Abstract
To analyze the clinical and genetic characteristics of Chinese patients with pyridox(am)ine-5′-phosphate oxidase (PNPO) deficiency. The clinical presentations and the responses to treatments were analyzed in 4 patients. Blood and urinary metabolic screenings, electroencephalogram (EEG), brain magnetic resonance imaging (MRI) and epilepsy-related genes detection were performed in all patients. Patient 1 and 2 were identical twin brothers, who were born at 35+5 w gestation with a sign of encephalopathy. Their seizures started within the first day and could not be controlled by pyridoxine or pyridoxal-5′-phosphate (PLP) completely. Patient 3 presented seizures at 5 months, responding well to pyridoxine. Seizures in patient 4 began at 40 days after birth and were controlled by valproic acid and topiramate. EEG showed atypical hypsarrhythmia or multifocal epileptiform discharges in 3 patients, and showed normality in patient 4. MRI showed nonspecific abnormality or normality. Blood metabolic screening showed multiple amino acids level abnormalities in all cases. Urinary metabolic screening showed vanillactic acid prominently elevated in 3 patients. Genetic analysis revealed 5 mutations of PNPO, three of which were novel. The mutation c.445_448del was carried by the twins and patient 3. Assessment of psychomotor development indicated severe delay in 3 patients and borderline to mild delay in patient 3. This is the first time to report patients with PNPO deficiency diagnosed by gene analysis in China. The novel clinical characteristics and novel mutations found here expanded the phenotypes and genotypes of this disease. Further, the frameshift mutation c.445_448del might be high prevalence in PNPO deficiency in Chinese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2002, Kuo and Wang (2002) first described a neonate whose seizures were controlled by pyridoxal-5′-phosphate (PLP) but resistant to pyridoxine. Then, this phenomenon was recognized as an epileptic disorder in which neonatal seizures did not respond to antiepileptic drugs (AEDs) and pyridoxine, but respond to PLP. The underlying genetic defect in the PNPO gene was identified in 2005 (Mills et al. 2005), identifying its autosomal recessive inheritance (OMIM 610090). In the past, it was named as PLP-dependent epilepsy or PLP-responsive epilepsy (Veerapandiyan et al. 2011; Veeravigrom et al. 2015). As a further study found that seizures could be completely or partially responsive to pyridoxine in some patients (Mills et al. 2014), the above two names became no longer appropriate. Then the name of pyridox(am)ine-5′-phosphate oxidase (PNPO) deficiency was used widely. PNPO is the rate-limiting enzyme in the synthesis of PLP from pyridoxine or pyridoxamine. PLP is the only active form of vitamin B6, and it is involved in a wide range of biochemical reactions, including the metabolism of amino acids and glycogen, the synthesis of nucleic acids, hemoglobin, sphingomyelin and neurotransmitters (Khayat et al. 2008). So in addition to epilepsy, other presentations including hepatomegaly, anaemia, hypoglycaemia and disturbance of amino acid metabolism have been seen in some PNPO deficiency patients (Ruiz et al. 2008). To date, there have been less than 50 cases reported world-widely (Coman et al. 2016). Here, we report 4 Chinese patients genetically diagnosed as PNPO deficiency, and find some novel atypical clinical presentations among them.
Materials and methods
Ethics statement
This study was approved by the Ethical Committee of Peking University First Hospital. The individuals’ parents in this manuscript have given written informed consent to publish the case details. All data were analyzed anonymously.
Methods
Biochemical studies, blood and urinary metabolic screenings, electroencephalography (EEG) and magnetic resonance imaging (MRI) were performed in all patients. Of them, the metabolic screenings were done in patient 1, 2 and 4 when they were on pyridoxine and in patient 3 before using pyridoxine. The psychomotor development was assessed according to clinical judgment and/or Gesell developmental scale.
Genetic analysis was performed through targeted next-generation sequencing, within a custom-designed panel capturing the coding exons of 470 genes associated with epilepsy, including PNPO, ALDH7A1 and so on (Xue et al. 2016). The pathogenicity of the identified missense substitutions was predicted based on the lack of such mutations in the control samples and on the conservation of substituted nucleotides and amino acids. We also predicted the functional alteration of the novel missense mutations using polymorphism phenotyping-2 (PolyPhen-2) and MutationTaster.
Results
Clinical presentation (Table 1)
Patient 1 and 2
Patient 1 and 2 were identical twin brothers. They were born at 35+5 weeks gestation to healthy non-consanguineous parents by caesarean section, with a birth weight of 1895 g and 1745 g respectively. Before the premature onset of labor, the pregnancy was uneventful. Labored and irregular breathing, and cold extremities were observed after birth in both, Apgar scores were 5–9-9 and 7–9-9 (at 1, 5 and 10 min) respectively.
The clinical manifestations of them were pretty similar. Seizures started within 24 h after birth and manifested as clusters of a sudden shouting with limbs stiff lasting for 1–2 s, which were speculated as myoclonus or spasms. Along with the progress of the disease, focal seizures, definite spasms and generalized tonic-clonic seizures were observed. Several kinds of AEDs, including phenobarbital, valproic acid, clonazepam, topiramate and levetiracetam were used in monotherapy or combination without seizure remission. Then, pyridoxine was used continuously in both patients from the age of 1 month, which obviously reduced the frequency of seizures from more than 10 times to 3–4 times a day. No temporary respiratory depression was observed during pyridoxine treatment. In the next few years, various AEDs were tried together with pyridoxine, without seizure control. From the age of 5 years, pyridoxine (300 mg/d) was used alone. Focal seizures were observed with a frequency ranging from one time per month to 3–4 times per day. At the age of 6 years 4 months, they were diagnosed as PNPO deficiency by gene analysis. Then PLP (30 mg/kg/d) was used instead of pyridoxine. Seizures worsened for the first few days after using PLP in both of them and returned to a similar frequency as before gradually. At the same time, due to the obvious side effects of PLP including frequent vomiting and poor appetite, pyridoxine was used again to replace PLP. At the last follow-up (6 years 9 months), seizures still existed in both. Their psychomotor development were severe delayed. They could sit independently, but could not speak, stand or walk.
Patient 3
Patient 3 was the second child of healthy non-consanguineous parents and had a healthy sister. He was born at term with a birth weight of 3800 g. Both the pregnancy and delivery were unremarkable. A seizure was first observed during a fever at the age of 5 months, manifesting as sudden loss of consciousness, staring, convulsive movements of limbs with fists clenched, teeth clenched and drooling, lasting 5 min approximately. No treatment was given at that time. Then, seizures aggravated gradually with a frequency of 1–2 times per month and were more frequently during infectious or febrile diseases. Valproic acid was used and no seizures were observed for about 7 months. Then seizures recurred and could not be controlled by adding other medicines such as phenobarbital, oxcarbazepine, carbamazepine and levetiracetam. At the age of 3 years 10 months, he was diagnosed as PNPO deficiency by gene analysis. Under a seizure frequency of 1–2 times per month, pyridoxine (180 mg/d, 10 mg/kg/d) was used together with valproic acid and oxcarbazepine, then seizures disappeared for two months. Whereafter, valproic acid was withdrawn gradually and he has remained seizure-free for more than 6 months to present. The next step, oxcarbazepine would be withdrawn. Gesell developmental scale assessment showed borderline to mild delay at 3 years 10 months, presenting borderline (76 ≤ DQ ≤ 85) in fine motor, and mild delay (55 ≤ DQ ≤ 75) in adaptability, gross motor, language and personal and social competence.
Patient 4
Patient 4 was the first child of healthy non-consanguineous parents with one spontaneous abortion history. He was born at term without problems. Feeding intolerance presented in the neonatal period. Seizures started at 40 days after birth, manifesting as elevation and rigidity of both legs during sleep, lasting about 20 s. Then, a manifestation of head turning to left with a stiff neck was observed during awake period, lasting about 20 s also. When he was 4 months old, convulsive symptoms closely following the above manifestations occurred, presenting as bilateral limbs twitching, ocular deviation, facial cyanosis, teeth and fists clenched, lasting 1 min approximately. The seizure frequency was from once every 4 months to 2–3 times per day. Without antiepileptic treatment, the frequency of seizures increased to 7–9 times per day at 10 months. Then valproic acid was used and seizures were controlled for nearly 2 years. When the seizures relapse, the dosage of valproic acid was increased (55 mg/kg/d) without effecacy and then topiramate was added (2.5 mg/kg/d). Seizures were controlled for more than 3 years under the above treatment and recurred again at 5.5 years old, which disappeared again after increasing the dosage of valproic acid (54 mg/kg/d) and topiramate (2.5 mg/kg/d). Up to the confirmation of PNPO deficiency (7.5 years old), he had no seizures for 2 years with the treatment of valproic acid (36 mg/kg/d) and topiramate (2 mg/kg/d). But considering the specific effect of PLP or pyridoxine on this syndrome, pyridoxine (180 mg/d, 6 mg/kg/d) was still added to the previous AEDs. During the short-term follow-up of 6 months, no side effects or relapses were observed. Next, whether or not the AEDs should be continued will be discussed according to the condition of seizure control and the will of parents. He had development delay since birth. He could sit from 1 year old, and walk from 2 years old. At the last follow-up (8 years old), he could not speak or self-care.
EEG, MRI and metabolic investigation
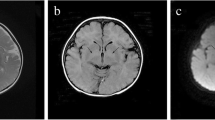
For patient 1 and 2, EEG showed multifocal epileptiform discharges at 7 months in both (Fig. 1a-b), and showed atypical hypsarrhythmia or posterior multifocal discharges respectively at 15 months (Fig. 1c-d). Several EEG examinations of patient 3 showed slow background activity with multifocal epileptiform discharges (Fig. 2). The only two EEGs of patient 4 at the age of 4 months and 10 months all showed normality during interictal periods. MRI showed periventricular white matter attenuation, bilateral ventricles widened mildly, sulci of frontal and temporal widened in the twins (at 15 months), and was normal in patient 3 and patient 4.
EEGs of patient 1 and patient 2. EEGs at 7 months: a sharp and slow waves in left anterior and right occipital and temporal areas in patient 1; and b spikes and spike waves in bilateral frontal and central regions in patient 2. EEGs at 1 year and 3 months: c atypical hypsarrhythmia in patient 1; and d spikes, spike waves and slow waves in posterior primarily in patient 2
Blood metabolic screening showed obviously decreased arginine (3.76 and 2.39 umol/L, normal range: 5–40 umol/L), aspartic acid (7.35 and 9.24 umol/L, normal range: 20–120 umol/L) and methionine (6.63 and 9.15 umol/L, normal range: 10–50 umol/L) in patient 1 and 2 respectively; showed mild elevated leucine (342 umol/L, normal range: 50–290 umol/L) and valine (306.5 umol/L, normal range: 60–290 umol/L) in patient 3; and showed mild decreased threonine (19.8 umol/L, normal range: 22–200 umol/L) in patient 4. Urinary metabolic screening showed vanillactic acid (VLA) marketly elevated in patient 1, 2 and 4 (44.8, 32.94 and 107.64 mmol/mol creatinine respectively, normal mean value: 0.9 mmol/mol creatinine), and was normal in patient 3.
Gene mutation analysis
Molecular genetic analysis revealed compound heterozygous mutations of PNPO gene (NM_018129) in all patients, including c.445_448del (p.Pro150ArgfsTer27) and c.481C > T (p.Arg161Cys) in the twins (Fig. 3); c.445_448del and c.413G > A (p.Arg138His) in patient 3 (Fig. 4); c.148G > A (p.Glu50Lys) and c.347G > A (p.Arg116Gln) in patient 4 (Fig. S1). No other gene variant was found in the genes represented on the panel. The parents of each patient had been available for testing and proved the autosomal recessive inheritance. All mutations were not detected in 100 control samples. Based on the Human Gene Mutation Database (HGMD), the pathogenicity of c.148G > A and c.347G > A had been reported in PNPO deficiency, and c.413G > A, c.481C > T and c.445_448del had never been reported before. All the 4 missense mutations here affected evolutionarily conserved residues (Fig. S2). The effects of c.148G > A and c.347G > A on PNPO activity were proved by Mills et al. (2014), decreasing PNPO activity by ~30% and ~20% respectively. The novel mutations c.413G > A and c.481C > T were predicted by PolyPhen-2 to be “probably damaging” on protein function with a probabilistic score of 1.000 in both, and predicted by MutationTaster to be “probably deleterious”.
Discussion
Dietary vitamin B6 enters the blood stream as pyridoxine, pyridoxamine and pyridoxal. Pyridoxine and pyridoxamine are converted to the active cofactor PLP through the activity of a kinase and then of PNPO (McCormick and Chen 1999). Dysfunction of PNPO leads to a deficiency of PLP and subsequently to epileptic seizures, the most common and cardinal symptom of PNPO deficiency. In this study, we report 4 cases diagnosed as PNPO deficiency clinically and genetically.
At present, some suggestive clinical presentations has been reported in patients with PNPO deficiency. For example, prematurity appeared in more than half of the patients; About 61% patients presented with seizures on the first day of life and 96% before 1 month; And myoclonus was the most common seizure type, and burst suppression in EEG was reported in 57% patients (Levtova et al. 2015). Consistently, the twins here had similar clinical presentations. They were premature and suffered from seizures during the first 24 h. A suspicious myoclonus was supposed by the clinical manifestations, though the EEG data at that time was unavailable. In addition, the twins had a sign of encephalopathy after birth, including labored and irregular breathing, and cold extremities. However, because these presentations could be observed in both birth asphyxia and some PNPO deficiency cases (Hatch et al. 2016), when we looked back to that, it was hard to discern whether it was due to true asphyxia or PNPO deficiency. None of the above presentations presented in other 2 patients, who were born at term and started seizures at 5 months and 40 days respectively. The later onset of seizures had been reviewed by Guerin et al. (2015) that 7% of the patients presented with late neonatal onset (up to 3 weeks of age), and 5% of the patients presented with early infantile onset (up to 5 months of age). Moreover, during the courses of these 2 patients, no myoclonic seizures occurred. Patient 3 was prone to seizures during fevers or infectious diseases, which was a feature commonly seen in pyridoxine dependent epilepsy (PDE) (Stockler et al. 2011; Xue et al. 2015), but was only reported in a few PNPO deficiency patients previously (Sudarsanam et al. 2014). Besides, patient 4 had frequent seizures easily controlled by AEDs, and the interictal EEG always showed normality. The normality of EEG was reported in only one PNPO deficiency patient before using PLP treatment by Hoffmann et al. (2007), but was reported in some patients with PDE (Xue et al. 2015).
PNPO deficiency was once described as neonatal seizures responding to PLP only. However, as the number of confirmed cases increased, the phenotype expanded to three subgroups: early-onset neonatal seizures responding to PLP; infantile spasms responding to PLP; and seizures beginning under 3 months of age and responding to pyridoxine (Mills et al. 2014). The twins here had a similar disease courses to the first subgroup above. But they had only partial response to both PLP and pyridoxine. A transient aggravation of seizures at the initial using PLP was observed in them, which might be because PLP had a strong inhibitory effect on PNPO activity and mutant PNPO protein might be inhibited by the administration of PLP (Plecko et al. 2014). One patient (patient 3) with onset of seizures at the age of 5 months here had a good response to pyridoxine, which expanded the onset age of seizures in the third subgroup. The mechanism of pyridoxine responsiveness is still unclear. A hypothesis proposed that the mutations carried by these patients had a small perturbation to the configuration of PNPO, allowing some pyridoxine phosphate being oxidized to PLP (Ware et al. 2014). However, Mills et al. (2014) reported that the same combination of mutations could be seen in both patients responding and not responding to pyridoxine, which suggested that pyridoxine responsiveness must be affected by some other factors, such as the age at the therapeutic trial. In addition, we found a relatively good response to AEDs in patient 4, who was controlled by valproic acid alone or combined with topiramate for 2 and 3 years respectively. Due to the good control of seizures by AEDs, there was no chance to identify the effect of pyridoxine. This might be a novel phenomenon in PNPO deficiency, but the mechanism is still unknown.
Up to now, more than 24 different disease-causing PNPO mutations have been reported, with the most common being missense/nonsense mutations (Veeravigrom et al. 2015). Here, we identified 5 different mutations, including 4 missense mutations and one deletion mutation. Among them, the novel mutation c.445_448del was expected to cause a frameshift in the protein sequence after Pro150, altering the next 27 amino acids before introducing an early stop codon, and further inducing a significant reduction in the PNPO activity. Its pathogenicity could be supported by the mutation c.448_451del reported by Guerin et al. (2015), which induces a similar change of amino acids (p.Pro150ArgfsTer27) with c.445_448del. This frameshift mutation identified in 3 of 4 patients here might indicate a possible high prevalence mutation in Chinese patients. The identical twin brothers carrying complete same mutations had very similar clinical characteristics, while the other patients had extremely different phenotypes. This suggested that the phenotype might be influenced, even decided, by the different genotypes. However, due to the limited number of cases up to now, further studies are needed to reveal the genotype-phenotype correlations in PNPO deficiency.
PLP is the cofactor for >140 enzyme-catalyzed reactions (Mills et al. 2014). The secondary dysfunction of PLP-dependent enzymes lead to abnormalities of amino acid, neurotransmitter and metabolites in cerebrospinal fluid (CSF), plasma or urine. Elevated glycine and threonine, and reduced arginine in plasma and CSF, as well as increased urinary VLA levels, had been reported in PNPO deficiency (Kuo and Wang 2002; Veeravigrom et al. 2015). However, these biomarkers were not specific, which were normal or even contrary to the descriptions above in some patients (Bagci et al. 2008; Khayat et al. 2008; Ware et al. 2014; Hatch et al. 2016). In our study, decreased plasma arginine and threonine, and elevated urinary VLA were confirmed again. In addition, decreased plasma aspartic acid and methionine, and elevated leucine and valine were also found, which were not reported in the literature. The mechanisms of these different plasma amino acids profiles are not clear yet. One possible explanation was that the level of plasma amimo acids might be more easily influenced by dietary intake and other factors. However, due to the condition limitations, its levels in CSF were not detected, which should be conducted in the future to confirm the hypothesis. Another possibility, the concentrations of metabolites above were decided by genotypes, that is to say, different gene mutations disturbed different functional domains of PNPO, leading to dysfunction of PLP-dependent enzymes in different types and severity.
As many rare diseases, it is difficult to assess the long-term outcome of PNPO deficiency. Various clinical outcomes have been reported, ranging from normal to severe development delay, even early death if left untreated. Patients who were diagnosed and treated appropriately earlier were more likely to have favorable outcomes (Veeravigrom et al. 2015). Three patients (twins and patient 4) in our study had severe development delay. For the twins, to some extent, it could be considered that intractable seizures might played a bad role on the poor outcomes. However, the above explanation might not be applied to patient 4, because only a relatively small number seizures occurred during his disease course. Besides, though experiencing a more than 3 years delay in controlling seizures with pyridoxine, patient 3 had only borderline to mild rather than severe development delay. All the above suggested that other than prompt treatment, some other factors such as genotypes might affect the disease severity also.
Conclusions
We reported 4 Chinese patients with PNPO deficiency here. The clinical characteristics of them differed greatly, including typical presentations but incomplete response to pyridoxine or PLP, later-onset seizures with a good response to pyridoxine, and seizures controlled by one or two kinds of AEDs for a few years, which expands the phenotypes of this syndrome. Besides, three novel mutations were found in this study, including the frameshift mutation c.445_448del identified in 3 of 4 patients, which might be high prevalence in PNPO deficiency in Chinese patients.
References
Bagci S, Zschocke J, Hoffmann GF et al (2008) Pyridoxal phosphate-dependent neonatal epileptic encephalopathy. Arch Dis Child Fetal Neonatal Ed 93(2):151–152
Coman D, Lewindon P, Clayton P et al (2016) PNPO deficiency and cirrhosis: expanding the clinical phenotype? JIMD Rep. 25:71–75
Guerin A, Aziz AS, Mutch C (2015) Pyridox(am)ine-5-phosphate oxidase deficiency treatable cause of neonatal epileptic encephalopathy with burst suppression: case report and review of the literature. J Child Neurol 30(9):1218–1225
Hatch J, Coman D, Clayton P et al (2016) Normal neurodevelopmental outcomes in PNPO deficiency: a case series and literature review. JIMD Rep 26:91–97
Hoffmann GF, Schmitt B, Windfuhr M et al (2007) Pyridoxal 5′-phosphate may be curative in early-onset epileptic encephalopathy. J Inherit Metab Dis 30(1):96–99
Khayat M, Korman SH, Frankel P et al (2008) PNPO deficiency: an under diagnosed inborn error of pyridoxine metabolism. Mol Genet Metab 94(4):431–434
Kuo MF, Wang HS (2002) Pyridoxal phosphate-responsive epilepsy with resistance to pyridoxine. Pediatr Neurol 26(2):146–147
Levtova A, Camuzeaux S, Laberge AM et al (2015) Normal cerebrospinal fluid Pyridoxal 5′-phosphate level in a PNPO-deficient patient with neonatal-onset epileptic encephalopathy. JIMD Rep 22:67–75
McCormick DB, Chen H (1999) Update on interconversions of vitamin B-6 with its coenzyme. J Nutr 129(2):325–327
Mills PB, Surtees RA, Champion MP et al (2005) Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5′-phosphate oxidase. Hum Mol Genet 14(8):1077–1086
Mills PB, Camuzeaux SS, Footitt EJ et al (2014) Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain 137(5):1350–1360
Plecko B, Paul K, Mills P et al (2014) Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology 82(16):1425–1433
Ruiz A, García-Villoria J, Ormazabal A et al (2008) A new fatal case of pyridox(am)ine 5′-phosphate oxidase (PNPO) deficiency. Mol Genet Metab 93(2):216–218
Stockler S, Plecko B, Gospe SM Jr et al (2011) Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol Genet Metab 104:48–60
Sudarsanam A, Singh H, Wilcken B et al (2014) Cirrhosis associated with pyridoxal 5′-phosphate treatment of pyridoxamine 5′-phosphate oxidase deficiency. JIMD Rep. 17:67–70
Veerapandiyan A, Winchester SA, Gallentine WB et al (2011) Electroencephalographic and seizure manifestations of pyridoxal 5′-phosphate-dependent epilepsy. Epilepsy Behav 20(3):494–501
Veeravigrom M, Damrongphol P, Ittiwut R et al (2015) Pyridoxal 5′-phosphate responsive epilepsy with novel mutations in the PNPO gene: a case report. Genet Mol Res 14(4):14130–14135
Ware TL, Earl J, Salomons GS et al (2014) Typical and atypical phenotypes of PNPO deficiency with elevated CSF and plasma pyridoxamine on treatment. Dev Med Child Neurol 56(5):498–502
Xue J, Qian P, Li H et al (2015) A cohort study of pyridoxine-dependent epilepsy and high prevalence of splice site IVS11+1G>a mutation in Chinese patients. Epilepsy Res 118:1–4
Xue J, Li H, Zhang Y et al (2016) Clinical and genetic analysis of two Chinese infants with Mabry syndrome. Brain Dev 38(9):807–818
Acknowledgments
We thank the patients and their families for participating.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the ethics committees of the Peking University First Hospital. For the genetic studies, written permissions were obtained from all patients’ parents or guardians.
Funding
This research was supported by grants from Beijing key laboratory of molecular diagnosis and study on pediatric genetic diseases (No: Z141107004414036).
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
PNPO gene analysis of patient 4 and his parents: c.148G > A inherited from his father, c.347G > A inherited from his mother. (GIF 212 kb)
Fig. S2
High conservation of the residues influenced by the four missense mutations here. (A) Arg161 influenced by c.481C > T in patient 1 and 2; (B) Arg138 influenced by c.413G > A in patient 3; (C) Glu50 influenced by c.148G > A and (D) Arg116 influenced by c.347G > A in patient 4. (GIF 63 kb)
Rights and permissions
About this article
Cite this article
Xue, J., Chang, X., Zhang, Y. et al. Novel phenotypes of pyridox(am)ine-5’-phosphate oxidase deficiency and high prevalence of c.445_448del mutation in Chinese patients. Metab Brain Dis 32, 1081–1087 (2017). https://doi.org/10.1007/s11011-017-9995-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-9995-2