Abstract
Cordymin is a peptide purified from the medicinal mushroom Cordyceps sinensis. The present study investigated the effects of Cordymin in prevention of focal cerebral ischemic/reperfusion (IR) injury. The right middle cerebral artery occlusion model was used in the study. The effects of Cordymin on mortality rate, neurobehavior, grip strength, glutathione content, lipid Peroxidation, glutathione peroxidase activity, glutathione reductase activity, catalase activity, Na+K+ATPase activity glutathione S transferase activity and on the regulation of C3 and C4 protein level, polymorphonuclear cells, interleukin-1β and tumor necrosis factor-α in a rat model were studied respectively. Treatment (orally) of Cordymin significantly boosted the defense mechanism against cerebral ischemia by increasing antioxidants activity related to lesion pathogenesis. Restoration of the antioxidant homeostasis in the brain after reperfusion may have helped the brain recover from ischemic injury. Moreover, Cordymin significantly inhibited infiltration of polymorphonuclear cells and IR-induced up-regulation of the brain production of C3 protein level, interleukin-1β and tumor necrosis factor-α. Cordymin significantly improved the outcome in rats after cerebral ischemia and reperfusion in terms of neurobehavioral function. Our findings suggest that cordymin have a neuroprotective effect in the ischemic brain, which is due to the inhibition of inflammation and increase of antioxidants activity related to lesion pathogenesis. Cordymin can be used as potential preventive agent against cerebral ischemia-reperfusion injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cordyceps sinensis (Caterpillar fungus) (CS) has been used as a tonic for longevity, endurance, and vitality for thousands of years by the Chinese (Guo et al. 2010, 2011; Zhu et al. 1998). Ischaemic brain injury is associated with inflammatory reaction, which consists of inflammatory cells (including the early accumulation of polymorphonuclear neutrophils and later monocytes/macrophages) and inflammatory mediators (including cytokines, chemokines, adhesion molecules and other inflammatory molecules) (Lindsberg and Grau 2003; Wang and Feuerstein 2000).Our former studies have shown that administration of Cordyceps sinensis extract significantly reduced focal cerebral ischemic/reperfusion (IR) injury (Liu et al. 2010), inhibited IR-induced up-regulation of NF-kappaB activation and the brain production of IL-1β, TNF-α, iNOS, ICAM-1, and COX-2 (Liu et al. 2011). However, there is little amount of information available about the constituents of CS which are responsible for the inhibition of IR injury. We undertook the present study to ascertain if cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, could be account for the inhibition of IR injury.
The N-terminal sequence of cordymin is AMAPPYGYRTPDAAQ, with a molecular mass of 10,906 Da (Wong et al. 2011). The present study investigated the effects of cordymin on mortality rate, neurobehavior, grip strength, glutathione content, lipid peroxidation, glutathione peroxidase activity, glutathione reductase activity, catalase activity, Na+K+ATPase activity and glutathione S transferase activity in a rat model. We also investigated the anti-inflammatory effect of cordymin in experimental middle cerebral artery occlusion/reperfusion (MCAO/R) model. These data may help in the development of effective and widely applicable pharmacological treatments for ischemic stroke patients with traditional medicines.
Materials and methods
Animals
Adult male wistar rats (2 months old and weighing 225 ± 25 g) were used in the study. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals. Care was taken to minimize discomfort, distress, and pain to the animals.
Preparation of cordymin
Cordymin was prepared by the way introduced by Wong et al. (2011). Briefly, Cultured CS mycelium was obtained from Shandong Lukang Pharmaceutical Co., Ltd. (Shandong, China). Crude C. sinensis mycelium (100 g) were homogenized in liquid nitrogen with a pestle, extracted in distilled water and centrifuged. To the resulting supernatant, ammonium acetate buffer (pH 4.5) was added until a final concentration of 20 mM was attained. The sample was loaded on an SP-Sepharose column. The adsorbed fraction was eluted with 1 M NaCl in 20 mM ammonium acetate buffer (pH 4.5), then dialyzed against distilled water and lyophilized. Then it was dissolved in 20 mM NH4OAc buffer (pH4.5) and applied on a Mono S column and eluted with the same buffer. The fraction containing Cordymin was concentrated and then purified on a Superdex 75 column in the same buffer. The single peak eluted constituted purified peptide designated as Cordymin.
Cordymin molecular mass determination
The molecular mass determination of cordymin was analyzed by means of SDS-PAGE and Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) in an Applied Biosystems 4700 Proteomics Analyzer (Wong et al. 2008).
Cordymin amino acid sequence analysis
The N-terminal amino acid sequence of cordymin was analyzed by means of automated Edman degradation using a Hewlett Packard 1000A protein sequencer equipped with an HPLC system (Wong et al. 2008).
Experimental design
The animals were separated into five groups of eight rats each. The first group served as sham (SHAM). The second group was the ischemic group (MCAO). Group I and group II were treated orally (gavage) by distilled water for 30 days respectively. Group III (Cordymin -1), Group IV (Cordymin -2) and Group V (Cordymin -4) were treated orally (gavage) by cordymin (1, 2and 4 mg/kg/day respectively) for 30 days followed by MCAO induced cerebral ischemia.
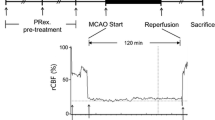
The right middle cerebral artery occlusion (MCAO) was performed using an intraluminal filament model and the method described by Longa et al. (Longa et al. 1989). In brief, the rats were anesthetized with chloral hydrate (400 mg/kg, i.p.), a 4-0 nylon monofilament with a blunt end was introduced into the external carotid artery (ECA) and advanced into the middle cerebral artery via the internal carotid artery (ICA) (17–20 mm), until a slight resistance was felt. Successful occlusion was confirmed by an 87–90% reduction in cerebral blood flow (CBF), as measured by laser-Doppler flowmetry (Shah et al. 2006).
Two hours after the induction of ischemia, the filament was slowly withdrawn and the animals were returned to their cages for a period of 22 h of reperfusion. Throughout the procedure, the body temperature was maintained at 37°C, with a thermostatically controlled infrared lamp. In sham rats, the ECA was surgically prepared for the insertion of the filament, but the filament was not inserted. The final number of rats was as follows: SHAM group n = 8; MCAO group n = 5; Cordymin -1 group n = 6; Cordymin -2 group n = 7 and Cordymin -4 group n = 8.
Neurobehavioral test
The sensorimotor integrity was conducted to assess the neurobehavior at 24 h after MCAO in rats (Lee et al. 2002). Five categories of motor neurological findings were scored: 0, no observable deficit; 1, forelimb flexion; 2, forelimb flexion and decreased resistance to lateral push; 3, forelimb flexion, decreased resistance to lateral push and unilateral circling; 4, forelimb flexion, unable or difficult to ambulate. Animals that showed the features of the higher scores also showed all the features of the lower grades.
Grip strength study
Grip strength in all the animals was measured for evaluation of neuromuscular strength, as described by Ali et al. (2004). The neuromuscular strength tests were carried out between 9:00 a.m. to 4:00 p.m. under standard laboratory conditions.
Tissue preparation
After grip strength measurement, blood samples were drawn from the tail vein from all the groups without anesthetizing the animal and serum was separated for biochemical estimations. Thereafter, the animals were sacrificed by cervical dislocation and their brains were taken out quickly for infarct size measurement. Then hippocampus (HIP) and Post-mitochondrial supernatant (PMS) was obtained for the estimation of various parameters related with oxidative stress and inflammatory cells and inflammatory mediators. The cerebral infarction was examined using 2-mm-thick slices of the cerebrum 24 h after MCAO reperfusion in rats through TTC staining.
Biochemical estimations
The PMS and HIP were used for the assay of glutathione (GSH) content, Lipid peroxidation (LPO), glutathione peroxidase (GPx) activity, glutathione reductase (GR) activity, catalase (CAT) activity, Na+-K+-ATPase activity and glutathione S transferase (GST) activity (Caliborne 1985; Svoboda and Mosinger 1981; Kamboj et al. 2008; Wheeler et al. 1990; Carlberg and Mannerviek 1975; Habig et al. 1974).
Inflammatory cells and inflammatory mediator estimations
Meloperoxidase (MPO) activity in the brain was measured to assess the extent of PMN infiltration in the ischemic cerebrum. The right middle cerebral artery (MCA) cerebrum was dissected and stored at −70°C until assay for MPO (Xu et al. 1990). The method of assaying MPO activity was according to the guide of the assay kit (Nanjing Jiancheng Bioengineering Co Ltd, China).
Protein concentration of IL-1β and TNF-α were measured by the way introduced by Cai et al. (2003). The brain concentration of IL-1β, TNF-α was then determined using a commercial ELISA kit as decscribed previously (Guo et al. 2009).
Measurement of C3 and C4 complement proteins
C3 and C4 complement proteins were measured using standard diagnostic tests and a semi-auto Biochemical Analyzer (SBA-733, Sunostik Medical Biotechnology, Jilin, China).
Statistical analysis
The data are expressed as mean ± SEM. Statistical differences between means were determined by one-way analysis of variance (anova) and multi-factor ANOVA respectively, followed by Dunnett t-test. The values of P < 0.05 were considered as significant.
Results
Molecular mass of Cordymin was determined by SDS-PAGE and mass spectrometry. It was 10,906 Da. The N-terminal sequence of Cordymin is AMAPPYGYRTPDAAQ. Both of them are consistent with the results of Wong et al. (2011).
In this study, the cerebroprotective effect of Cordymin on ischemic neuronal damage was demonstrated using focal ischemia model rats. The behavioral tasks adopted in this study were designed to assess impairments consistent with the known functional architecture of the rat brain. Twenty-four hours after MCAO in rats, neurological deficit scores were reduced in Cordymin -2 -treated rats and Cordymin-4 -treated rats. The neurobehavior for the SHAM group was 0.9 (0.6–1.1), the MCAO group was 3.7 (2.6–5.3), the Cordymin -1 group was 3.2 (2.8–4.5), the Cordymin -2 group was 2.3 (1.6–4.1) and the Cordymin -4 group was 2.5(1.6–4.5). It is clear that the behavioral abnormality was significantly developed in the MCAO group as compared with the sham (Fig. 1). In contrast, the Cordymin -2 group and Cordymin -4 significantly suppressed the development of behavioral abnormality as compared with the MCAO group (P < 0.05 and P < 0.01 respectively).
The grip strength in the SHAM group was found to be 0.963 ± 0.006 kg units. A significant decrease in the grip strength was observed in the MCAO group, as compared to the sham rats (P < 0.01). Cordymin -1, Cordymin -2 and Cordymin -4 treated rats showed a (P > 0.05, P < 0.05 and P < 0.01 respectively) increase in grip strength, as compared to the MCAO group (Table 1).
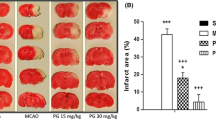
The infarction in representative sections from different groups is shown in Fig. 2. In the MCAO group (Fig. 2b ), a marked infarction (white unstained tissue) was found in the cerebrum, as compared to the sham group (Fig. 2a). The Cordymin -4 group (Fig. 2c) showed significant reduction in the infarct size (pink stained tissue), as compared to the MCAO group.
Reduced glutathione (GSH) is one of the primary endogenous antioxidant defense systems in the brain, which removes hydrogen peroxide and lipid peroxides. Decline in GSH levels could either increase or reflect oxidative status (Coyle and Puttfarcken 1993; Bains and Shaw 1997). Cordymin produced the increase in the level of GSH. Concentrations of GSH were lower in MCAO group than those in SHAM group (Table 2). It can be attributed to several factors such as cleavage GSH to cysteine, decrease in the synthesis of GSH and the formation of mixed disulfides, causing their cellular stores to be depleted (Shivakumar et al. 1995).
Lipid peroxidation (LPO) can inhibit the function of cell membrane bound receptors and enzymes. The level of LPO content adds to the proof of the increased peroxidative damage during cerebral ischemia (Love 1999). A significant increase (P < 0.01) in the content of LPO was observed in the MCAO group when compared with the SHAM group. In the Cordymin -2 and Cordymin -4 group, a significant decrease (P < 0.05) was seen in the level of LPO when compared with the MCAO group (Table 3).
Measurement of endogenous antioxidants enzymes i.e. GPx, GR, CAT and GST as well as Na+K+ATPase had been performed to estimate the amount of oxidative stress. Activities of various antioxidant enzymes and Na+K+ATPase of different groups have been listed in Table 4. The activity of endogenous antioxidant enzymes was decreased significantly (P < 0.01) in the MCAO group, as compared to the sham group, whereas in the Cordymin -2 and Cordymin -4 group, Cordymin treatment showed a significant (P < 0.05–0.01) restoration in the level of various enzyme as compared with MCAO group.
The present study was undertaken to determine whether Cordymin reduce the number of polymorphonuclear cells (PMN) in the ischemia/reperfusion-injured cerebral tissue. The activity of MPO was determined as an indicator of PMNs migration. In this study, the MPO activity was relatively low in the sham group, and significantly increased in the MCAO group. Treatment with Cordymin -2 and Cordymin -4 significantly reduced MPO activity in the ischemia/reperfusion injured cerebral tissue (Table 5). Thus, cordymin resulted in a substantial decrease in the extent of PMN infiltration in the cerebral ischemia/reperfusion.
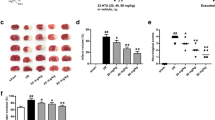
Cytokines are thus upregulated in the brain in a variety of diseases, including ischaemic brain injury. Among these inflammatory mediators, IL-1β and TNF-α are of particular importance because they play a major role in coordinating mechanisms that command pro-inflammation. Fig. 3 shows that cerebral ischemia/reperfusion significantly increased protein concentration of IL-1β in the brain. Cordymin -2 and Cordymin -4 treatment decreased the level of IL-1β by 55.6% and 58.3% as compared to the MCAO group respectively (P < 0.01). As shown in Fig. 4, the levels of TNF-α elevated significantly after cerebral ischemia/reperfusion. Cordymin -2 and Cordymin -4 suppressed this response (P < 0.05). However the same result did not occur in the Cordymin -1 treated group.
A significant rise in C3 in the MCAO group relative to the sham group is observed. However, C3 drops in the Cordymin -2 and Cordymin -4 groups (Table 6). On the contrary, the C4 value drops in the MCAO group relative to the sham group (P < .005). However, statistical analysis does not demonstrate an essential difference in the variation of cordymin doses (Table 6).
Discussion
Ischemic hypoxic brain injury often causes irreversible brain damage. The cascade of events leading to neuronal injury and death in ischemia includes the release of cytokines and free radicals, and induction of inflammation, apoptosis, and excitotoxicity (Kuroda and Siesjo 1997). Reperfusion of ischemic areas could exacerbate ischemic brain damage through the generation of reactive oxygen species. A great deal of effort has been directed toward searching for a new drug that can be used for protection of cerebral ischemia-reperfusion injury.
In the current study cordymin -treatment showed protective effects on brain injuries induced by middle cerebral artery occlusion followed by reperfusion in rats. Here we showed supplementation of cordymin significantly boosted the defense mechanism against cerebral ischemia by increasing antioxidants activity related to lesion pathogenesis. Restoration of the antioxidant homeostasis in the brain after reperfusion may have helped the brain recover from ischemic injury.
Inflammatory processes not only have fundamental roles in the pathophysiology of cerebral ischaemia but also are considered to be a risk or trigger factor for human stroke (Lindsberg and Grau 2003). Ischaemic brain injury is associated with inflammatory reaction, which consists of inflammatory cells (including the early accumulation of polymorphonuclear neutrophils and later monocytes/macrophages) and inflammatory mediators (including cytokines, chemokines, adhesion molecules and other inflammatory molecules) (Wang and Feuerstein 2000). In the current study cordymin -treatment showed protective effects on brain injuries induced by middle cerebral artery occlusion followed by reperfusion in rats by inhibiting IR-induced up-regulation of inflammatory cytokines IL-1β and TNF-α, blocking a substantial decrease in the extent of PMN infiltration in the ischemic brain.
C3 and C4 proteins are complement and acute phase proteins. The concentration of these proteins changes during inflammation and tissue damage. The C3 level rises during the inflammatory reaction. The C4 concentration changes in sinusoid way, which is a result of C4 synthesis and consumption processes (Cakosiñski et al. 2009). In our studies, cordymin significantly reduced the level of C3 protein. We hypothesized that it is the result of inhibit the inflammatory process by cordymin. However, statistical analysis does not demonstrate an essential difference in the variation of cordymin doses on the level of C4 protein. It probably need more hours to show the C4 concentration changes in sinusoid way.
Our findings suggest that cordymin have a neuroprotective effect in the ischemic brain, which is due to the inhibition of inflammation and increase of antioxidants activity related to lesion pathogenesis. Cordymin can be used as potential preventive agent against cerebral ischemia-reperfusion injury.
References
Ali A, Ahmad FJ, Pillai KK, Vohora D (2004) Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav 5:322–328
Bains JS, Shaw CA (1997) Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 25:335–338
Cai Z, Pang Y, Lin S, Rhodes PG (2003) Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res 975:37–47
Cakosiñski I, Dobrzyñski M, Cakosiñska M, Seweryn E, Bronowicka-Szydeko A, Dzierzba K, Ceremuga I, Gamian A (2009) Characterization of an inflammatory response (Polish). Post Hig Med Doœw 63:395–408
Caliborne A (1985) Catalase activity. In: Wakd G (ed) CRC hand book of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–294
Carlberg I, Mannerviek B (1975) Glutathione reductase levels in rat brain. J Biol Chem 250:5475–5480
Coyle JT, Puttfarcken PO (1993) Oxidative stress, glutamate and neurodegenera-tive disorders. Science 262:689–695
Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F (2009) Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol 612:54–60
Guo JY, Han CC, Liu YM (2010) A contemporary treatment approach to both diabetes and depression by Cordyceps sinensis, rich in vanadium. Evid Based Complement Alternat Med 7:387–389
Guo J, Li C, Wang J, Liu Y, Zhang J (2011) Vanadium-Enriched Cordyceps sinensis, a Contemporary Treatment Approach to Both Diabetes and Depression in Rats. Evid Based Complement Alternat Med 2011:450316
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–139
Kamboj SS, Chopra K, Sandhir R (2008) Neuroprotective effect of N-acetylcysteine in the development of diabetic encephalopathy in streptozotocin-induced diabetes. Metab Brain Dis 23:427–443
Kuroda S, Siesjo BK (1997) Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci 4:199–212
Lee EJ, Chen HY, Wu TS, Chen TY, Ayoub IA, Maynard KI (2002) Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J Neurosci Res 68:636–645
Lindsberg PJ, Grau AJ (2003) Inflammation and infections as risk factors for ischemic stroke. Stroke 34:2518–2532
Liu Z, Li P, Zhao D, Tang H, Guo J (2010) Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav Brain Funct 6:61
Liu Z, Li P, Zhao D, Tang H, Guo J (2011) Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation 34(6):639–644
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9:119–131
Shah ZA, Namiranian K, Klaus J, Kibler K, Dore S (2006) Use of an optimized transient occlusion of the middl cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis 15:133–138
Shivakumar BR, Kolluri SV, Ravindranath V (1995) Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J Pharmacol Exp Ther 274:1167–1173
Svoboda P, Mosinger B (1981) Catecholamines and the brain microsomal Na,K-adenosinetriphosphatase-I. Protection against lipoperoxidative damage. Biochem Pharmacol 30:427–432
Wang X, Feuerstein GZ (2000) Role of immune and inflammatory mediators in CNS injury. Drug News Perspect 13:133–140
Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW Jr (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activity. Anal Biochem 184:193–199
Wong JH, Wang HX, Ng TB (2008) Marmorin, a new ribosome inactivating protein with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the mushroom Hypsizigus marmoreus. Appl Microbiol Biotechnol 81:669–674
Wong JH, Ng TB, Wang H, Sze SC, Zhang KY, Li Q, Lu X (2011) Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 18:387–392
Xu JA, Hsu CY, Liu TH, Hogan EL, Perot PL Jr, Tai HH (1990) Leukotriene B4 release and polymorphonuclear cell infiltration in spinal cord injury. J Neurochem 55:907–912
Zhu JS, Halpern GM, Jones K (1998) The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis. J Altern Complem Med 4:289–303
Acknowledgments
This work was supported by National Natural Science Foundation of China(No.81173166);China Postdoctoral Science Foundation Special Project(No.2010030224);China Postdoctoral Science Foundation (No.20090450546), the project from Key Laboratory of Mental Health, Chinese Academy of Sciences, NNSF grant (30800301, 31170992), the Key New Drugs Innovation project from Ministry of Science and Technology (2010ZX09102-201-018) and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-254, KSCX2-EW-Q-18 and KSCX2-EW-J-8).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, J., Liu, YM., Cao, W. et al. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab Brain Dis 27, 159–165 (2012). https://doi.org/10.1007/s11011-012-9282-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9282-1