Abstract
Maple Syrup Urine Disease (MSUD) is an inborn error of metabolism caused by a deficiency of the branched-chain α-keto acid dehydrogenase complex activity. This blockage leads to accumulation of the branched-chain amino acids leucine, isoleucine and valine, as well as their corresponding α-keto acids and α-hydroxy acids. The affected patients present severe neurological symptoms, such as coma and seizures, as well as edema and cerebral atrophy. Considering that the mechanisms of the neurological symptoms presented by MSUD patients are still poorly understood, in this study, protein levels of apoptotic factors are measured, such as Bcl-2, Bcl-xL, Bax, caspase-3 and −8 in hippocampus and cerebral cortex of rats submitted to acute administration of branched-chain amino acids during their development. The results in this study demonstrated that BCAA acute exposure during the early postnatal period did not significantly change Bcl-2, Bcl-xL, Bax and caspase-8 protein levels. However, the Bax/Bcl-2 ratio and procaspase-3 protein levels were decreased in hippocampus. On the other hand, acute administration of BCAA in 30-day-old rats increase in Bax/Bcl-2 ratio followed by an increased caspase-3 activity in cerebral cortex, whereas BCAA induces apoptosis in hippocampus through activation and cleavage of caspase-3 and −8 without changing the Bax/Bcl-2 ratio. In conclusion, the results suggest that apoptosis could be of pivotal importance in the developmental neurotoxic effects of BCAA. In addition, the current studies also suggest that multiple mechanisms may be involved in BCAA-induced apoptosis in the cerebral cortex and hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maple syrup urine disease (MSUD; branched-chain keto aciduria) is an autosomal recessive disorder caused by the deficiency of branched chain α-ketoacid dehydrogenase complex (BCKAD, E.C. 1.2.4.4) activity. The metabolic defect leads to accumulation of the branched-chain amino acids (BCAA) leucine (Leu), isoleucine (Ile) and valine (Val) and the corresponding branched-chain α-ketoacids (BCKA), α-ketoisocaproic acid (KIC), α-keto-β-methylvaleric acid (KMV) and α-ketoisovaleric acid (KIV) in tissues and body fluids (Chuang and Shih 2001; Treacy et al. 1992).
MSUD presents as heterogeneous clinical and molecular phenotypes, characterized by ketoacidosis, hypoglycemia, poor feeding, apnea, ataxia, convulsions, coma, psychomotor delay and mental retardation, as well as generalized edema in the central nervous system (CNS), atrophy of the cerebral hemispheres, white matter spongy degeneration and delayed myelinization (Chuang and Shih 2001; Schonberger et al. 2004). The mechanisms of brain damage in MSUD are still poorly understood; it has been postulated that the metabolites accumulating in MSUD may affect energy metabolism in rat brain (Howell and Lee 1963; Land et al. 1976; Pilla et al. 2003; Ribeiro et al. 2008; Sgaravatti et al. 2003) and may cause significant alterations of the concentrations of the neurotransmitters glutamate, aspartate and aminobutyric in the brain (Dodd et al. 1992; Prensky and Moser 1966; Tavares et al. 2000; Yudkoff et al. 1994). The brain injury in this disorder may also be related to reduction of brain uptake of essential amino acids (Araujo et al. 2001), apoptosis of neural cells (Jouvet et al. 1998, 2000a, b) oxidative stress (Barschak et al. 2008a, b, 2009; Bridi et al. 2003, 2005; Fontella et al. 2002; Mescka et al. 2011) and DNA damage (Mescka et al. 2014, 2015; Scaini et al. 2012).
Studies have also reported altered apoptotic factors and their mediated responses in MSUD. Changes include DNA damage, mitochondrial dysfunction and enhanced oxidative stress, as described above. Additionally, magnetic resonance imaging studies in children with MSUD have confirmed both white matter and neuronal injury, including extensive brain edema and pathological changes in the basal ganglia (Brismar et al. 1990; Steinlin et al. 1998). At the histopathological level, deficiencies in myelination of major tracts in the pons and spinal cord, widespread areas of spongy change in the white matter, focal areas of astrocytosis, and binucleated neurons have also been reported (Langenbeck 1984). These studies implicate the involvement of cell death in the pathophysiology of neurological dysfunction in MSUD. To further clarify the possible involvement of apoptosis in MSUD, protein levels of apoptotic factors are measured, such as Bcl-2, Bcl-xL, Bax, caspase-3 and −8 in hippocampus and cerebral cortex of rats submitted to acute administration of BCAA during their development. For this purpose, a chemically-induced model of MSUD, which produce high sustained levels of BCAA that are similar to those found in plasma of MSUD patients, is used.
Materials and methods
Animals
Male Wistar rats at 10 and 30 days old (weighing 20–25 g, and 80–100 g, respectively) were obtained from the Central Animal House of the Universidade do Extremo Sul Catarinense. Ten-day-old rats were left with their dams until the day of the experiment, and the 30-day-old rats were weaned at 21 days of life. All rats were caged in groups of 5 with free access to food and water and were maintained on a 12-h light/dark cycle (lights on 7:00 am) at a temperature of 23 ± 1 °C. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior recommendations for animal care, with the approval of the Ethics Committee of the Universidade do Extremo Sul Catarinense (protocol number 60/2010).
Acute administration of BCAA pool
The animals received three subcutaneous administrations of BCAA pool (15.8 μL/g body weight at 1-h intervals) containing 190 mmol/L leucine, 59 mmol/L isoleucine, and 69 mmol/L valine in saline solution (0.85 % NaCl) or saline alone (control group). The BCAA pool and saline solution were given to rats on postnatal day (PD) 10 or PD 30 (n = 6). One hour after the last injection, the animals were sacrificed by decapitation, the brain was rapidly removed, and the hippocampus and cerebral cortex were collected. The choice of doses of BCAA and age of animals were based on a previous study (Bridi et al. 2006) showing that the administration of BCAA pool to rats (doses and ages similar to those used in the present study) resulted in increased levels of leucine, isoleucine and valine in blood and brain, mimicking the main biochemical finding observed in MSUD patients during crises.
Immunoblotting
To perform the immunoblot experiments, the samples were first homogenized in Laemmli-sample buffer (62.5 mM Tris–HCl, pH 6.8, 1 % (w/v) SDS, 10 % (v/v) glycerol). Equal amounts of protein (30 μg/well) were fractionated by SDS-PAGE and electro-blotted onto nitrocellulose membranes. The protein loading and electro-blotting efficiency were verified with Ponceau S staining. The membranes were blocked in Tween-Tris buffered saline (TTBS: 100 mM Tris–HCl, pH 7.5, containing 0.9 % NaCl and 0.1 % Tween-20) containing 5 % albumin. The membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody against Bcl-2 Antibody (Cell Signaling - 2876), Bcl-xL (Cell Signaling - 2762), Bax (Cell Signaling - 2772), caspase-3 and −8 (Cell Signaling – 9662, 4927, respectively). The primary antibody was then removed and the membranes were washed 4 times for 15 min. After washing, an anti-rabbit IgG peroxidase linked secondary antibody was incubated with the membranes for 1 h (1:10000 dilution) and the membranes were washed again. Finally, the immunoreactivity was detected using an enhanced chemiluminescence ECL Plus kit. After exposure, the membranes were then stripped and incubated with a mouse monoclonal antibody to β-Actin (sigma - A2228) in the presence of 5 % milk. An anti-mouse IgG peroxidase linked secondary antibody was incubated with the membranes for 1 h (1:10000 dilution) and the membranes were washed again. The immunoreactivity was detected using an enhanced chemiluminescence ECL Plus kit. Densitometry was performed using Image J v.1.34 software. SeeBlue ® Plus2 Prestained Standard (Invitrogen) was used as a molecular weight marker to make sure that the correct bands were analyzed for Bcl-2, Bcl-xL, Bax, caspase-3, caspase-8 and β-Actin.
Caspase activity measurement
The caspase-3 and −8 activities in the hippocampus and cerebral cortex [homogenised in lysis buffer (50 mM HEPES, pH 7.5, 0.1 % CHAPS, 2 mM dithiothreitol, 0.1 % Nonidet P-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leu-peptin, and 2 μg/ml pepstatin] were determined by monitoring proteolysis of the appropriate fluorochrome substrates. DEVD-AMC and Z-IETD-AFC served as substrates employed to detect caspase-3 and −8 enzyme activities, respectively. Briefly, 50 μg of tissue homogenate protein was added to a buffer containing 100 μl of caspase buffer with 50 μM of appropriate caspase-specific substrate (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 1 h at 37 °C. Fluorescence was read on a SpectraMax® M2 Microplate Reader (Molecular Devices, Sunnyvale, CA) at an excitation of 355 nM and an emission of 460 nM.
Statistical analysis
Results are presented as the mean ± standard deviation. All assays were performed in duplicate, and the mean was used for statistical analysis. Tests for determination of normality and equal variances were performed to examine whether the data qualified for parametric statistical tests. The data were normally distributed (Shapiro–Wilk, P > 0.05) with equal variances among samples (equal variances test, P > 0.05). Thus, Student’s t test was used for the comparison of two means. Differences between the groups were considered to be significant at p < 0.05. All analyses were carried out on an IBM-compatible PC computer using the Statistical Package for the Social Sciences software (Armonk, New York, USA).
Results
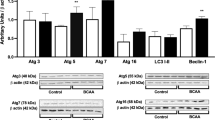
To determine whether BCAA leads to changes in Bcl-2 family protein levels in hippocampus and cerebral cortex after acute administration of BCAA in 10 and 30-day-old rats, the Bcl-xL, Bcl-2 and Bax protein levels were examined. Figure 1a shows that there was no significant difference in protein level of pro-apoptotic factor Bax and anti-apoptotic factors Bcl-2 and Bcl-xL among groups in the hippocampus and cerebral cortex after acute administration of BCAA in 10-day-old rats. In addition, the Bax/Bcl-2 ratio was lower in hippocampus and cerebral cortex in BCAA group when compared with control (Fig. 1b). On the other hand, the acute administration of BCAA in 10-day-old rats decreased protein levels of procaspase-3 without altering the activity of caspase-3 in hippocampus, but not in cerebral cortex (Fig. 2a). Moreover, BCAA administration did not change protein levels of procaspase-8 and caspase-8 activity in hippocampus and cerebral cortex of 10-day-old rats (Fig. 2b).
Effect of acute administration of branched-chain amino acids on Bax, Bcl-2 and Bcl-xL (a) and Bax/Bcl-2 ratio (b) in hippocampus and cerebral cortex of 10-day-old rats. Data are ratios of optical density of Bcl-2, Bcl-xL and Bax to β-actin, expressed as arbitrary units/ β-actin. Data are expressed as mean ± standard deviation for 5–6 animals per group. Different from control, *p < 0.05 (Student’s t test)
Effect of acute administration of branched-chain amino acids on protein level of procaspase-3 and −8 (a), and enzyme activity levels for caspase-3 and −8 (b) in hippocampus and cerebral cortex of 10-day-old rats. Data are expressed as mean ± standard deviation for 5–6 animals per group. Different from control, *p < 0.05 (Student’s t test)
According to the data presented in Fig. 3a, the protein levels of Bax were increased significantly in the cerebral cortex of 30-day-old rats after acute administration of BCAA, while protein levels of Bcl-2 and Bcl-xL were not affect. On the other hand, in the hippocampus of 30-day-old rats the acute administration of BCAA increased the protein levels of Bcl-2 and Bcl-xL, and did not affect the protein levels of Bax. Additionally, the BCAA administration increased significantly the Bax/Bcl-2 ratio in cerebral cortex when compared with control (Fig. 3b).
Effect of acute administration of branched-chain amino acids on Bax, Bcl-2 and Bcl-xL (a) and Bax/Bcl-2 ratio (b) in hippocampus and cerebral cortex of 30-day-old rats. Data are ratios of optical density of Bcl-2, Bcl-xL and Bax to β-actin, expressed as arbitrary units/ β-actin. Data are expressed as mean ± standard deviation for 5–6 animals per group. Different from control, *p < 0.05 (Student’s t test)
Western blot analysis showed that the levels of the procaspase-3 and −8 were significantly decreased in hippocampus of 30-day-old rats after acute administration of BCAA. However, the activity of caspase-3 and −8 were increased significantly (Fig. 4a). Moreover, a significant increase in the levels of procaspase-3 accompanied by an increase in caspase-3 activity was found in cerebral cortex of 30-day-old rats submitted to acute administration of BCAA (Fig. 4b).
Effect of acute administration of branched-chain amino acids on protein level of procaspase-3 and −8 (a), and enzyme activity levels for caspase-3 and −8 (b) in hippocampus and cerebral cortex of 30-day-old. Data are ratios of optical density of caspase-3 and caspase-8 to β-actin, expressed as arbitrary units/ β-actin. Data are expressed as mean ± standard deviation for 5–6 animals per group. Different from control, *p < 0.05 (Student’s t test)
Discussion
Acute neurological deterioration in children is often associated with increased plasma and cerebrospinal fluid concentrations of BCAA and BCKA (Levin et al. 1993; Riviello et al. 1991). In neurological disorders, cell death occurs by necrosis or apoptosis depending on the specific disease or insult (Friedlander 2003; Kanazawa 2001; Vajda 2002). The pathway for these processes can vary and the same players may participate in both pathways. Neurological sequelae are common in MSUD patients. Brain imaging methods have structurally defined the pathophysiology seen in persons with uncontrolled MSUD, but not the cellular events that cause the dysmorphology (Brismar et al. 1990; Holmes et al. 1997; Korein et al. 1994; Ong et al. 1998; Riviello et al. 1991; Uziel et al. 1988).
In this study, it was demonstrated that BCAA acute exposure during the early postnatal period (postnatal day 10) did not significantly change Bcl-2, Bcl-xL, Bax and procaspase-8 protein levels and caspase-8 activity. On the other hand, the procaspase-3 protein levels were decreased without altering the activity of caspase-3 in hippocampus. In addition, BCAA administration in infant rats decreased Bax/Bcl-2 ratios in hippocampus and cerebral cortex. The results suggest that acute administration of BCAA during early postnatal period does not induce apoptosis by mechanisms evaluated in this study, but a possibility that other pathway may lead to cell death at this stage of development cannot be ruled out. Corroborating this hypothesis, Jouvet et al. (2000b) showed that direct intracerebral injection of KIC leads to neuronal apoptosis in the hippocampus of neonatal rats in a dose-dependent manner.
Briefly, the proapoptotic Bax and antiapoptotic Bcl-2 are membrane-bound pore-forming proteins that interact through heterodimerization. Together, they regulate the mitochondrial transmembrane passage of cytochrome c, which in turn activates caspase proteins. The Bax/Bcl-2 ratio appears more important than the individual Bax or Bcl-2 levels on determining a cell vulnerability to apoptosis; high Bax/Bcl-2 ratios lead to greater apoptotic activity (Oltvai et al. 1993). Neuronal apoptosis in the CNS is significantly lower than normal in Bax-deleted mice and in Bcl-2-overexpressing mice, while in Bcl-2-deleted mice and in Bax-overexpressing mice, apoptosis is greater than normal (Vekrellis et al. 1997). Furthermore, the ratio between Bax/Bcl-2 appears to be essential in deciding the survival of a cell (Korsmeyer 1999; White 1996). In this study we showed that BCAA administration in 30-day-old rats induces increase in Bax/Bcl-2 ratio followed by an increased caspase-3 activity in cerebral cortex, suggesting that apoptosis is activated after BCAA exposure in 30-day-old rats and may contribute to secondary cell injury and cell death. The increased activities of caspase-3 induced by BCAA suggests that BCAA-induced apoptosis in the cerebral cortex was likely mediated by mitochondria/cytochrome c pathway.
On the other hand, our results shown that BCAA induces apoptosis in hippocampus through activation and cleavage of caspase-3 and −8 without changing the Bax levels. Caspase-8 may be involved in death receptor-mediated apoptosis pathway (Ashkenazi and Dixit 1998). Following trimerization of the Fas receptor, procaspase-8, one of the most proximal members of caspases, is recruited to the Fas receptor–FADD complex and is physically associated with Fas-Associated protein with Death Domain (FADD) via a death effector domain (DED) homology interaction (Chinnaiyan and Dixit 1997), resulting in the activation of procaspase-8 to activated caspase-8 and finally to the activation of caspase-3. In this line, we suggest that BCAA induces apoptosis in hippocampus through extrinsic (death receptor) pathways. Supporting this hypothesis Jouvet et al. (2000b) showed that apoptosis induced by BCAA was associated with a reduction in cell respiration but without impairment of respiratory chain function, without early changes in mitochondrial membrane potential and without cytochrome c release into the cytosol.
Based on the results, we suggest that BCAA can induce apoptosis in the cerebral cortex and hippocampus by multiple mechanisms, through activation of both intrinsic and extrinsic pathways, and these mechanisms can be negatively regulated by antiapoptotic proteins Bcl-2 and Bcl-xL. However, the acute administration of BCAA during early postnatal period does not induce apoptosis by mechanisms evaluated in this study. This raises questions regarding the potential age-related differences in apoptotic response to BCAA. Further study will be required to identify the apoptotic pathways and mediators, which cause the resistance to BCAA-induced apoptosis in neonate rats. In conclusion, these results suggest that during crises of metabolic decompensation, as observed in untreated MSDU patients, when brain is exposed to high concentrations of BCAA (at millimolar concentrations) and the metabolites, various deleterious mechanisms might be triggered, including apoptosis. These observations may explain, at least in part, the neurological sequelae associated with high plasma concentrations of MSUD metabolites.
References
Araujo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Wajner M (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281:1305–1308
Barschak AG, Marchesan C, Sitta A, Deon M, Giugliani R, Wajner M, Vargas CR (2008a) Maple syrup urine disease in treated patients: biochemical and oxidative stress profiles. Clin Biochem 41:317–324
Barschak AG, Sitta A, Deon M, Barden AT, Dutra-Filho CS, Wajner M, Vargas CR (2008b) Oxidative stress in plasma from maple syrup urine disease patients during treatment. Metab Brain Dis 23:71–80
Barschak AG, Sitta A, Deon M, Busanello EN, Coelho DM, Cipriani F, Dutra-Filho CS, Giugliani R, Wajner M, Vargas CR (2009) Amino acids levels and lipid peroxidation in maple syrup urine disease patients. Clin Biochem 42:462–466
Bridi R, Araldi J, Sgarbi MB, Testa CG, Durigon K, Wajner M, Dutra-Filho CS (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, Dutra-Filho CS (2005) alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis 20:155–167
Bridi R, Fontella FU, Pulrolnik V, Braun CA, Zorzi GK, Coelho D, Wajner M, Vargas CR, Dutra-Filho CS (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Brismar J, Aqeel A, Brismar G, Coates R, Gascon G, Ozand P (1990) Maple syrup urine disease: findings on CT and MR scans of the brain in 10 infants. AJNR Am J Neuroradiol 11:1219–1228
Chinnaiyan AM, Dixit VM (1997) Portrait of an executioner: the molecular mechanism of FAS/APO-1-induced apoptosis. Semin Immunol 9:69–76
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, Johnston GA (1992) Glutamate and gamma-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem 59:582–590
Fontella FU, Gassen E, Pulrolnik V, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2002) Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348:1365–1375
Holmes E, Foxall PJ, Spraul M, Farrant RD, Nicholson JK, Lindon JC (1997) 750 MHz 1H NMR spectroscopy characterisation of the complex metabolic pattern of urine from patients with inborn errors of metabolism: 2-hydroxyglutaric aciduria and maple syrup urine disease. J Pharm Biomed Anal 15:1647–1659
Howell RK, Lee M (1963) Influence of alpha-ketoacids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Jouvet P, Rustin P, Felderhoff U, Pocock J, Joashi U, Mazarakis ND, Sarraf C, Edwards AD, Mehmet H (1998) Maple syrup urine disease metabolites induce apoptosis in neural cells without cytochrome c release or changes in mitochondrial membrane potential. Biochem Soc Trans 26:S341
Jouvet P, Kozma M, Mehmet H (2000a) Primary human fibroblasts from a maple syrup urine disease patient undergo apoptosis following exposure to physiological concentrations of branched chain amino acids. Ann N Y Acad Sci 926:116–121
Jouvet P, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H (2000b) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 11:1919–1932
Kanazawa I (2001) How do neurons die in neurodegenerative diseases? Trends Mol Med 7:339–344
Korein J, Sansaricq C, Kalmijn M, Honig J, Lange B (1994) Maple syrup urine disease: clinical, EEG, and plasma amino acid correlations with a theoretical mechanism of acute neurotoxicity. Int J Neurosci 79:21–45
Korsmeyer SJ (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59:1693s–1700s
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and beta-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Langenbeck U (1984) Pathobiochemical and pathophysiologic analysis of the MSUD phenotype. In: Adibi SA, Fekl W, Langenbeck U, Schauder P (eds) Branched chain amino and keto acids in health and disease. Karger, Basel, pp 315–334
Levin ML, Scheimann A, Lewis RA, Beaudet AL (1993) Cerebral edema in maple syrup urine disease. J Pediatr 122:167–168
Mescka C, Moraes T, Rosa A, Mazzola P, Piccoli B, Jacques C, Dalazen G, Coelho J, Cortes M, Terra M, Regla Vargas C, Dutra-Filho CS (2011) In vivo neuroprotective effect of L-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26:21–28
Mescka CP, Wayhs CA, Guerreiro G, Manfredini V, Dutra-Filho CS, Vargas CR (2014) Prevention of DNA damage by L-carnitine induced by metabolites accumulated in maple syrup urine disease in human peripheral leukocytes in vitro. Gene 548:294–298
Mescka CP, Guerreiro G, Hammerschmidt T, Faverzani J, de Moura CD, Mandredini V, Wayhs CA, Wajner M, Dutra-Filho CS, Vargas CR (2015) L-Carnitine supplementation decreases DNA damage in treated MSUD patients. Mutat Res 775:43–47
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Ong LC, Khoo TB, Zulfiqar A, Zarida H, Ruzana A (1998) Computed tomography findings in maple syrup urine disease. Singapore Med J 39:370–372
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem Res 28:675–679
Prensky AL, Moser HW (1966) Brain lipids, proteolipids, and free amino acids in maple syrup urine disease. J Neurochem 13:863–874
Ribeiro CA, Sgaravatti AM, Rosa RB, Schuck PF, Grando V, Schmidt AL, Ferreira GC, Perry ML, Dutra-Filho CS, Wajner M (2008) Inhibition of brain energy metabolism by the branched-chain amino acids accumulating in maple syrup urine disease. Neurochem Res 33:114–124
Riviello JJ Jr, Rezvani I, DiGeorge AM, Foley CM (1991) Cerebral edema causing death in children with maple syrup urine disease. J Pediatr 119:42–45
Scaini G, Jeremias IC, Morais MO, Borges GD, Munhoz BP, Leffa DD, Andrade VM, Schuck PF, Ferreira GC, Streck EL (2012) DNA damage in an animal model of maple syrup urine disease. Mol Genet Metab 106:169–174
Schonberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82:69–75
Sgaravatti AM, Rosa RB, Schuck PF, Ribeiro CA, Wannmacher CM, Wyse AT, Dutra-Filho CS, Wajner M (2003) Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Steinlin M, Blaser S, Boltshauser E (1998) Cerebellar involvement in metabolic disorders: a pattern-recognition approach. Neuroradiology 40:347–354
Tavares RG, Santos CE, Tasca CI, Wajner M, Souza DO, Dutra-Filho CS (2000) Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J Neurol Sci 181:44–49
Treacy E, Clow CL, Reade TR, Chitayat D, Mamer OA, Scriver CR (1992) Maple syrup urine disease: interrelations between branched-chain amino-, oxo- and hydroxyacids; implications for treatment; associations with CNS dysmyelination. J Inherit Metab Dis 15:121–135
Uziel G, Savoiardo M, Nardocci N (1988) CT and MRI in maple syrup urine disease. Neurology 38:486–488
Vajda FJ (2002) Neuroprotection and neurodegenerative disease. J Clin Neurosci 9:4–8
Vekrellis K, McCarthy MJ, Watson A, Whitfield J, Rubin LL, Ham J (1997) Bax promotes neuronal cell death and is downregulated during the development of the nervous system. Development 124:1239–1249
White E (1996) Life, death, and the pursuit of apoptosis. Genes Dev 10:1–15
Yudkoff M, Daikhin Y, Nissim I, Pleasure D, Stern J, Nissim I (1994) Inhibition of astrocyte glutamine production by alpha-ketoisocaproic acid. J Neurochem 63:1508–1515
Acknowledgments
We thank the authors who have provided relevant clarification on their articles. Laboratory of Bioenergetics (Brazil) are one of the centers of the National Institute for Molecular Medicine (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research was supported by grants from CNPq (ELS), FAPESC (ELS), and UNESC (ELS). ELS is a 1D CNPq Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thais C. Vilela and Giselli Scaini contributed equally to this work.
Rights and permissions
About this article
Cite this article
Vilela, T.C., Scaini, G., Furlanetto, C.B. et al. Apoptotic signaling pathways induced by acute administration of branched-chain amino acids in an animal model of maple syrup urine disease. Metab Brain Dis 32, 115–122 (2017). https://doi.org/10.1007/s11011-016-9892-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9892-0