex tissues of animals were rapidly dissected, thoroughly washed with isotonic saline and then weighed. Each brain tissues (frontal cortex area) were homogenized in 75% aqueous HPLC grade methanol (10% w/v). The homogenate was spun at 4000 r.p.m. for 10 min for the determination of monoamines and their metabolites, while for the estimation of the other biochemical investigations, brain tissue was homogenized in an ice-cold medium of 50 mM Tris-HCl (pH 7.4) to give a 10% (w/v) homogenate. After being centrifuged at 1000×g for 10 min at 4 °C, the supernatants obtained from the homogenates was separated and stored at −80 °C. The total protein content of the frontal cortex homogenate in all experiments was estimated by the method of Lowry et al. (1951).
Determination of oxidative stress markers in the frontal cortex
Malondialdehyde
It was determined by HPLC. The MDA standard (reference standard was purchased from Sigma Chemical Co.) was prepared by dissolving 25 ml 1,1,3,3 tetraethoxypropane (TEP) in 100 mL of water to give a 1 mM stock solution. The working standard was prepared by hydrolysis of 1 ml TEP stock solution in 50 mL 1% sulphuric acid and incubation for 2 h at room temperature. The resulting MDA standard of 20 nmol/mL was further diluted with 1% sulphuric acid to yield the final concentration of 1.25 nmol/mL, used as the standard for the estimation of total MDA (Lowry et al. 1951). The samples were analyzed with the Agilent HP 1200 series HPLC apparatus (USA). The analytical column was Supelcosil C18 (5 μm particle and 80 AO Pore size) (250X4.6 ID). The mobile phase consisted of 30 mmol potassium dihydrogen phosphate-methanol HPLC grade (65%–35% H3PO4, pH 4), with a flow rate of 1.5 mL/min and wavelength of 250 nm, according to the method of Karatas et al. (2002).
Nitric oxide (NO)
It was determined as the ratio of nitrites/nitrate by HPLC according to the method of Papadoyannis et al. (1999). Sodium nitrite and sodium nitrate were used for the reference standard preparation with stock concentration 1 mg/mL. The samples were analyzed with the Agilent HP 1100 series HPLC apparatus (USA). The analytical column was an anion exchange PRP-X100 Hamilton, 150 and 4.1 mm by 10 mm. The mobile phase was a mixture of 0.1 M sodium chloride – methanol (45:55 v/v), with a flow rate 2 mL/min and wavelength of 230 nm.
Reduced and oxidized glutathione (GSH and GSSG)
Both of them were determined by HPLC according to the method of Jayatilleke and Shaw (1993). The GSH and GSSG reference standard was purchased from Sigma Chemical Co., dissolved in 75% methanol in stock 1 mg/mL and diluted before application to HPLC. The samples were analyzed with the Agilent HP 1100 series HPLC apparatus (USA). The analytical column was m-Bondapak column (15 cm and 3.9 mm). The mobile phase consisted of 25 nmol sodium dihydrogen phosphate containing 5 mmol tetrabutyl ammonium phosphate and methanol (87:13% H3PO4, pH 3.5), with a flow rate of 1 mL/min and wave-length adjusted at 190 nm.
Determination of 8 -hydroxy-2 –deoxyguanosine (8-OHdG) in the frontal cortex
Isolation and hydrolysis of brain DNA was performed using the method of Lodovici et al. (1997). The hydrolyzed mixture was finally centrifuged and the supernatant were injected into the HPLC. The separation of 8-OH-2DG was performed with an LC/Agilent 1200 series HPLC apparatus (USA) using UV detector. For chromatographic separation we used C18 reverse phase columns in series (Supelco, 5 pm, I.D. 0.46 × 25 cm); the eluting solution was H2O /CH3OH (85: 15 v/v) with 50 mM KH2PO4, pH 5.5 at a flow rate of 0.68 mL/min. The UV detector was set at 245 nm. The resulting chromatogram identified the concentration from the sample as compared to that of the standard purchased from Sigma Aldrich.
Superoxide dismutase activity (SOD)
It was assayed in the brain tissue by the method of Marklund and Marklund (1974) at 420 nm for 1 min on a UV Shimadzu spectrophotometer (2450). Activity was expressed as the amount of enzyme that inhibits the auto oxidation of pyrogallol by 50%, which is equal to 1 U/mg protein.
Catalase (CAT) and glutathione perodixase (GPx) activities
It was measured by spectrophotometric method based on the decomposition of H2O2 Aebi (1984), whereas GPx activity was measured by applying the method of Paglia and Valentine (1967) using H2O2 as a substrate.
Determination of tumor necrosis factor (TNF-α) and interlukin-1β (IL-1β) in the frontal cortex
IL-6 and TNF-α levels in the rats cortex were estimated using a rat-specific immunoassay ELISA kit (Rat TNF-α and IL-1β) from Glory Science (Del Rio, Texas, USA) according to the manufacturer’s protocol. The intensity of the colored product was directly proportional to the concentration of rat TNF-α and IL-1β, as evaluated using a microplate reader (Biotech ELx800; Biotech Instruments) set at 450 nm. The sample concentration was determined against a standard curve and is expressed in ng of TNF-α and IL-1β per gram of brain tissues.
Determination of the frontal cortex neurotransmitters and metabolites
The HPLC system consisted of quaternary pump; a column oven, Rheodine injector and 20 μl loop, UV variable wavelength detector. The report and chromatogram taken from data acquisition program purchased from chemstation. The sample was immediately extracted from the trace elements and lipids by the use of solid phase extraction CHROMABOND column NH2 phase cat. No.730031. the sample was then injected directly into an AQUA column 150 mm 5 μ C18, purchased from Phenomenex, USA under the following conditions: mobile phase 20 mM potassium phosphate, pH 2.5, flow rate 1.5 mL/min, UV 190 nm. Norepinephrine (NE), dopamine DA), and serotonin (5-HT) were separated after 12 min. The resulting chromatogram identified each monoamine position and concentration from the sample as compared to that of the standard purchased from Sigma Aldrich, and finally, the determination of the content of each monoamine as μg per gram brain tissue was calculated according to Pagel et al. (2000).
Determination of acetylcholinesterase (AChE) activity in the frontal cortex
The colorimetrically procedure used for the determination of acetylcholinesterase activity in the brain samples of rats is a modification of Ellman et al. (1961) method as described by Gorun et al. (1978).
Determination of purinergic metabolites in the frontal cortex
Brain adenine nucleotides adenosine triphosphate (ATP) was assayed by HPLC [Agilent] according to the method of Teerlink et al. (1993). The analysis was employed using gradient elution and UV detection at 254 nm. ATP was quantified by measurement of peak height compared to corresponding standard with each set of experiments. Results were expressed as μmol/g wet tissue.
Statistical analysis
The values were expressed as the mean ± S.E. for the 6 rats in each group. Differences between groups were assessed by one way analysis of variance (ANOVA) using SPSS (version 13.0). Significant differences among means were evaluated using Duncan’s Multiple Range Test.
Results
MLE prevents Cd-induced oxidative stress in cerebral cortex
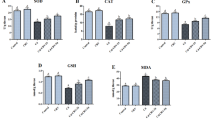
The oral administration of CdCl2 (5 mg/kg b.wt) once a day for 28 days induced oxidative stress in the tissue of frontal cortex of male albino rats, as evidenced by the increase in the levels of MDA, NO, GSSG and 8-OHdG and depleted the GSH content as compared to the control group. Meanwhile, the treated rats with MLE (100, 200 and 300 mg/kg b.wt) showed the ability of the extract to counteract the oxidative stress induced following the CdCl2 treatment by elevating the content of GSH and decreasing the levels of MDA, NO, GSSG and 8-OHdG when compared to Cd-exposed rats (Fig. 1).
Mitigation effects of the oral administration of Mangifera indica leaf extract (MLE) on cortical MDA (a), NO (b), GSSG (c), GSH (d) and 8-OHdG (e) exposed to cadmium chloride (CdCl2) for 28 days. Data are expressed as Mean ± S.E. for 6 rats/group. a significant difference from control group at the same column with one way ANOVA at p < 0.05. b significant difference from Cd group at the same column with one way ANOVA at p < 0.05. c significant difference from MLE 100 group at the same column with one way ANOVA at p < 0.05
The activity of the cellular antioxidant defense system namely; SOD, CAT and GPx activities have been declined significantly in Cd-intoxicated rats (p < 0.05) when compared to the control values as illustrated in Fig. 2. The treatment with different doses of MLE enhanced the activities of these antioxidant enzymes to be higher than the Cd treated rats.
Ameliorative effects of Mangifera indica leaf extract (MLE) on the activities of SOD (a), CAT (b) and GPx (c) in the frontal cortex after the treatment with cadmium chloride (CdCl2) for 28 days. Data are expressed as Mean ± S.E. for 6 rats/group. a significant difference from control group at the same column with one way ANOVA at p < 0.05. b significant difference from Cd group at the same column with one way ANOVA at p < 0.05. c significant difference from MLE 100 group at the same column with one way ANOVA at p < 0.05
MLE attenuated Cd-induced inflammation in cerebral cortex
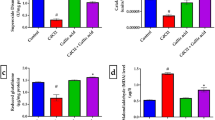
To explore the possible anti-inflammatory activity of MLE, cortical level of TNF-α and IL-1β has been estimated. Cd treated animals showed a significant (p < 0.05) elevation in these cytokines, while the administration of MLE reversed these changes to be near the control levels (Fig. 3).
Immunomodulatory effect of Mangifera indica leaf extract (MLE) on cortical IL-1β (a) and TNF-α (b) after the administration of cadmium chloride (CdCl2) for 28 days in male albino rats. Data are expressed as Mean ± S.E. for 6 rats/group. a significant difference from control group at the same column with one way ANOVA at p < 0.05. b significant difference from Cd group at the same column with one way ANOVA at p < 0.05. c significant difference from MLE 100 group at the same column with one way ANOVA at p < 0.05
The neuromodulatory activity of MLE against Cd-induced neurotoxicity
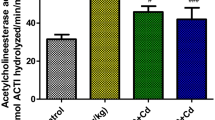
To study the neuromodulatory activity of MLE against Cd toxicity, the monoaminergic transmitters and some of their metabolites, the activity of acetylcholine esterase and the levels of ATP were examined. Cd-exposed rats showed a marked and significant (p < 0.05) suppression in the contents of NE, DA, 5-HT, the activity of AChE and ATP, while the tested biogenic metabolites including DOPAC, HVA and 5HIAA were elevated as compared to the control group. In contrast, the orally treated animals with MLE restored significantly (p < 0.05) all these changes in the frontal cortex when compared to Cd-intoxicated rats (Fig. 4).
Neuroprotective effect of Mangifera indica leaf extract (MLE) on cortical AChE (a), DA (b), NE (c), 5HT (d), ATP (e), HVA (f), DOPAC (g) and 5-HIAA (h) of male albino rats treated with cadmium chloride (CdCl2) for 28 days. Data are expressed as Mean ± S.E. for 6 rats/group. a significant difference from control group at the same column with one way ANOVA at p < 0.05. b significant difference from Cd group at the same column with one way ANOVA at p < 0.05. c significant difference from MLE 100 group at the same column with one way ANOVA at p < 0.05
Discussion
Cd-induced neurotoxicity mechanisms are still unknown. However, several reports proposed that Cd may mediate its toxicity through ROS production, disturbs differentiation and neurochemical alterations. In addition, Cd has been reported to be positively associated with DNA oxidation. Cadmium has been identified as one of the most dangerous environmental hazards because it changes the integrity and permeability of the BBB and enters the brain tissue (Nishimura et al. 2006). Once accumulated in the brain tissue, it causes lesions and produces a disturbance in the cellular defense mechanism causing neuronal damage (Goncalves et al. 2010; Shukla and Chandra 1987). Cerebral cortex has been identified as targets to Cd-mediated toxicity (Yuan et al. 2013). There are data confirming the involvement of Cd in the development of several neurological and psychological problems including mood disorders, depression, anxiety, learning disability, attention deficit hyperactivity disorder and schizophrenia (Ciesielski et al. 2012). In the present study, we observed a severe biochemical and neurochemical changes in the function of the frontal cortex as a result of Cd-intoxication. The treated rats with CdCl2 (5 mg/kg b.wt) for 28 consecutive days induced imbalance between the oxidant and the antioxidant systems causing stressed condition in the frontal cortex, that was clear through the increase in the levels of MDA, NO, GSSH and 8-OHdG and the decrease of the activities of SOD, CAT, GPx and GSH content. These findings are consistent with previous studies that showed that Cd exposure generates ROS, leading to the elevation of lipid peroxidation, decline of sulfhydryls, alterations of antioxidant cellular defenses and DNA damage (Abdel Moneim 2014; Karaca and Eraslan 2013; Shagirtha et al. 2011). In addition, oxygen radicals and NO can interact and exert a cytotoxic effect by causing lipid peroxidation, which results in the formation of MDA (Guzik et al. 2003). The increase in the lipid peroxidation may be due to the overproduction of the superoxide anions which suppress the antioxidant enzymatic system (Amara et al. 2011). Moreover, the increase in the levels of GSSG could be due to the increase of H2O2 metabolism. Stohs et al. (2001) stated that the toxicity of Cd is partly due to oxidative DNA damage associated with the increased production of ROS, such as superoxide ion, hydroxyl radicals and hydrogen peroxide. Furthermore, Cd elevate the level of 8-OHdG which is biomarker for the endogenous oxidative DNA damage and carcinogenesis (Martinez Sanchez et al. 2001). The decreased activities of SOD, CAT and GPx in the present experiment might be attributed to the binding of Cd with the sulfhydryl group of these enzymes and the replacement of endogenous redox metals which alters these enzyme structures leading to their inhibition (Shagirtha et al. 2011). In addition to this effect, Cd potentiates the up regulation of some stress genes which probably affect the activity of the antioxidant enzymes (Wang and Du 2013). The depletion in the GSH content may be due to the binding of heavy metals with GSH forms glutathione-S-conjugates, which ultimately forms mercapturic acids which may deplete GSH (Gohil et al. 1988). Also, this decrease may be due to the utilization of GSH in scavenging the produced free radicals (Rana and Verma 1996). Overall, the combined effect of elevated MDA and the decrease of SOD, CAT, and GPx in the current study may produce neurodegeneration as result of Cd exposure. On the other hand, the obtained results suggested that treatment with different doses of MLE (100, 200 & 300 mg/kg b.wt) attenuated significantly the cerebral oxidative status produced by Cd-exposure. These findings are in agreement with Wattanathorn et al. (2014) who found that the different doses of ethanolic extract of Mangifera indica decreased the levels of MDA and enhanced the activities of SOD and GPx in hippocampus of mild cognitive impairment model. The antioxidant activity of MLE may be due to the powerful antioxidant activity of mangiferin which is the major active constituent in Mangifera indica (Garrido et al. 2008). The antioxidant properties of mangiferin is due to its ability to enhance GSH content (Amazzal et al. 2007), overwhelm free radicals or ROS (Viswanadh et al. 2010) and inhibit lipid peroxidation (Leiro et al. 2003). Magniferin was also found to decrease the level of MDA and elevate the activity of SOD and GSH content in the brain tissue of rats with the cerebral ischemia-reperfusion injury (Yang et al. 2016). Elevated exposure to Cd causes oxidative status which could lead to causes lesions including decrease in total cortical volume, white matter, enlargement of cerebroventricular system, changes in gray and white matter, impaired hippocampus-dependent learning and memory, abnormal laminar organization; these severe alterations in the brain tissue was found to be linked with decreased attention and reading difficulties, behavioral problems, memory and cognitive impairments in animal and humans (Gao et al. 2008; Orisakwe 2014; Wang et al. 2018). Cd disrupts calcium homeostasis as a result of ROS production, leading to apoptosis in cortical neurons and primary murine neurons. Cd can also impair the homeostasis of Cu, Zn, and Co which leads to the dysfunction of neuronal cells in the central nervous system (Wang and Du 2013).
Levels of cortical TNF-α and IL-1β were elevated after Cd administration in the present study. Several studies identified Cd as immune-suppressor, causing elevation in the levels of TNF-α and IL-1β in different experimental models, attributing this increase to the production of ROS (Abbes et al. 2007; Freitas and Fernandes 2011; Lag et al. 2010). Moreover, it has been reported that oxidative stress was found to be associated with the overproduction of the inflammatory cytokines (Liu et al. 2011). The excessive release of TNF-α and IL-1β following the activation of microglia promotes learning and memory deficits and synaptic memory mechanisms in Alzheimer’s disease (Wang et al. 2015). In addition, neutralizing these mediators was found to protect against cognitive decline. Levels of the tested inflammatory mediators in the current study restored to be near the control level, indicating the immunomodulatory impact of MLE. Previous reports found that Mangifera indica extracts have the ability to inhibit the pro inflammatory cytokines in different experimental protocols (Garrido-Suarez et al. 2010; Marquez et al. 2010; Rivera et al. 2011). This anti-inflammatory response correlated with modulating the activity of macrophage which considers the main source of cytokines (Marquez et al. 2010).
A disturbance in the monoaminergic and cholinergic neurotransmission has been recorded in this experiment, which was obvious through the decrease in the content of NE, DA, 5-HT and AChE activity and the increase of some tested metabolites including DOPAC, HVA and 5HIAA in the frontal cortex. The decrease in the level of NE, DA, 5-HT and the increase of DOPAC, HVA and 5HIAA might be due to the oxidative stress which increase the rate of their degradation, prevent the re-uptake and suppressing the activity of the enzymes involved in the synthesis of these neurotransmitters (Andersson et al. 1997; Pillai et al. 2003; Zhao et al. 2009). This decrease in the tested neurotransmitters may alter the learning and memory processes and the progress of Parkinson’s disease (Biradar et al. 2012). Furthermore, the deficiency of DA, NE and 5-HT and the pathophysiological changes in their receptors are thought to be responsible for motor symptoms, disturbing the autonomic sympathetic functions, behavioral disturbances, cognitive dysfunction and associated with sleep disorders, therefore, they considers as the main target for the treatment of these neurological disorders (Xu et al. 2012). Moreover, the release of NE, DA and 5-HT was found to modulate the motor activities and cognition (Dishman et al. 2006).
The cholinergic system including acetylcholine esterase is playing a crucial role in the cognitive processes and it considers as an important physiological and pathological biomarker for various neurological diseases such as Alzheimer disease (Soreq and Seidman 2001). The inhibition of AChE might be due to that Cd competes with metal cofactors and causing conformational changes in the active site leading to its deactivation (Casalino et al. 1997). Furthermore, free radicals produced by Cd in the brain interferes with the cellular antioxidant system eliciting alterations in the structural integrity of lipids and affects the activity of Na+/K+-ATPase and AChE (Mendez-Armenta and Rios 2007; Shukla et al. 1996). Desi et al. (1998) demonstrated that the change in Ca2+ metabolism is associated with the deactivation of AChE. The change in AChE activity has been found to be associated with alterations in the behavior and caused learning and memory impairment in rats following Cd intoxication (Goncalves et al. 2010). In addition, high levels of Cd and lead in children’s hair were associated with learning disabilities. Motor and perceptual abilities of children exposed to Cd in uteri were significantly affected (Goncalves et al. 2010). Furthermore, Cd administration enhances the development of senile plaques and impairs the memory function in transgenic mice (Li et al. 2012). Moreover, cholinergic dysfunction was linked with motor coordination and locomotion alterations (Kassab and El-Hennamy 2017).
In the current experiment, different doses of MLE alleviated the changes in the levels of the tested neurochemical transmitters in the frontal cortex, indicating its neuroprotective impact against cadmium-intoxication. Magniferin was shown to improve the learning and memory capabilities through modulating the levels of NE and DA in Alzheimer’s disease model in rats (Biradar et al. 2012).
A decrease in ATP content was observed after Cd exposure in the frontal cortex. The treatment with MLE reversed these changes in the level of the tested purinegic mediator to the normal values. ATP is a neurotransmitter released by neurons and astrocytes. It has multiple functions such as regulating the formation and development of synaptic vesicles and regulation of the ionic gradients; also controlling the metabolism, structural plasticity and ageing. The alteration in the level of adenosine is associated with a disturbance in the brain activity (Burnstock 2008; Verkhratsky and Burnstock 2014; Zhang et al. 2015). Furthermore, ATP regulates cytoplasmic Ca2+ and cyclic adenosine monophosphate (Burnstock 2016), both messengers play a vital role in the release of different neurotransmitters (Neher and Sakaba 2008), the alterations in the levels of these messengers would affect the neurotransmission. The activation of purinergic receptors in the cortical neurons have been recorded to be involved in cognitive-related neurological diseases and using antagonists to these receptors such as TNP-ATP may use in the treatment of cognitive impairments (Koch et al. 2015; Guzman and Gerevich 2016).
Our study confirmed the involvement of oxidative stress in cadmium-induced neurotoxicity and showed a significant neuroprotective impact of Mangifera Indica leaves extract as evidenced by restoring the balance between generation and clearance of ROS, mending antioxidant homeostasis, inhibiting the increased pro-inflammatory cytokines, reversing the alterations in the biogenic, cholinergic and purinergic transmission in the frontal cortex of rats. However, further investigation into the deep mechanism of action of MLE is required.
References
Abbes S et al (2007) Inactivation of cadmium induced immunotoxicological alterations in rats by Tunisian montmorillonite clay. Int Immunopharmacol 7:750–760. https://doi.org/10.1016/j.intimp.2007.01.013
Abdel Moneim AE (2014) Citrus peel extract attenuates acute cyanide poisoning-induced seizures and oxidative stress in rats. CNS Neurol Disord Drug Targets 13:638–646
Abdel Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF (2014) The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol Trace Elem Res 160:392–399. https://doi.org/10.1007/s12011-014-0066-9
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Afifi OK, Embaby AS (2016) Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. JMAU 4:36–45. https://doi.org/10.1016/j.jmau.2015.10.001
Amara S, Douki T, Garrel C, Favier A, Ben Rhouma K, Sakly M, Abdelmelek H (2011) Effects of static magnetic field and cadmium on oxidative stress and DNA damage in rat cortex brain and hippocampus. Toxicol Ind Health 27:99–106. https://doi.org/10.1177/0748233710381887
Amazzal L, Lapotre A, Quignon F, Bagrel D (2007) Mangiferin protects against 1-methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A cells. Neurosci Lett 418:159–164. https://doi.org/10.1016/j.neulet.2007.03.025
Andersson H, Petersson-Grawe K, Lindqvist E, Luthman J, Oskarsson A, Olson L (1997) Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol Teratol 19:105–115
Ashok A, Rai NK, Tripathi S, Bandyopadhyay S (2015) Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143:64–80. https://doi.org/10.1093/toxsci/kfu208
Bernard A (2008) Cadmium & its adverse effects on human health. Indian J Med Res 128:557–564
Bhowmik A, Khan LA, Akhter M, Rokeya B (2009) Studies on the antidiabetic effects of Mangifera indica stem-barks and leaves on nondiabetic, type 1 and type 2 diabetic model rats. Bangladesh. Aust J Pharm 4:110–114. https://doi.org/10.3329/bjp.v4i2.2488
Biradar SM, Joshi H, Chheda TK (2012) Neuropharmacological effect of Mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer's disease. Eur J Pharmacol 683:140–147. https://doi.org/10.1016/j.ejphar.2012.02.042
Burnstock G (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7:575–590. https://doi.org/10.1038/nrd2605
Burnstock G (2016) Purinergic signalling and neurological diseases: an update. CNS Neurol Disord Drug Targets 16(3):257–265. https://doi.org/10.2174/1871527315666160922104848
Casalino E, Sblano C, Landriscina C (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346:171–179. https://doi.org/10.1006/abbi.1997.0197
Ciesielski T, Weuve J, Bellinger DC, Schwartz J, Lanphear B, Wright RO (2012) Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ Health Perspect 120:758–763. https://doi.org/10.1289/ehp.1104152
Desi I, Nagymajtenyi L, Schulz H (1998) Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J Appl Toxicol 18:63–70
Dishman RK et al (2006) Neurobiology of exercise. Obesity (Silver Spring) 14(3):345–356
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Freitas M, Fernandes E (2011) Zinc, cadmium and nickel increase the activation of NF-kappaB and the release of cytokines from THP-1 monocytic cells. Metallomics 3:1238–1243. https://doi.org/10.1039/c1mt00050k
Gao et al (2008) Trace element levels and cognitive function in rural elderly Chinese. J Gerontol A Biol Sci Med Sci 63:635–641
Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, Delporte C, Núñez-Sellés AJ, Delgado R (2004) In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res 50:143–149. https://doi.org/10.1016/j.phrs.2003.12.003
Garrido G, González D, Romay C, Núñez-Sellés AJ, Delgado R (2008) Scavenger effect of a mango (Mangifera indica L.) food supplement’s active ingredient on free radicals produced by human polymorphonuclear cells and hypoxanthine–xanthine oxidase chemiluminescence systems. Food Chem 107:1008–1014
Garrido-Suarez BB, Garrido G, Delgado R, Bosch F, del CRM (2010) A Mangifera indica L. extract could be used to treat neuropathic pain and implication of mangiferin. Molecules 15:9035–9045. https://doi.org/10.3390/molecules15129035
Gohil K, Viguie C, Stanley WC, Brooks GA, Packer L (1988) Blood glutathione oxidation during human exercise. J Appl Physiol (1985) 64:115–119
Goncalves JF et al (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60. https://doi.org/10.1016/j.cbi.2010.04.011
Gorun V, Proinov I, Baltescu V, Balaban G, Barzu O (1978) Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal Biochem 86:324–326
Guha S, Ghosal S, Chattopadhyay U (1996) Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 42:443–451
Guzik TJ, Korbut R, Adamek-Guzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54:469–487
Guzman SJ, Gerevich Z (2016) P2Y receptors in synaptic transmission and plasticity: therapeutic potential in cognitive dysfunction. Neural Plast 2016:1207393
Infante-Garcia C, Jose Ramos-Rodriguez J, Marin-Zambrana Y, Teresa Fernandez-Ponce M, Casas L, Mantell C, Garcia-Alloza M (2016) Mango leaf extract improves central pathology and cognitive impairment in a type 2 diabetes mouse model. Brain Pathol 27:499–507. https://doi.org/10.1111/bpa.12433
Jarup L et al (2000) Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 57:668–672
Jayatilleke E, Shaw S (1993) A high-performance liquid chromatographic assay for reduced and oxidized glutathione in biological samples. Anal Biochem 214:452–457. https://doi.org/10.1006/abio.1993.1522
Karaca S, Eraslan G (2013) The effects of flaxseed oil on cadmium-induced oxidative stress in rats. Biol Trace Elem Res 155:423–430. https://doi.org/10.1007/s12011-013-9804-7
Karatas F, Karatepe M, Baysar A (2002) Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal Biochem 311:76–79
Kassab RB, El-Hennamy RE (2017) The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rats. Egy J Basic Appl Sci 4:160–167
Kawpoomhae K, Sukma M, Ngawhirunpat T, Opanasopit P, Sripattanaporn A (2010) Antioxidant and neuroprotective effects of standardized extracts of Mangifera indica leaf. Thai J Pharm Sci 34:32–43
Koch H, Bespalov A, Drescher K, Franke H, Krugel U (2015) Impaired cognition after stimulation of P2Y1 receptors in the rat medial prefrontal cortex. Neuropsychopharmacology 40:305–314
Lag M, Rodionov D, Ovrevik J, Bakke O, Schwarze PE, Refsnes M (2010) Cadmium-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol Lett 193:252–260. https://doi.org/10.1016/j.toxlet.2010.01.015
Leiro JM, Alvarez E, Arranz JA, Siso IG, Orallo F (2003) In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-alpha and transforming growth factor-beta genes. Biochem Pharmacol 65:1361–1371
Lemus-Molina Y, Sanchez-Gomez MV, Delgado-Hernandez R, Matute C (2009) Mangifera indica L. extract attenuates glutamate-induced neurotoxicity on rat cortical neurons. Neurotoxicology 30:1053–1058. https://doi.org/10.1016/j.neuro.2009.06.012
Li X, Lv Y, Yu S, Zhao H, Yao L (2012) The effect of cadmium on a beta levels in APP/PS1 transgenic mice. Exp Ther Med 4:125–130. https://doi.org/10.3892/etm.2012.562
Li M, Pi H, Yang Z, Reiter RJ, Xu S, Chen X, Chen C, Zhang L, Yang M, Li Y, Guo P, Li G, Tu M, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Yu Z, Zhou Z (2016) Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J Pineal Res 61:353–369. https://doi.org/10.1111/jpi.12353
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238:209–214. https://doi.org/10.1016/j.taap.2009.01.029
Liu Z, Li P, Zhao D, Tang H, Guo J (2011) Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation 34:639–644. https://doi.org/10.1007/s10753-010-9273-5
Lodovici M, Casalini C, Briani C, Dolara P (1997) Oxidative liver DNA damage in rats treated with pesticide mixtures. Toxicology 117:55–60
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Marquez L et al (2010) Anti-inflammatory effects of Mangifera indica L. extract in a model of colitis. World J Gastroenterol 16:4922–4931
Martinez Sanchez G, Candelario-Jalil E, Giuliani A, Leon OS, Sam S, Delgado R, Nunez Selles AJ (2001) Mangifera indica L. extract (QF808) reduces ischaemia-induced neuronal loss and oxidative damage in the gerbil brain. Free Radic Res 35:465–473
Mendez-Armenta M, Rios C (2007) Cadmium neurotoxicity. Environ Toxicol Pharmacol 23:350–358. https://doi.org/10.1016/j.etap.2006.11.009
Nair AR, Degheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14:6116–6143. https://doi.org/10.3390/ijms14036116
Neher E, Sakaba T (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59:861–872. https://doi.org/10.1016/j.neuron.2008.08.019
Nishimura Y, Yamaguchi JY, Kanada A, Horimoto K, Kanemaru K, Satoh M, Oyama Y (2006) Increase in intracellular Cd(2+) concentration of rat cerebellar granule neurons incubated with cadmium chloride: cadmium cytotoxicity under external Ca(2+)-free condition. Toxicol in Vitro 20:211–216. https://doi.org/10.1016/j.tiv.2005.06.006
Núñez Sellés AJ, Vélez Castro HT, Agüero-Agüero J, González-González J, Naddeo F, De Simone F, Rastrelli L (2002) Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J Agric Food Chem 50:762–766. https://doi.org/10.1021/jf011064b
Okuda B, Iwamoto Y, Tachibana H, Sugita M (1997) Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg 99:263–265
Orisakwe OE (2014) The role of lead and cadmium in psychiatry. N Am J Med Sci 6(8):370–376. https://doi.org/10.4103/1947-2714.139283
Pagel P, Blome J, Wolf HU (2000) High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson's disease. J Chromatogr B Biomed Sci Appl 746:297–304
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Papadoyannis IN, Samanidou VF, Nitsos CC (1999) Simultaneous determination of nitrite and nitrate in drinking water and human serum by high performance anion-exchange chromatography and UV detection. J Liq Chromatogr Relat Technol 22:2023–2041. https://doi.org/10.1081/jlc-100101783
Pillai A, Priya L, Gupta S (2003) Effects of combined exposure to lead and cadmium on the hypothalamic-pituitary axis function in proestrous rats. Food Chem Toxicol 41:379–384
Prasad S, Kalra N, Shukla Y (2007) Hepatoprotective effects of lupeol and mango pulp extract of carcinogen induced alteration in Swiss albino mice. Mol Nutr Food Res 51:352–359. https://doi.org/10.1002/mnfr.200600113
Rana SV, Verma S (1996) Protective effects of GSH, vitamin E, and selenium on lipid peroxidation in cadmium-fed rats. Biol Trace Elem Res 51:161–168. https://doi.org/10.1007/BF02785435
Rivera DG, Hernández I, Merino N, Luque Y, Álvarez A, Martín Y, Amador A, Nuevas L, Delgado R (2011) Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J Pharm Pharmacol 63:1336–1345. https://doi.org/10.1111/j.2042-7158.2011.01328.x
Rocha Ribeiro SM, Queiroz JH, Lopes Ribeiro de Queiroz ME, Campos FM, Pinheiro Sant'ana HM (2007) Antioxidant in mango (Mangifera indica L) pulp. Plant Foods Hum Nutr 62:13–17. https://doi.org/10.1007/s11130-006-0035-3
Sairam K, Hemalatha S, Kumar A, Srinivasan T, Ganesh J, Shankar M, Venkataraman S (2003) Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J Ethnopharmacol 84:11–15
Sarkar A, Sreenivasan Y, Ramesh GT, Manna SK (2004) beta-D-Glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor kappaB but potentiates apoptosis. J Biol Chem 279:33768–33781. https://doi.org/10.1074/jbc.M403424200
Shagirtha K, Muthumani M, Prabu SM (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 15:1039–1050
Shukla GS, Chandra SV (1987) Concurrent exposure to lead, manganese, and cadmium and their distribution to various brain regions, liver, kidney, and testis of growing rats. Arch Environ Contam Toxicol 16:303–310
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405
Soreq H, Seidman S (2001) Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci 2:294–302. https://doi.org/10.1038/35067589
Sreeramulu D, Reddy CV, Chauhan A, Balakrishna N, Raghunath M (2013) Natural antioxidant activity of commonly consumed plant foods in India: effect of domestic processing. Oxidative Med Cell Longev 2013:1–12. https://doi.org/10.1155/2013/369479 369479
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20:77–88
Teerlink T, Hennekes M, Bussemaker J, Groeneveld J (1993) Simultaneous determination of creatine compounds and adenine nucleotides in myocardial tissue by high-performance liquid chromatography. Anal Biochem 214:278–283. https://doi.org/10.1006/abio.1993.1488
Thevenod F, Lee WK (2013) Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol 87:1743–1786. https://doi.org/10.1007/s00204-013-1110-9
Verkhratsky A, Burnstock G (2014) Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 36:697–705. https://doi.org/10.1002/bies.201400024
Viswanadh EK, Rao BN, Rao BS (2010) Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum Exp Toxicol 29:409–418. https://doi.org/10.1177/0960327110361752
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:1–12. https://doi.org/10.1155/2013/898034 898034
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Wang H, Zhang L, Abel GM, Storm DR, Xia Z (2018) Cadmium exposure impairs cognition and olfactory memory in male C57BL/6 mice. Toxicol Sci 161:87–102. https://doi.org/10.1093/toxsci/kfx202
Wattanathorn J, Muchimapura S, Thukham-Mee W, Ingkaninan K, Wittaya-Areekul S (2014) Mangifera indica fruit extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in animal model of mild cognitive impairment. Oxidative Med Cell Longev 2014:132097–132097. https://doi.org/10.1155/2014/132097
Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y, Wang Q (2012) Neurotransmitter receptors and cognitive dysfunction in Alzheimer's disease and Parkinson's disease. Prog Neurobiol 97(1):1–13. https://doi.org/10.1016/j.pneurobio.2012.02.002
Yang Z, Weian C, Susu H, Hanmin W (2016) Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol 771:145–151. https://doi.org/10.1016/j.ejphar.2015.12.003
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One 8:e64330. https://doi.org/10.1371/journal.pone.0064330
Zhang P, Bannon NM, Ilin V, Volgushev M, Chistiakova M (2015) Adenosine effects on inhibitory synaptic transmission and excitation-inhibition balance in the rat neocortex. J Physiol 593:825–841. https://doi.org/10.1113/jphysiol.2014.279901
Zhao P, Zhou T, Zhu WW, An L, Peng J, Sun DQ (2009) WITHDRAWN: the changes of apoptotic signaling in cerebral cortex and hippocampus of cadmium-treated rats. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2009.02.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Al omairi, N.E., Radwan, O.K., Alzahrani, Y.A. et al. Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab Brain Dis 33, 1121–1130 (2018). https://doi.org/10.1007/s11011-018-0222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0222-6