Abstract
The present study was designed to investigate the effects of LY294002 on Tourette syndrome (TS) in rats. TS model was induced in rats by DOI (the selective 5-HT2A/2C agonist 1- (2, 5- dimethoxy −4 - iodophenyl) -2- aminopropane). Behavior was assessed by stereotypic score and autonomic activity. Inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in serum and striatum were detected. The protein levels of PI3K/Akt/NF-B in striatum were detected by Western Blot. LY294002 treatment significantly reduced IL-6, IL-1β and TNF-α in serum and striatum of TS rats, Also, highly expressed P-PI3K, P-Akt, P-NF-κBp65, P-IκBα in TS rats were restored respectively by LY294002 treatment as indicted in western blot analysis and immunohistochemistry analysis. Thus, it was supposed that the protective effect of LY294002 against TS in rat might be associated with the regulation of PI3K/Akt/NF-B pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourette syndrome is a severe, recurrently neurological disorder that occurs in patients before the age of 18 years. The illness is characterized by chronic muscle movements and phonic tics for over a year (Leckman 2002). Sometimes, patients with Tourette syndrome are associated with sensory symptoms, such as premonitory urges, which incessantly promote tics and feelings of momentary relief that follow representation of tic expression (Peterson and Leckman 1998). More often, a large proportion of patients with Tourette syndrome have co-occurring symptoms of behavioral difficulties, such as dis-inhibited conduct or speech, impulsivity, motoric hyperactivity, distractibility, and obsessive–compulsive symptoms, which worse functional outcomes and impair family life and social acceptance (Bloch et al. 2011). Although the etiology of Tourette syndrome has not yet fully elucidated, a genetic component and abnormalities of central inflammation and oxidative stress are strongly suggested.
Inflammation has been suggested to participate in the pathophysiological process during Tourette syndrome. Elevated inflammatory factors, such as interleukin-1β, interferon-γ, and interleukin-2 in basal ganglia of patients with Tourette syndrome were reported in recent research (Morer et al. 2010). The indirect evidence of inflammation was recorded in basal ganglia by a longitudinal imaging method, with acute basal ganglia enlargement and obsessive-compulsive symptoms (Giedd et al. 1996). It has been known that the PI3K/Akt pathway is implicated in the negative regulation of NF-κB activation and expression inflammatory cytokines which is believed to be implicated in the development of neuroinflammations and neuropathology of central nervous system diseases (Lv et al. 2017; Zhao et al. 2014). LY294002, a PI3K inhibitor, is believed to be implicated in the development of nervous system diseases (Tu et al. 2016; Zhao et al. 2016). Inhibition of PI3K/Akt pathway showed beneficial effects in central nervous system diseases (Zhang et al. 2016; Wang et al. 2015).
This work was aimed to further illustrated the mechanisms underling the protective effects of LY294002 against Tourette syndrome.
Material and methods
Chemicals and reagents
LY294002 was purchased from Tianjin Chase Sun Pharmaceutical. Co., Ltd. (Tianjin, China). DOI (the selective 5-HT2A/2C agonist 1-(2,5-dimethoxy-4-iodophenyl) -2-aminopropane) was obtained from Sigma-Aldrich (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for the detection of IL-6, IL-1β and TNF-α were produced by Nanjing KeyGEN Biotech. CO., LTD. (Nanjing, China).
Animals
Forty male Wistar rats, weighting 180–200 g, were purchased from Beijing vital river co., LTD (License number: SCXK (Jing) 2012–0001) and housed under a 12 h/12 h light/dark cycle environment. Rats were allowed to adapt the environment for 1 week at a temperature of 22 ± 1 °C and 40–70% humidity, with free to standard food and water. All of the experiments were performed in full compliance with the guidelines of the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH Publication No. 85–23, revised 1996). Animal care and experimental protocols were approved by the Nanjing University of Chinese Medicine Committee.
Experimental protocols
Rats were randomly separated into four groups (with 10 rats in each group): control group, DOI treated group, DOI treated with tiapride (25 mg/kg) group, DOI treated with LY294002 (PI3K inhibitor, 25 μg/kg dissolved in 10% dimethyl sulfoxide) group, Tourette Syndrome was induced in rats by DOI intraperitoneal injection at dosage of 1 mg/kg, once daily for 21 days continuously. Control group and DOI group were intraperitoneal injection with normal saline or DOI, while tiapride group was treated with intracerebroventricularly injected tiapride orally as the positive. LY294002 group was treated with intracerebroventricularly injected LY294002.
Behavioral testing
Stereotypy recording
Stereotypy recording was conducted by two trained observers who were familiar with the measurements but blind to the group condition. For evaluating the stereotypy, each animal was observed for 2 min after DOI injection and drug administrations. The average score was calculated for each rat.
Autonomic activity test
Autonomic activity test was conducted. We connected the animal behavior analysis system with a spontaneous activity video analysis system. The sequence of each group was random. One rat was placed in every autonomic activity box. Before recording, the rat was allowed to adapt to the environment for 5 min. Then, the activity of each rat was recorded for 5 min. We chose the total distance as the objective indicator to judge the autonomic activity of the rat. The box was kept in shade, and the environment was quiet.
Evaluations of inflammatory cytokines in serum and striatum
Rats striatum were carefully dissected out under magnifying glass on ice. The level of IL-1β, IL-6, TNF-α in serum and striatum were detected by enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Nanjing KeyGEN Biotech. CO., LTD., Nanjing, China). The results of the concentrations of inflammatory cytokines were expressed as pictograms per milligram protein.
Western blot
Striatum tissue and primary neuron cultures were homogenized in ice-cold RIPA buffer containing 0.1% phenylmethylsulfonyl fluoride. The total protein content was quantified by Bicinchoninic acid (BCA) protein assay kits (Beyotime, Nanjing, China) Equal amounts of protein were loaded on 8% to 12% SDS-polyacrylamide gel electrophoresis. The transferred PVDF membranes from SDS-polyacrylamide gel electrophoresis were blocked in skim milk at room temperature over 2 h. Then the PVDF membranes were incubated with the appropriate concentration of specific antibodies overnight at 4 °C. On the second day, PVDF membranes were incubated with second antibody at room temperature for 1 h after washing three times by TBST. The immunoreactive bands were interacted with an enhanced chemiluminescence (ECL) kit and visualized on a gel imaging system (Tanon Science & Technology Co., Ltd., China).
Immunohistochemistry
The expressions of P-PI3K and p-NF-κBp65 in the striatum were evaluated by immunohistochemistry staining. In brief, the striatum tissues were embedded in paraffin and sectioned. Then, the paraffin sections were deparaffinized in xylene, rehydrated by ethanol and incubated with 3% hydrogen peroxide. Striatum samples were blocked with 3% BSA and incubated with respective primary antibody at 4 °C overnight. After incubated with secondary and three antibodies, samples were stained with DAB and observed under a microscope.
Results
The effects of LY294002on stereotypy score
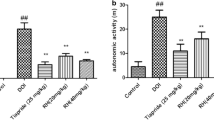
As shown in Fig.1a, Rat model groups with TS induced by LY294002 showed abnormal stereotypes in different degrees compared with the control group, LY294002 showed a significant decrease compared with the model group.
The effects of LY294002 on autonomic activity
As shown in Fig.1b, the total distance of rat model groups with TS induced by DOI showed a significant increase compared with the control group, LY294002 showed a significant decrease compared with the model group.
Effects of LY294002 on inflammatory cytokines in serum and striatum
To investigate inflammatory responses triggered by DOI stimulation, we detected the concentrations of IL-1β, IL-6, TNF-α both in serum and striatum. As expected, the release of inflammatory cytokines IL-1β, IL-6, TNF-α were significantly increased serum and striatum in DOI-treated rats compared with the control group. LY294002 or Tiapride significantly decreased the levels of inflammatory cytokines in serum and stratum induced by DOI exposure (Fig. 2).
Effects of LY294002 on PI3k/Akt/NF-κB pathway in striatum
To further investigate the possible mechanisms of LY294002, the expressions of PI3k/Akt/NF-κB pathway were detected in striatum. As shown in Fig. 3, increased levels of P-PI3K, P-Akt, p-NF-κBp65, p-IκBα were observed in DOI rats compared with those in the control group. In contrast, administration of LY294002 or tiapride treatment were able to reverse the effects of DOI injection o inflammation related proteins.
Effects of LY294002 on P-PI3Kand P-NF-кB pathway in striatum
The data depicted in Fig. 4. demonstrated the highly expressed P-PI3K and p-NF-κBp65 in DOI-stimulated rats from immunohistochemistry. LY294002 reduced the expressions of P-PI3K and p-NF-κBp65, which further confirmed the involvement of inflammation pathway in DOI-induced inflammation in striatum.
Discussion
Tourette Syndrome is a childhood-onset, relapsing disorder diagnosed by involuntary motor and phonic tics, with a high comorbidity with obsessive-compulsive disorder. Although up to 0.1 to 1% of the population has been affected by Tourette syndrome, especially males diagnosed more often than females (Peterson and Leckman 1998), the scientific evidence and research of Tourette syndrome is relative scare compared with other central neural system diseases. The lack of information about Tourette syndrome possibly because this illness is a non-fatal disorder, mostly outgrown in late adolescence (Leckman 2002). TS has been associated with dysfunctional signaling by the neuromodulator dopamine, which is strongly linked to mechanisms of reinforcement learning. Other biochemical pathways, including histaminergic neurotransmission and amino acid neurotransmission, are likely to be involved TS (Cox et al. 2016), and our studies have reported LY294002 could regulate histaminergic neurotransmission in TS model (Hongyan and Zhangpiao 2010; Piao et al. 2010). In this study, we focused on stress and inflammation of striated in TS model. LY294002, a inhibitor of PI3K which exerts beneficial effects to Tourette syndrome patients. Our present research further evidence that LY294002 administration is favorable to Tourette syndrome, which significantly attenuated DOI-induced injuries. The therapeutic effects of LY294002 might involve with PI3K/Akt mediated neuroinflammation pathway, which provided further evidence for its clinical application
Neuroinflammation is referred to the activation of immune responses in the brain, during which, immune cells are activated and, as a consequence, inflammatory cytokines are over-produced, including IL-6 and TNF-α (Streit 2006; Dobos et al. 2010). Neuroinflammation has been investigated in a list of central nervous system diseases, such as major depression, Alzheimer’s disease, stroke and so forth (Chen et al. 2016; Deng et al. 2015a; Zhu et al. 2015; Chen et al. 2015). However, the investigations on inflammatory responses in central neural system of Tourette syndrome are few. The lack of data about inflammation in brain of Tourette syndrome patients is most likely due to ethical constrains for obtaining cerebrospinal fluid from pediatric patients (Morer et al. 2010). As manifested in the study, DOI stimulation caused brain inflammatory responses as indicated by the increased levels of IL-1β, TNF-α and IL-6 in striatum and serum of model mice. These data suggested that peripheral and central inflammatory were both emerged during the progression of Tourette symptom. Further evidence can be seen from the results in vitro, which DOI challenge increased the concentrations of inflammatory cytokines. LY294002 administration significantly alleviated DOI-induced elevation of inflammatory cytokines.
The PI3K/Akt and NF-κB pathway are conserved mechanisms in maintaining cellular homeostasis. In our present study, we showed that PI3K/Akt and NF-κB pathway are activated in DOI-induced animal model of Tourette symptom. LY294002 administration or tiapride significantly inhibited the expression of PI3K/Akt patway. Under basal conditions, NF-κB is distributed in the cytosol binding to its inhibitor IκB. Upon activation, IκBα is degraded by the IκB kinase (IKK) complex, which drives NF-κB translocates into the nucleus and induces the transcription and expression of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α (Rutledge and Adeli 2007; Deng et al. 2015b). The ROCK regulated NF-κB signaling was examined in the present study, our findings showed that LY294002 administration or tiapride treatment inhibited IkBα degradation. Also, we found that DOI-induced nuclear translocation of NF-kB p65 was inhibited in response to LY294002 administration. Our results were consistent with previous studies that NF-κB pathway in response to stimuli could be regulated by PI3K/Akt, and suggested a previously unidentified regulatory system for the effects of LY294002 in Tourette symptom models.
In sum, LY294002 treatment significantly relieved Tourette syndrome induced by DOI, 5HT2A/c receptor agonist, indicating a therapeutic application of LY294002 in tics. Moreover, LY294002 treatment significantly attenuated the symptoms of Tourette syndrome and reduced PI3K/Akt/NF-κB mediated neuroinflammation, further suggesting the pharmacology actions of this clinical efficient agent.
References
Bloch M, State M, Pittenger C (2011) Recent advances in Tourette syndrome. Curr Opin Neurol 24:119–125
Chen T, Ma Z, Zhu L, Jiang W, Wei T, Zhou R et al. (2015) Suppressing Receptor-Interacting Protein 140: a New Sight for Salidroside to Treat Cerebral Ischemia. Mol Neurobiol
Chen J, Deng X, Liu N, Li M, Liu B, Fu Q et al (2016) Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J Funct Foods 22:463–476
Cox JH, Seri S, Cavanna AE (2016) Histaminergic modulation in Tourettesyndrome. Expert Opin Orphan Drugs 4(2):205–213
Deng XY, Li HY, Chen JJ, Li RP, Qu R, Fu Q et al (2015a) Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res 291:12–19
Deng XY, Chen JJ, Li HY, Ma ZQ, Ma SP, Fu Q (2015b) Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem Biol Interact 240:22–28
Dobos N, Korf J, Luiten PGM, Eisel ULM (2010) Neuroinflammation in Alzheimer's disease and major depression. Biol Psychiatry 67:503–504
Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE (1996) Case study: acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry 35:913–915
Hongyan L, Zhangpiao TX (2010) Effect of Jian oral liquid on Tourette's behavior and content of 5-HT in brain and plasma in rats of Tourette syndrome. Chin J Basic Med Tradit Chin Med 16:8
Leckman JF (2002) Tourette's syndrome. Lancet 360:1577–1586
Lv J, Wei Y, Chen Y, Zhang X, Gong Z, Jiang Y, Gong Q, Zhou L, Wang H, Xie Y (2017) Dexmedetomidine attenuates propofol-induce neuroapoptosis partly via the activation of the PI3k/Akt/GSK3β pathway in the hippocampus of neonatal rats. Environ Toxicol Pharmacol 52:121–128
Morer A, Chae W, Henegariu O, Bothwell ALM, Leckman JF, Kawikova I (2010) Elevated expression of MCP-1, IL-2 and PTPR-N in basal ganglia of Tourette syndrome cases. Brain Behav Immun 24:1069–1073
Peterson BS, Leckman JF (1998) The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry 44:1337–1348
Piao Z, Hongyan L, Tan X, Lingling D (2010) Effect of Jinan oral liquid on opamine and homovanillic acid in plasma and brain tissue of rats with Tourette syndrome. Chin J Exp Tradit Med Form 16(1)
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23
Streit WJ (2006) Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci 29:506–510
Tu XK, Zhang HB, Shi SS, Liang RS, Wang CH, Chen CM, Yang WZ (2016) 5-LOX inhibitor Zileuton reduces inflammatory reaction and ischemic brain damage through the activation of PI3K/Akt signaling pathway. Neurochem Res 41(10):2779–2787
Wang YK, Deng F, Miao J, Xie H, Feng JC (2015) Neuroprotection by Carbenoxolone against ischemia injury involves PI3K/Akt pathway. Clin Lab 61(10):1561–1568
Zhang H, Xiong X, Liu J, Gu L, Li F, Wan Y, Xu S (2016) Emulsified Isoflurane Protects Against Transient Focal Cerebral Ischemia Injury in Rats via the PI3K/Akt Signaling Pathway. Nesth Analg 122(5):1377–1384
Zhao M, Zhou A, Xu L, Zhang X (2014) The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Oncotarget Neurosci 269:93–101
Zhao W, Pan X, Li T, Zhang C, Shi N (2016) Lycium barbarum Polysaccharides protect against Trimethyltin chloride-induced apoptosis via sonic hedgehog and PI3K/Akt signaling pathways in mouse neuro-2a cells. Oxidative Med Cell Longev 2016:982672
Zhu L, Wei T, Gao J, Chang X, He H, Luo F et al (2015) The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis 20:1433–1443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No potential conflicts of interest were disclosed.
Grant support
This project was supported by Program for the Human Resources and Social Security Department of Jiangsu Province–“Six Talent Summit”(WSN-061)
Rights and permissions
About this article
Cite this article
Hongyan, L., Chunyan, W. & Yue’e, Y. LY294002, a PI3K inhibitor, attenuates Tourette syndrome in rats. Metab Brain Dis 32, 1619–1625 (2017). https://doi.org/10.1007/s11011-017-0051-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0051-z