Abstract
Pyridoxine-dependent epilepsy (PDE) is a pharmacoresistant epileptogenic encephalopathy controlled by pyridoxine supplementation at pharmacological doses. Despite supplementation, the long-term outcome is often poor possibly because of recurrent seizures and developmental structural brain abnormalities. We report on five patients with PDE from three unrelated families. The diagnosis was confirmed by ALDH7A1 sequencing, which allowed for the characterization of two homozygous variations [NM_001182.3:c.1279G > C - p.(Glu427Gln) and c.834G > A - p.(Val278Val)]. Brain autopsy was conducted for one untreated patient with molecularly confirmed antiquitin deficiency. Macroscopic and histological examination revealed a combination of lesions resulting from recurrent seizures and consisting of extensive areas of cortical necrosis, gliosis, and hippocampic sclerosis. The examination also revealed developmental abnormalities including corpus callosum dysgenesis and corticospinal pathfinding anomalies. This case is the second to be reported in the literature, and our findings show evidence that antiquitin is required for normal brain development and functioning. Despite prophylactic prenatal pyridoxine supplementation during the last trimester of pregnancy in one of the three families and sustained pyridoxine treatment in three living patients, the clinical outcome remained poor with delayed acquisition of neurocognitive skills. Combined therapy (pyridoxine/arginine supplementation and lysine-restricted diet) should be considered early in the course of the disease for a better long-term outcome. Enhanced knowledge of PDE features is required to improve treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyridoxine-dependent epilepsy (PDE) is a rare autosomal recessive disease (OMIM#266,100) related to deficiency of alpha aminoadipic acid semialdehyde (α-AASA) dehydrogenase, or antiquitin (EC 1.2.1.31). The main clinical feature is a pharmacoresistant epileptic encephalopathy that can be controlled by pyridoxine supplementation at pharmacological doses. A high mortality rate occurs in the absence of specific treatment (Striano et al. 2009). Seizure onset usually occurs at birth, but antenatal and late-onset presentations have also been reported (Mills et al. 2010, 2006). Multiple types of seizures have been described, ranging from focal to myoclonic or generalized tonic-clonic seizures. Electroencephalograms (EEGs) may vary from focal discharges to burst-suppression patterns (Hellstrom-Westas et al. 2002; Mills et al. 2010). Postmortem brain abnormalities have been reported in very few cases (Baxter et al. 1996; Jansen et al. 2014; Miyasaki et al. 1978). Jansen et al. recently demonstrated that antiquitin is a glial protein expressed during embryonic brain development and is therefore likely to play roles in neurogenesis and neuronal migration (Jansen et al. 2014). Antiquitin is involved in the catabolism of lysine, which results in acetyl-CoA as an end product, and its deficiency is responsible for the accumulation of metabolic intermediates including α-AASA, δ1-piperideine-6-carboxylate, and pipecolic acid. The chemical condensation of piperideine-6-carboxylic acid (P6C) with pyridoxal phosphate (PLP), the active form of pyridoxine, underlies the pathophysiology of PDE, at least in part. In addition, α-AASA is a reactive compound that could be involved in multiple cellular reactions, and its accumulation may alter brain development. Pyridoxine withdrawal test was commonly used to prove PDE, however it may be unsafe and not reliable in some cases by inducing nonspecific EEG responses (Bok et al. 2010). This test is replaced by non-invasive and reliable urinary (or plasma) screening for α-AASA/P6C accumulation (Stockler et al. 2011).
PLP is a coenzyme for 145 enzymes involved in numerous metabolic pathways (Percudani and Peracchi 2009). Five of six general classes of enzymes include PLP-dependent enzymes (oxidoreductases, transferases, hydrolases, lyases, isomerases). Importantly, PLP-dependent enzymes catalyze steps of neurotransmitter metabolism (e.g., glutamic acid decarboxylase, which leads to the conversion of glutamate to γ-aminobutyric acid), and PLP deficiency may alter neurotransmitter function and induce epilepsy. However, it is worth noting that even though pyridoxine supplementation succeeds in controlling clinical and electrical seizure activity, clinical outcome in PDE is usually poor, with 75 % of treated patients experiencing developmental delay and/or intellectual disability (IQ < 70) (Bok et al. 2012; van Karnebeek and Jaggumantri 2015). A new combined therapeutic approach using pyridoxine, a lysine-restricted diet, and L-arginine supplementation has recently been reported (Coughlin et al. 2015; van Karnebeek and Jaggumantri 2015; van Karnebeek et al. 2014). Arginine, which limits the lysine influx into the brain, and a lysine-restricted diet may reduce the concentration of neurotoxic lysine metabolites and could improve neurodevelopmental outcome. The present study aims at describing five patients from three unrelated families. It includes the initial clinical presentations of the patients, long-term follow-up for one patient (16 years), a shorter term follow-up for two patients (9 and 4 years), biochemical and molecular data, and autopsy findings in one patient.
Patients and methods
Patients
Family 1
The first child (P1) was born at 37 weeks of gestation (WG) to consanguineous parents of the third degree. Forceps delivery was necessary due to abnormal fetal heart rhythm. Routine antenatal ultrasound (US) performed at 22 WG revealed complete isolated agenesis of the corpus callosum, confirmed by fetal magnetic resonance imaging (MRI) performed at 30 WG (T1, T2, and FLAIR sequences). There were no associated abnormalities in the intermediate zone. The neonate weighed 3985 g at birth (normal at 37–38 WG: 2813.1 ± 395.3 g, >97th percentile), and height was 51 cm (normal at 37–38 WG: 49.3 ± 2.5 cm 75th percentile). He had macrocrany (head circumference of 38 cm; normal at 37–38 WG: 32.8 ± 1.6 cm, >97th percentile), according to (Guihard-Costa et al. 2002). Apgar scores were 10/10. At 1 h of life, he presented eye rolling, jitteriness, mouthing movements, and cyanotic spells, which rapidly resolved after phenobarbital infusion. Three seizures lasting 1 to 15 min occurred again within the first 2 days of life despite intravenous administration of phenobarbital and phenytoin. The neonate required intubation and assisted ventilation for 5 days. On day 1, biological tests for blood glucose levels were normal, and lactate level and pH were measured at 2.8 mmol/L and 7.34, respectively. EEG examination performed at 1 and 2 days of life showed background discontinuity with no modulation of sleep stages or seizures. At 4 days of life, EEG displayed continuity of the background but with a low voltage. Antiepileptic treatment was replaced by valproic acid. Brain MRI performed at 9 days of life revealed extracellular vasogenic edema of the deep white matter that appeared as a hyposignal on diffusion sequences as well as hypersignal on T1 sequences corresponding to right transverse sinus thrombosis. The patient’s status gradually improved, and he was discharged from the hospital at the age of 13 days. Two months later, he developed recurrent seizures consisting of mouthing, abnormal eye movements, and generalized stiffening of the limbs. The child was hypotonic, EEG was normal, and no seizures were registered. Lamotrigine and clobazam were introduced in addition to valproic acid. One week later EEG showed focal right or left seizures with secondary generalization. Brain MRI showed delayed myelination on T1 sequences. Clonazepam treatment was introduced and then replaced by vigabatrin. At 1 year of age, he presented tonic seizures refractory to high doses of clonazepam, phenobarbital, phenytoin, midazolam, nesdonal, lorazepam, and xylocaine. He required intubation and assisted ventilation. EEG showed extreme background discontinuity. Brain MRI revealed cortical and basal ganglia edema that appeared in hypersignal on T2 sequences, and on T1 sequences partial agenesis of the corpus callosum with Probst bundles (Supplementary Fig. 1A). The child died at the age of 14 months. He had never been given pyridoxine, and the diagnosis was made in his sister (P2) born 3 years later at 36 WG. Fifteen hours after birth, the neonate presented irritability, abdominal distension, myoclonic jerks, and respiratory distress. Biological tests revealed metabolic acidosis (lactacte level at 11 mmol/L) and severe hypoglycemia (0.6 mmol/L). EEG showed background discontinuity with no epileptic seizures. Myoclonic jerks ceased within 5 min after pyridoxine administration (100 mg intravenously). Normalization of EEG profile was observed 2 days later. Brain MRI performed at 4 days of life revealed diffuse white matter hypersignal on T2 sequences in both cerebral hemispheres and cortical edema (Supplementary Fig. 1B). A 15 mg/kg/d (p.o.) pyridoxine treatment was introduced, with a gradual increase to 20 mg/kg/d at 3 weeks of life and 25 mg/kg/d at 6 weeks of life. At 9 months of life, physical examination was normal. At 1 year of age, delayed psychomotor acquisitions were noted, with sitting difficulties that persisted until 14 months of age. Speech acquisition was initially normal, but speech therapy was introduced at 3 years of age. She was able to walk without any help at the age of 24 months. Since then, motor and cognitive development have progressed normally, while mild psychomotor retardation persisted. The child is currently 4 years old and being treated with a pyridoxine dosage of 500 mg/d (p.o.).
Family 2
The propositus (P3) was the second male child born at term to consanguineous parents of the first degree following an uneventful pregnancy. One hour after birth he presented with respiratory distress, along with lid spasms, mouthing, and stereotypic jerks of the superior limbs. EEG displayed multiple left hemispheric spike-wave discharges. MRI analysis revealed corticospinal tract abnormalities. Seizures were initially treated with intravenous pyridoxine (100 mg) infusion without any improvement. They were eventually controlled using a combination of phenobarbital, phenytoin, and clonazepam. At 4 months of age, the patient was admitted for status epilepticus, which was treated by antiepileptic drugs including valproate (30 mg/kg/d) and vigabatrin (100 mg/kg/d). At 5 months of age, he was again hospitalized for status epilepticus refractory to antiepileptic treatment. Pyridoxine (15 mg/kg/d p.o.) paired with phenobarbital allowed for clinical improvement. At 8 months of age, he presented respiratory sepsis with recurrent seizures and died despite intensive resuscitation and treatment reinforcement. A third pregnancy was interrupted owing to antenatal discovery of the same mutation while the fourth daughter was healthy. Family history study revealed that the first female child had presented a similar clinical history and died at 9 months of life (Supplementary Fig. 2A patient VI:1). A female maternal cousin also died at 2 years of age from epilepsy (Supplementary Fig. 2A V:7).
Family 3
A female child (P4) was born at 36 WG to unrelated parents. Her birth weight, height, and head circumference were 2400 g (normal at 35–36 WG: 2460.8 ± 342.1 g, 50th percentile), 43.5 cm (normal at 35–36 WG: 47.1 ± 2.4 cm, 10th percentile), and 34.5 cm (normal at 35–36 WG: 31.6 ± 1.6 cm, 95th percentile), respectively. The pregnancy had been uneventful, and the mother did not report any abnormal fetal movements. Routine US examination performed during the third trimester revealed an isolated mild cerebral ventricular dilatation. At birth, the child presented neonatal respiratory distress. At 1 day of life, she presented proximal myoclonic jerks with spontaneous flexion of the four limbs highly suggestive of seizures. She had global hypotonia and almost no archaic reflexes. EEG showed alterations consisting of discontinuous background activity with no epileptic activity. Brain US displayed moderate ventricular dilatation and a small right periventricular hemorrhage. MRI carried out at day 5 revealed a right frontal pseudocyst of germinolysis on T1 and T2 sequences and a hypersignal on T2 sequences in the left periventricular area related to calcification and confirmed subependymal hemorrhage visible as a hyposignal on T1 sequences. Gyration and corpus callosum were normal. At 6 weeks of age, she presented tonic-clonic seizures with secondary generalization confirmed by EEG, which were treated with phenobarbital (20 mg/kg), phenytoin (8 mg/kg per 8 h), sodium valproate (40 mg/kg), and vigabatrin (until 150 mg/kg). Initial treatment with pyridoxine (15 mg/kg/d, p.o.), corresponding to a standard dose of 50 mg/d for a child weighing 3.5 kg, failed to stop seizures with withdrawal after 48 h. In addition, she had macrocrany (head circumference measured at 40 cm; normal at 6 weeks: 37.5 cm, >97th percentile) with a high forehead, but no facial dysmorphism. Due to neurological distress as well as persisting seizures, pyridoxine was reinitiated at 15 mg/kg/d (p.o.). Amino acid chromatography, organic acid chromatography, ammonemia, and lactic acid concentration were normal, and carbohydrate-deficient glycoprotein syndrome testing was negative. At 5 months of age an attempt at therapy withdrawal was made, but 36 h later she presented nausea, horizontal nystagmus of the right eye, and loss of consciousness. Facial cyanosis was noted, along with clonic movements of the right hand and both lower limbs. Pyridoxine reintroduction at 7 mg/kg/d (p.o.), corresponding to 50 mg/d for a child weighing 7 kg, allowed for the disappearance of all neurological signs, and the diagnosis of PDE was made. At 8 months of age, the pyridoxine dosage was increased to 11 mg/kg/d (p.o.) (100 mg/d for a child weighing 9 kg) but failed to control seizures. A chronic pyridoxine dosage of 500 mg/d (p.o.) was then started. At 5 years of age, she presented with delayed acquisition of neurocognitive skills, particularly for graphics, and growth retardation, with a height at −2 SD. At 9 years old, she experienced difficulties in mathematics. At the age of 16, executive function disabilities persisted so that school difficulties worsened. The second and third sisters are in good health.
The fourth female child (P5) was born at term 9 years after her affected sister. The mother had been supplemented with pyridoxine (500 mg/d p.o.) during the last trimester of pregnancy. At 8 days of life, the child was admitted to the hospital for seizures. Neurological examination and EEGs were normal. She was immediately given vitamin B6 therapy at high doses from seizure onset (70 mg/kg/d or 250 mg/d p.o. for a child weighing 3.5 kg), and no recurrent seizures were observed. At 12 months, she had mild macrocrany with a head circumference > 3 SD without significant ventricular dilatation. Neurological examination was normal, although she was not able to sit without support. At 18 months of age, she was able to walk by herself, but she presented delayed speech acquisition. At 3 years of age, understanding was normal but expressive language was delayed. A mild craniofacial dysmorphism was noted, which consisted of down-slanting palpebral fissures; her hands were short with thick fingers. Six years later, growth parameters remained normal, and she was at an appropriate classroom level at ordinary school, but delayed language acquisition persisted, along with dyspraxia and executive function and attention difficulties.
Methods
Autopsy procedures
An autopsy restricted to the brain was performed in the first child of family 1 (P1) with the informed consent of both parents and according to French law. The brain was fixed in a 10 % formalin-zinc buffer solution for 1 month. Brain biometric dates were evaluated according to (Ludwig 2002). Multiple 7-μm sections obtained from paraffin-embedded blocks were stained using hematoxylin–eosin to identify the lesions and phloxin-luxol fast blue to evaluate brain myelination. Routine immunohistochemical studies were also carried out, using antibodies directed against glial fibrillary acidic protein (1:300; Dakopatts, Trappes, France) to evaluate reactive gliosis intensity and location.
Brain MRI
The MRI used in Rouen University Hospital for families 1 and 3 was DXT 1.5 Tesla 2008. Family 2 was investigated in the Military Hospital of Tunis using Siemens 3 Tesla.
Molecular analyses
Genomic DNA was extracted from venous blood using QIAamp DNA Blood Mini Kit® Qiagen and was amplified in vitro by PCR. Multiple pairs of primers were synthesized to amplify each of the ALDH7A1 exonic regions, including intron/exon boundaries and the promoter. Primers used to amplify the genomic sequences were designed according to the sequence NM_001182.3 (primer sequences are available upon request). PCR amplifications were carried out in 1X Thermo Scientific Buffer IV (75 mM Tris-HCl pH 8.8, 20 mM (NH4)2SO4, 0.01 % Tween 20), 1.5 mM MgCl2, 100 μM dNTPs, 0.025 U/μL Taq polymerase (Thermo Scientific), 0.6 μM of each primer). Touchdown PCR consisted of one cycle of 95 °C for 5 min for the initial denaturation step followed by 12 cycles of denaturation at 95 °C for 25 s, varying annealing (60 °C to 48 °C) for 25 s, and extension at 72 °C. Then, 35 cycles were performed as follows: denaturation at 95° for 25 s, annealing at 48° for 25 s, and extension at 72 °C for 25 s. PCR was terminated after a final cycle at 72 °C for 5 min. Direct sequencing of DNA fragments was performed with an ABI Prism BigDye Terminator cycle Sequencing Ready Reaction Kit (PE Applied Biosystem and ABI model 3130xl Genetic Analyzer). The patient’s genomic sequence was compared with the reference sequence by using Variant Reporter software (Applied Biosystem). The identified variations were mined by ALAMUT software (Interactive-Biosoftware). The described variations were named according to the current nomenclature recommendations (http://www.hgvs.org/mutnomen).
Results
Molecular findings
The clinical diagnosis of PDE in the reported patients was confirmed by genetic studies. ALDH7A1 sequencing revealed two deleterious homozygous variations: ALDH7A1: Chr5(GRCh37):g.125887751C > G; NM_001182.3:c.1279G > C - p.(Glu427Gln) in patients P1, P2 and P3 from families 1 and 2 and ALDH7A1; Chr5(GRCh37):g.125903988C > T; NM_001182.3:c.834G > A - p.(Val278Val) in patients P4 and P5 from family 3 (Supplementary Fig. 2). These variations were present in a heterozygous state in the unaffected carrier parents from families 1 and 2.
The p.(Glu427Gln) variant, located in exon 14, accounts for 30 % of the reported mutated alleles (Bennett et al. 2009; Mills et al. 2006; Salomons et al. 2007). The synonymous variation in exon 9, p.Val278Val, is located within a cryptic splicing site, 40 nucleotides upstream of the authentic donor site of intron 9. Its presence favors the use of the cryptic site and induces alternate splicing (Salomons et al. 2007).
Two prenatal diagnoses were performed in family 2 by molecular analysis using trophoblast samples. The first fetus homozygous variant p.(Glu427Gln) was identified, leading to medical termination of the pregnancy while the fourth daughter was healthy.
Autopsy findings
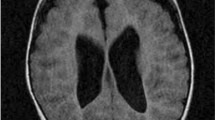
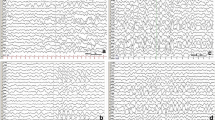
The child displayed no cranio-facial dysmorphism. Brain weight was 1114 g (normal at 14 months: 944 g). External examination of the brain revealed diffuse swelling of the convolutions with narrowing of the sulci (Fig. 1a). On coronal sections, all brain structures were macroscopically normal, except for the corpus callosum, which was severely dysgenetic, with hypoplasia in its anterior part and absence in its posterior part, with Probst bundles on the left side and fragmentation on the right side (Fig. 1b). A mild asymmetric ventricular dilatation was also noted (Fig. 1b), along with a diffuse congestion of infratentorial structures. Histological examination of the brainstem revealed bilateral vacuolization of the pons, with dysmorphic, misplaced, and fragmented pyramidal tracts (Fig. 1c), but pyramidal decussation was identifiable. No olivary pachygyria was observed, and cranial nerve nuclei were normal. No lesions were present in the cerebellum. Otherwise, brain myelination was in accordance with the age. At the supratentorial level, diffuse vacuolization of the cortex and neuronal loss were observed. Cortical lamination was almost indiscernible in some areas, particularly in the frontal cortex, where reactive gliosis associated with neocapillaries replaced the necrotic cortical ribbon (Fig. 1d). Areas of ischemic necrosis with gliosis were observed in the white matter of the temporal poles, but no migrational abnormalities were observed in the hemispheric white matter or in the periventricular areas. No calcified neurons were observed in the basal ganglia or thalami. Hippocampic sclerotic lesions were also observed, consisting of hypotrophic and discontinuous dentate gyrus with a complete loss of pyramidal cells (Fig. 1e). Right dysgenetic and interrupted corpus callosum and left Probst bundles were histologically confirmed (Fig. 1f).
Main macroscopic and histological lesions hallmarks of pyridoxine-dependent epilepsy observed in patient P1, family 1. a Macroscopic superior view of the brain displaying diffuse swelling of convolutions. b Coronal section passing through the thalami, showing mild asymmetric ventricular dilatation, left Probst bundle (thin arrow) and right dysgenesis (thick arrow). c Histological section passing through the pons exhibiting a symmetric vacuolization of the central tegmental tracts and superior cerebellar peduncles (asterisks), with aberrant dorsally placed corticospinal tract fascicles (thick arrow) and dysmorphic fragmented fascicles (thin arrow) (Phloxin-Luxol fast blue staining, OM × 25). d Diffuse neuronal necrosis and cortical lamination loss replaced by neocapillaries and gliosis in the temporal cortex (hematoxylin and eosin staining, OM × 25). e Hippocampal sclerotic lesions with almost complete disappearance of the dentate gyrus (arrow) and massive loss of pyramidal cells in the CA1 and CA3 fields of the Ammon’s horn with relative sparing of CA2 field (hematoxylin and eosin staining, OM × 100). f Histological view of the left Porbst bundle, made of disorganized callosal fibers (hematoxylin and eosin staining, OM × 100) OM = original magnification
Discussion
The classification of epilepsy is based not only on the semiology but also, and mainly, on its etiology. Symptomatic secondary epilepsy results from an independent primary pathology, monogenic disorders (e.g. channelopathy or inborn error of metabolism) tumor, trauma, infectious process, and antenatal cerebrovascular insufficiency, regardless of the cause leading to hypoxic-ischemic injury. Conversely, seizures occurring in the context of inborn errors of metabolism that have a primary effect on the nervous system typically manifest with epilepsy and fall into the group of primary epileptic encephalopathies. In this group of diseases, epilepsy may occur during the neonatal period, or later, even during adolescence. Some of them are at least partly treatable, particularly PDE, provided that it is recognized as early as possible. In PDE, a variety of seizure types have been described, in addition to various types of lesions revealed in imaging studies; however, none of the signs or symptoms have been sufficiently specific to alert the clinician and provide some diagnostic clues prior to biochemical and genetic results.
Based on imaging studies, two main categories of changes coexist in PDE: lesions secondary to epilepsy and brain structural abnormalities. In the original report of six patients by Baxter et al., lesions resulting from epilepsy consisted of mild generalized atrophy of gray and white matter, with ventricular dilatation, whereas structural brain anomalies consisted of cerebellar hypoplasia and focal parietal cortical dysplasia (Baxter et al. 1996). Three recent studies (Bok et al. 2012; Mills et al. 2010; Perez et al. 2013) reported a total of 44 from patients with PDE. These scans were performed during the neonatal period and later in life and in children for whom pyridoxine supplementation prior to birth was used. MRI results were considered to be normal in 10 cases (25 %). Corpus callosum abnormalities including hypoplasia, partial agenesis, or dysplasia represented the most frequent lesion (15/44 patients, 34 %), and white matter anomalies (delayed myelination, white matter signal intensity and density on T2-weighted images) and ventricular dilatation/hydrocephalus were observed in 7/44 patients (16 %). Posterior fossa abnormalities including mega cisterna magna and cerebellar hypoplasia were found in nine patients (20 %). Intriguingly, excitotoxic/hypoxic-ischemic encephalopathy and periventricular leukomalacia were detected in only five patients (11 %), and hippocampal sclerosis was not documented in any of the cases, although some of these patients had presented with status epilepticus. In addition, a systematic evaluation of corpus callosum morphology and cross-sectional cerebral area in 30 individuals with PDE versus a control group revealed a markedly reduced callosal area in the entire group with PDE (Friedman et al. 2014).
To our knowledge, only one exhaustive postmortem neuropathological study of confirmed PDE has been reported so far (Jansen et al. 2014), and only 12 autopsy cases of suspected PDE were reported before 2001, with no genetic confirmation of the disease (Baxter 2001; Lott et al. 1978; Miyasaki et al. 1978). In these reports, lesions consisted of gliosis, cerebral edema, small intracerebral hemorrhages, neuronal loss in the thalamus and decreased white matter volume. Brain structural lesions consisted of thinning of the corpus callosum and irregular lobulation of the cerebellum. In a recent case described by (Jansen et al. 2014), a surgical resection of right occipital and posterior parietal lobe had been performed at the age of 15 months, and the examination revealed severe dyslamination of the cortex with microcolumnar focal cortical dysplasia. Postmortem neuropathological examination performed at the age of 9 years revealed patchy cortical gliosis with an increased number of interstitial neurons in the white matter and small neurons heterotopias in the insular area, which were not observed in our case. By contrast, lesions related to status epilepticus were more diffuse in our case, with extended areas of cortical necrosis and gliosis predominating in the frontal and temporal cortices. Hippocampic sclerosis, which was observed in our case, was also reported by Jansen et al. and is known to result from status epilepticus. Neuronal loss involves neuron groups that are vulnerable, particularly cortical neurons (especially pyramidal cell layer III) and hippocampal neurons (CA1 and CA3 fields and sometimes affecting the full length of the hippocampus). As reported by Jansen et al., histological examination of infratentorial structures revealed a combination of secondary lesions to epilepsy with developmental anomalies (Jansen et al. 2014). Taken together, our findings and those of Jansen, which are summarized in Table 1, provide evidence that antiquitin is most likely required for normal brain development and functioning. To our knowledge, very little is known about the roles of antiquitin during development, apart from severe developmental anomalies of the optic cup in ALDH7A1 knock-down zebrafish (Babcock et al. 2014). Antiquitin has been also shown to be necessary for proper development of the inner ear (Skvorak et al. 1997). More recently, Jansen et al. found that antiquitin is expressed in the radial glia, the common progenitor that gives rise to different cell lineages in the developing central nervous system (CNS), but not in mature neurons. This finding suggests that antiquitin most likely acts during the early events of CNS development. Corticospinal tracts and corpus callosum develop during the 10th and 20th week of gestation, that argues in favor of its key role in the formation of decussating and projecting fibers (as observed in patient P1).
Despite early pyridoxine treatment in patients P2, P4, and P5, and even an antenatal supplementation in patient P5, the outcome was poor with a mild developmental delay. Of note, the prenatal pyridoxine supplementation in the pregnant mother was initiated during the last trimester. Afterwards, early supplementation was recommended with variable effect; indeed, Bok et al. reported good developmental outcome after prenatal treatment in three patients (Bok et al. 2012), although this treatment did not prevent poor cognitive outcome in two other patients (Rankin et al. 2007). These findings are in accordance with the variable clinical outcomes reported in patients treated with pyridoxine; few reports describe a favorable outcome (Oliveira et al. 2013; Proudfoot et al. 2013; Riikonen et al. 2015), and a high proportion of treated patients present with intellectual disability (Bok et al. 2012; van Karnebeek and Jaggumantri 2015). This could be explained by the potential importance of antiquitin for brain development. To control seizures and to prevent neurodevelopmental impacts, early diagnosis of PDE and new treatment strategies including a lysine-restricted diet with pyridoxine and arginine supplementations are required.
References
Babcock HE et al. (2014) aldh7a1 regulates eye and limb development in zebrafish. PLoS One 9:e101782. doi:10.1371/journal.pone.0101782
Baxter P (2001) Pyridoxine dependent and pyridoxine responsive seizures. In: Baxter P (ed) Vitamin responsive conditions in paediatric neurology. edn. MacKeith Press., London, pp 109–165
Baxter P, Griffiths P, Kelly T, Gardner-Medwin D (1996) Pyridoxine-dependent seizures: demographic, clinical, MRI and psychometric features, and effect of dose on intelligence quotient. Dev Med Child Neurol 38:998–1006
Bennett CL, Chen Y, Hahn S, Glass IA, Gospe SM Jr (2009) Prevalence of ALDH7A1 mutations in 18 North American pyridoxine-dependent seizure (PDS) patients. Epilepsia 50:1167–1175. doi:10.1111/j.1528-1167.2008.01816.x
Bok LA et al. (2010) The EEG response to pyridoxine-IV neither identifies nor excludes pyridoxine-dependent epilepsy. Epilepsia 51:2406–2411. doi:10.1111/j.1528-1167.2010.02747.x
Bok LA et al. (2012) Long-term outcome in pyridoxine-dependent epilepsy. Dev Med Child Neurol 54:849–854. doi:10.1111/j.1469-8749.2012.04347.x
Coughlin CR 2nd et al. (2015) Triple therapy with pyridoxine, arginine supplementation and dietary lysine restriction in pyridoxine-dependent epilepsy: neurodevelopmental outcome. Mol Genet Metab 116:35–43. doi:10.1016/j.ymgme.2015.05.011
Friedman SD et al. (2014) Callosal alterations in pyridoxine-dependent epilepsy. Dev Med Child Neurol 56:1106–1110. doi:10.1111/dmcn.12511
Guihard-Costa AM, Menez F, Delezoide, AL (2002) Organ weights in human fetuses after formalin fixation: standards by gestational age and body weight Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 5:559–578 doi:10.1007/s10024-002-0036-7
Hellstrom-Westas L, Blennow G, Rosen, I (2002) Amplitude-integrated encephalography in pyridoxine-dependent seizures and pyridoxine-responsive seizures. Acta Paediatr. (Oslo, Norway : 1992) 91:977–980
Jansen LA, Hevner RF, Roden WH, Hahn SH, Jung S, Gospe SM Jr (2014) Glial localization of antiquitin: implications for pyridoxine-dependent epilepsy. Ann Neurol 75:22–32. doi:10.1002/ana.24027
Lott IT, Coulombe T, Di Paolo RV, Richardson EP Jr, Levy HL (1978) Vitamin B6-dependent seizures: pathology and chemical findings in brain. Neurology 28:47–54
Ludwig, J (2002) Handbook of autopsy practice. third edition edn
Mills PB et al. (2006) Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med 12:307–309. doi:10.1038/nm1366
Mills PB et al. (2010) Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency). Brain J Neurol 133:2148–2159. doi:10.1093/brain/awq143
Miyasaki K, Matsumoto J, Murao S, Nakamura K, Yokoyama S, Hayano M, Nakamura H (1978) Infantile convulsion suspected of pyridoxine responsive seizures. Acta Pathol Jpn 28:741–749
Oliveira R et al. (2013) Pyridoxine-dependent epilepsy due to antiquitin deficiency: achieving a favourable outcome Epileptic disorders: international epilepsy journal with videotape 15:400–406 doi:10.1684/epd.2013.0610
Percudani R, Peracchi A (2009) The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Biochem 10:273. doi:10.1186/1471-2105-10-273
Perez B et al. (2013) Clinical, biochemical, and molecular studies in pyridoxine-dependent epilepsy. Antisense therapy as possible new therapeutic option. Epilepsia 54:239–248. doi:10.1111/epi.12083
Proudfoot M, Jardine P, Straukiene A, Noad R, Parrish A, Ellard S, Weatherby, S (2013) Long-term follow-up of a successfully treated case of congenital pyridoxine-dependent epilepsy JIMD Rep 10:103–106 doi:10.1007/8904_2012_210
Rankin PM, Harrison S, Chong WK, Boyd S, Aylett SE (2007) Pyridoxine-dependent seizures: a family phenotype that leads to severe cognitive deficits, regardless of treatment regime. Dev Med Child Neurol 49:300–305. doi:10.1111/j.1469-8749.2007.00300.x
Riikonen R, Mankinen K, Gaily, E (2015) Long-term outcome in pyridoxine-responsive infantile epilepsy European journal of paediatric neurology : EJPN: official journal of the European Paediatric Neurology Society 19:647–651 doi:10.1016/j.ejpn.2015.08.001
Salomons GS et al. (2007) An intriguing "silent" mutation and a founder effect in antiquitin (ALDH7A1). Ann Neurol 62:414–418. doi:10.1002/ana.21206
Skvorak AB et al. (1997) An ancient conserved gene expressed in the human inner ear: identification, expression analysis, and chromosomal mapping of human and mouse antiquitin (ATQ1). Genomics 46:191–199. doi:10.1006/geno.1997.5026
Stockler S et al. (2011) Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol Genet Metab 104:48–60. doi:10.1016/j.ymgme.2011.05.014
Striano P et al. (2009) Two novel ALDH7A1 (antiquitin) splicing mutations associated with pyridoxine-dependent seizures. Epilepsia 50:933–936. doi:10.1111/j.1528-1167.2008.01741.x
van Karnebeek CD, Jaggumantri S (2015) Current treatment and management of pyridoxine-dependent epilepsy. Curr Treat Options Neurol 17:335. doi:10.1007/s11940-014-0335-0
van Karnebeek CD et al. (2014) Lysine-restricted diet as adjunct therapy for pyridoxine-dependent epilepsy: the pde consortium consensus recommendations. JIMD Rep 15:1–11. doi:10.1007/8904_2014_296
Acknowledgments
The authors are grateful to Hélène DRANGUET and Carine TARDIVEL-PILON for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Electronic supplementary material
Supplementary Figure 1
Main imaging lesions observed in family 1. (A) T1 weighted images of coronal section passing through the hippocampi, displaying absent commissuration of callosal fibers with Probst bundles (thin white arrow) and aberrantly dispersed callosal fibers in the cincular gyrus (thick white arrow). (B) T2 weighted images of coronal section passing through the parieto-occipital lobes, showing diffuse white matter edema, with narrowing of the sulci (white arrow). (GIF 120 kb)
Supplementary Figure 2
Family trees and disease-causing variations. (A) Family trees: (red star) patients with documented epilepsy. (B) c.1279G > C - p.(Glu427Gln), control and patient P2 sequence. The mutated base is indicated by an arrow. This variation has been identified in families 1 and 2 (patients P1, P2 and P3). (C) c.834G > A - p.(Val278Val) control and patient P4 sequence. The mutated base is indicated by an arrow. This variation was identified in family 3 (patients P4 and P5). (GIF 255 kb)
Rights and permissions
About this article
Cite this article
Marguet, F., Barakizou, H., Tebani, A. et al. Pyridoxine-dependent epilepsy: report on three families with neuropathology. Metab Brain Dis 31, 1435–1443 (2016). https://doi.org/10.1007/s11011-016-9869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9869-z