Abstract
23-Hydroxytormentic acid (23-HTA) is an important herbal medicine purified from immature fruits of African Rubus aceae (Rosaceae). This study was carried out to examine the protection properties and potential mechanisms of 23-HTA against cerebral ischemia/reperfusion (I/R) damage. Rats underwent middle cerebral artery occlusion/reperfusion (MCAO/R) 2/24 h. All animals were euthanized 24 h after reperfusion. Rats were injected with various concentrations of 23-HTA intraperitoneally. Evaluations of infarct volumes, neurological deficit, and brain water contents were carried out to assess the outcome of 23-HTA treatment. The results showed that 23-HTA reduced infarct volumes, brain water content, and neurological deficit in a dosage-dependent manner. 23-HTA can also significantly reduce the numbers of TUNEL-positive cells, the expression levels of Bax, caspase-3, lipid peroxidation, Sod 1, Sod 2, catalase, and pro-inflammatory cytokines TNF and IL-1β and increase the expression levels of Bcl-2 and p-Akt. 23-HTA has a neuroprotective effect due to its anti-apoptotic, antioxidant, and anti-inflammatory effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemic vascular disease has become a severe disease with high morbidity, disability, and mortality worldwide (Faris et al. 2020; Murphy and Werring 2020; Shukla et al. 2017). Timely restoration of blood supply to the brain tissue is the best way to save the life of patients with brain ischemia (Faris et al. 2020; Mayor and Tymianski 2018). However, the restoration of blood flow perfusion brings new damage to the brain tissue, which is ischemia-reperfusion (I/R) damage (Xiao et al. 2017). Searching for effective approaches to reduce reperfusion damage has become a popular research topic (LeBaron et al. 2019; Sarkar et al. 2019; Yang et al. 2019). The mechanism of I/R damage is complex (Chen et al. 2018; Liao et al. 2020). At present, it is believed that multiple biologically active molecules and intracellular signal transduction pathways are involved, such as reperfusion-caused bursts of reactive oxygen species (ROS), nitric oxide (NO), cellular inflammation, autophagy, and apoptosis. Irregular changes of these factors may eventually aggravate brain damage and endanger patients’ lives (Chumboatong et al. 2017). Extensive efforts have been made on exploring the mechanisms of I/R damage. A variety of prevention and treatment methods have been developed to reduce cerebral I/R damage, such as drug pretreatment and ischemic post-treatment (Arslan et al. 2019; He et al. 2017).
It is well-established that inflammation, oxidation, and cell apoptosis could further damage brain cells and tissues. Previous studies have revealed a variety of antioxidants to attenuate the cerebral I/R damage in animals, such as Clostridium butyricum pretreatment (Sun et al. 2016), thalidomide (Palencia et al. 2015), hydrogen sulfide (Gheibi et al. 2014). The extract and effective compound from herbal medicine has also been demonstrated to have protective effects on cerebral I/R damage, including Danhong injection (He et al. 2012), Qishiwei Zhenzhu pills (Xu et al. 2020), and Bunao Fuyuan decoction (Gao and Gu 2020). Chinese herbal medicine monomer refers to a single compound extracted from Chinese herbal medicine. An increasing number of Chinese herbal medicines have been shown to be effective in treating cerebral I/R damage. For example, galuteolin, salidroside, and apigenin have protective effects on cerebral I/R damage (Ling et al. 2020; Yang et al. 2020b; Zhu et al. 2020). In the present study, we aimed to explore a new drug for the ischemic post-treatment.
Rubiaceae are a family of flowering plants, such as coffee, madder, or bedstraw family, which consist of terrestrial trees, shrubs, lianas, and herbs (Della et al. 2018). African Rubus aceae is a genus of Rosaceae in the Rosaceae family, including blackberries and raspberries (Henderson 2006). 23-Hydroxytormentic acid (23-HTA) is an important herbal medicine purified from immature fruits of African Rubus aceae (Rosaceae) (Nam et al. 2006). In a previous study, it was observed that 23-HTA, isolated from unripe fruits of Rubus coreanus, had anti-nociceptive, anti-inflammatory, anti-gastropathic, anti-oxidation, and anti-rheumatic effects in animal models (Kim et al. 2011a; Nam et al. 2006; Youn et al. 2017). Besides, it was also revealed that 23-HTA could ameliorate cisplatin-related toxicity by modulating the expression of antioxidant enzymes (Kim et al. 2011a). Considering its superior ability in anti-inflammation and anti-oxidation, we investigated the protective effect of 23-HTA on cerebral I/R damage in rats and the possible mechanisms underlying these effects.
Materials and methods
Rats
Adult male Sprague-Dawley rats (around 200 g) were obtained from the Animal Center of Shandong University. Rats were kept at 24 °C with a 12/12-h day/night cycle. Rats had free access to eat or drink. All animal experiments were approved by the Animal Usage Committee of 80th Army Hospital of the Chinese People’s Liberation Army and carried out at this hospital. All experimental procedures were conducted following the National Institute of Health Laboratory Animal Protection and Use Guidelines.
Focal cerebral I/R model
Briefly, rats were anesthetized intraperitoneally (IP) with 3% sodium pentobarbital (30 mg/kg body weight, P3761, Sigma, USA, USA), and the temperature was kept at 37 °C throughout the experiment. After skin and muscle incisions, the left common carotid artery (CCA) was exposed and clamped with arterial clamps to isolate and ligate the external carotid artery (ECA). A nylon monofilament with a heparin-coated tip (standard diameter of 0.25–0.28 mm) (Beijing Shunsheng Biotechnology Co., Ltd., Beijing, China) was inserted from the CCA to the internal carotid artery (ICA) until resistance was encountered. A laser Doppler flowmeter (LDF, PeriFlux5000, China) reduced regional cerebral blood flow (rCBF) to less than 20% of baseline in the cerebral cortex region supplied by the midbrain, indicating successful occlusion. After 2 h of MCAO (Li et al. 2017; Wen et al. 2018), reperfusion was conducted by taking out the monofilament. The sham rats had MCAO surgery but without monofilament.
Drug injection
A total of 150 rats were randomly divided into 5 groups: (1) In the sham operation group (n = 30), except that no line was inserted in CCA, all were treated with MCAO surgery. (2) In the I/R group (n = 30), ligated for 2 h and reperfusion for 24 h. (3–5) In the low-dosage 23-HTA (SMB00242, Sigma, purity ≥ 98%; Fig. 1a) (20 mg/kg) (n = 30), medium-dosage 23-HTA (40 mg/kg) (n = 30), and high-dosage 23-HTA (80 mg/kg) (n = 30) group, rats were given a slow intraperitoneal injection of 23-HTA (20, 40, 80 mg/kg) 2 h after the ischemic attack. In this study, we examined the effect of 23-HTA (5–160 mg/kg) on focal cerebral ischemic damage and explored the optimal dosage that provides the greatest protective effects against brain I/R damage (data not available display). 23-HTA was dissolved in ethanol and diluted with physiological saline to a final ethanol concentration of 5% (Li et al. 2018). Rats in the sham operation group and the I/R group received an equal volume of vehicle (5% ethanol in normal saline) simultaneously. After 24 h of reperfusion, rats were euthanized. For euthanasia, rats were deeply anesthetized intraperitoneally with 3% sodium pentobarbital (30 mg/kg body weight). Sacrifice was through cervical dislocation. The specific experimental design of this study is shown in Fig. 1b.
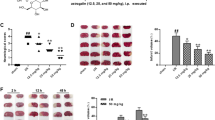
Effect of 23-HTA on infarct volumes, neurobehavioral outcome, and brain water contents in rats with brain I/R. a Chemical structure of 23-HTA (C30H48O6, molecular weight: 504.70). b Intraperitoneal injection of vehicle (5% ethanol in normal saline) and 23-HTA (20, 40, 80 mg/kg). The sham group and I/R group had the same amount of simultaneously 2 h after ischemic attack. c, d Effect of 23-HTA on infarct volume in brain I/R rats. e Effect of 23-HTA on neurobehavioral prognosis in rats with brain I/R. f Effect of 23-HTA on the brain water content in rats with brain I/R. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Evaluations of infarct volumes, neurological deficit, and brain water contents
Cerebral infarction volume was measured with 2,3,5-triphenyltetrazole chloride (TTC, T8877, Sigma) staining. Briefly, after reperfusion, rats (n = 6) were anesthetized and decapitated. The brain was dissected and sectioned. Five 1.5-mm-thick sections were sliced and stained with 1% TTC for 30 min and then fixed with 4% paraformaldehyde. Infarcted tissue was unstained (white), while normal tissue was stained red. The stained sections were digitally photographed and the volume of each section was determined using the ImageJ software (National Institutes of Health, Bethesda, MD). Percent infarct was calculated by dividing the infarct volume by the total volume of all sections.
According to the method described previously, a single observer blinded to group assignment performed neurological testing (Longa et al. 1989) as described: 0 points, rats behaved normally; 1 point, rats were unable to fully extend the left foreleg; 2 points, rats turned around; 3 points, rats fell to the left; and 4 points, rats could not move by itself and loses consciousness.
Brain water content was measured 24 h after reperfusion (n = 6). The infarcted cerebral hemispheres were quantified with an electronic scale (wet weight), dried in a drying cabinet at 105 °C overnight, and then weighed (dry weight). The total brain fluid was calculated as [(wet-dry weight)/wet weight] × 100% (Swanson et al. 1990).
TUNEL staining
The TUNEL kit was used to detect apoptosis in the cerebral cortex (coronal slices, cortex, mainly III–IV, bregma, − 1.0 to 1.0 mm range, n = 6) following the manufacturer’s instructions (C1091, Beyotime). The frozen tissue sections (5 μm) were dried and immersed in 1% sodium hydroxide 80% ethanol buffer for 5 min, rinsed in 70% ethanol for 2 min, and then rinsed in distilled water. After washing with phosphate-buffer saline (PBS) three times, sections were stained with TUNEL following the manufacturer’s instructions. The number of TUNEL-positive cells was counted in 6 randomly selected visual fields under × 400 magnification.
TNF and IL-1β immunoassay
The infarcted areas of the cortex were homogenized by a protease inhibitor (Sigma, USA) in PBS and centrifuged. The supernatant was stored at − 80 °C prior to use. The expressions of TNF and IL-1β were determined using TNF and IL-1β ELISA kits (RTA00, RLB00, R&D Systems, USA).
Lipid hydroperoxide
The degree of lipid peroxidation was measured using an LPO assay kit (705002, Cayman Chemical Company, Ann Arbor, MI, USA), and the level of lipid hydrogen peroxide (LPO) in the infarcted area was measured. Results were represented in μM lipid hydroperoxide per milligram of protein.
Western blotting analysis
The infarct area organization came from the left cerebral hemispheres (the damaged hemisphere for MCAO) (n = 6). The protein from the damaged area was separated on SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked with 3% skimmed milk in PBS IX and blocked with corresponding primary antibodies (Akt, sc-377457; pAkt, sc-377556; SOD1, sc-17767; SOD2, sc-137254; catalase, sc-271803; Bcl-2, sc-7382; Bax, sc-70405; caspase-3, sc-7272; 1: 500, and β- Actin, sc-8432; 1: 2000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and inducible nitric oxide synthase (iNOS) (ab178945; 1:500, Abcam) at 4 °C for 24 h. The membrane was then treated with HRP-conjugated secondary antibodies (1:1000) (sc-2004, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signals were detected using the ECL kit (Santa Cruz Biotech., USA). The optical density of the bands was quantitatively analyzed using the ImageJ program (Scion, Frederick, MD, USA).
Data analyses
Data were expressed as mean ± stand deviation (SD). The normality of the distribution of data was tested by the Kolmogorov-Smirnov test. Differences between means were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test if data conformed to normality and homogeneity of variance, or by a non-parametric method (Kruskal-Wallis test) followed by the Mann-Whitney U test using the Bonferroni correction if not. The difference was considered significant when p < 0.05.
Results
Effect of 23-HTA on infarct volumes, behavioral prognosis, and water contents after brain I/R damage
To assess the effect of 23-HTA on brain I/R damage in MCAO/R rat models, rats were treated with three different dosages of 23-HTA. The infarct volume of rat brain was evaluated using TTC staining. As shown in Fig. 1 c and d, in contrast to the sham operation group, the infarct volume in the I/R rats was markedly enhanced (p < 0.01). Administration of 23-HTA at 2 h after occlusion could significantly reduce the infarct volume caused by three dosages of I/R (p < 0.05, p < 0.01). Moreover, neurological score and brain water contents were also quantified to assess the effect of 23-HTA after focal I/R. The results showed that brain I/R greatly elevated neurological score and brain water content (P < 0.01), but 23-HTA at 20, 40, and 80 mg/kg greatly decreased these outcomes (p < 0.05, p < 0.01) (Fig. 1 e and f). These data demonstrated the effect of 23-HTA against brain I/R damage.
Effect of 23-HTA on neuronal apoptosis
As shown in Fig. 2 a and b, cell apoptosis was elevated greatly in the infarcted areas of the I/R rats (p < 0.01). In contrast, in the 23-HTA-treated rats, TUNEL-positive staining was markedly decreased (p < 0.05, p < 0.01), indicating that 23-HTA reduced cell mortality.
Effect of 23-HTA on neuronal death using TUNEL staining. Rats undergo 2 h of ischemia and 24 h of reperfusion. 23-HTA (20, 40, 80 mg/kg) was given 2 h after ischemia. a Representative pictures of TUNEL staining in each group (× 400 magnification, Bar = 50 μm.). b Quantitative analysis of neuronal apoptosis. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
To examine the effect of 23-HTA on I/R-caused neuronal apoptosis, the expressions of Bcl-2, Bax, and caspase-3 were measured by Western blotting (Fig. 3a). It showed that the expression levels of Bcl-2 protein were enhanced, while the expression levels of Bax and caspase-3 were reduced in the 23-HTA-treated group compared to that in the I/R group (P < 0.05, P < 0.01) (Fig. 3b–d). The Bcl-2/Bax ratio increased in the 23-HTA treatment group (data not shown). These results indicated that 23-HTA inhibited I/R-caused apoptosis.
Effect of 23-HTA on I/R-caused apoptosis. Rats undergo 2 h of ischemia and 24 h of reperfusion. 23-HTA (20, 40, 80 mg/kg) was given 2 h after ischemia. a Western blotting analysis of the expression of Bcl-2, Bax, and caspase-3. b Bcl-2, c Bax, and d caspase-3 normalized to β-actin levels. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Akt is a Ser/Thr protein kinase that can be activated through phosphatidylinositol 3-kinase. Akt could promote cell survival and prevent cell death through the inactivation of pro-apoptosis protein and activation of anti-apoptosis protein. To assess the effect of 23-HTA on Akt activities from I/R, Akt phosphorylation was detected by Western blotting. It showed increased Akt phosphorylation in the 23-HTA-treated group compared to that in the I/R group (p < 0.01) (Fig. 4 a and b). These results indicated that 23-HTA induced Akt activation.
Effect of 23-HTA on Akt activation. Rats undergo 2 h of ischemia and 24 h of reperfusion. 23-HTA (20, 40, 80 mg/kg) was given 2 h after ischemia. a Western blotting determination of p-Akt and Akt in sham rat and rat under treatment of vehicle or 23-HTA 24 h after reperfusion. b Histogram showing the relative amount of p-Akt/Akt. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Effects of 23-HTA on I/R-caused oxidation stress
Oxidation stress is important in the initiation and development of cerebral ischemic damage. To evaluate the effect of 23-HTA on I/R-caused oxidative stress, LPO analysis was used to quantify lipid hydroperoxides produced by lipid peroxidation in the infarcted area. It showed that the administration of 23-HTA significantly reduced hydroperoxide levels relative to the I/R group (p < 0.05, p < 0.01) (Fig. 5a). Anti-oxidation activation may be related to the factors in the prevention of neural damage resulted from ischemia-related oxidation stress. Western blotting was conducted to examine the expression of Sod1, Sod2, and catalase (Fig. 5b). It showed that the administration of 23-HTA increased the expression levels of Sod 1 (Fig. 5c), Sod 2 (Fig. 5d), and catalase (Fig. 5e) compared to that in the I/R group (p < 0.05, p < 0.01). These data indicated that 23-HTA reduced I/R-caused oxidative stress.
Effect of 23-HTA treatment on I/R-caused oxidative stress. Rats undergo 2 h of ischemia and 24 h of reperfusion. 23-HTA (20, 40, 80 mg/kg) was given 2 h after ischemia. a Lipid peroxidation by lipid peroxidation in the infarcted area was detected by lipid hydrogen peroxide assay in sham rats and rats under treatment of excipients or 23-HTA 24 h after reperfusion, the level of hydrogen. b Western blotting analysis of the expression of Sod 1, Sod 2, and catalase. c Levels of Sod 1, Sod 2, and catalase normalized to β-actin levels. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Effect of 23-HTA on I/R-caused inflammations
To assess the effect of 23-HTA on I/R-caused inflammations, the concentrations of the pro-inflammation cytokines IL-1β and TNF-α were detected using ELISA. 23-HTA injection markedly reduced the concentrations of TNF-α (p < 0.05, p < 0.01) (Fig. 6a), as well as IL-1β (p < 0.05, p < 0.01) (Fig. 6b) compared to that in the I/R rats.
Effect of 23-HTA treatment on I/R-caused inflammation. Rats undergo 2 h of ischemia and 24 h of reperfusion. 23-HTA (20, 40, 80 mg/kg) was given 2 h after ischemia. a TNF and b IL-1β level in sham rats and rats under treatment of vehicle or 23-HTA 24 h after reperfusion. n = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Effect of 23-HTA on I/R-caused oxidative stress
To assess the effect of 23-HTA on I/R-caused oxidative stress, the concentrations of inducible nitric oxide synthase (iNOS) were detected using Western blot. 23-HTA injection markedly reduced the expression levels of iNOS protein (p < 0.05, p < 0.01) (Fig. 7) compared to that in the I/R rats.
Effect of 23-hydroxytormentic acid on inducible nitric oxide synthase (iNOS) protein expression in the brain tissues of MCAO/R rats. a Western blotting analysis of iNOS. b Levels of iNOS normalized to β-actin levels. N = 6. Compared with the sham operation group, ##p < 0.01; compared with the I/R group, *p < 0.05, **p < 0.01
Discussion
Therapeutic treatment is involved in the prevention and treatment of cerebral I/R injury that usually focuses on relieving oxidative stress, inflammatory response, Na+/H+ exchangers, NO metabolism, and metabolic additives (Hao et al. 2020). It is widely accepted that infarct volumes, behavioral prognosis, and water contents after brain I/R damage could be effective evaluation indicators for the effects of a therapeutic treatment (Cao et al. 2007; Li et al. 2020; Wang et al. 2021; Yang et al. 2020a). In a previous study, it was observed that 23-hydroxytormentic acid (23-HTA), isolated from unripe fruits of Rubus coreanus, had anti-nociceptive, anti-inflammatory, anti-gastropathic, anti-oxidation, and anti-rheumatic effects in animal models (Kim et al. 2011b; Sohn et al. 2011; Youn et al. 2017). In our study, we firstly assessed the effect of 23-HTA on brain I/R damage in MCAO/R rat models using different dosages of 23-HTA. We found that in the I/R rats, the infarct volume was markedly enhanced, infarct volume was greatly suppressed, and neurological score and brain water content were greatly elevated. Treatment of 23-HTA 2 h after occlusion can significantly attenuate these effects caused by I/R. In comparison with I/R rats and the sham group, it is obvious that 23-HTAs have protective effects over brain I/R damage. Cell assay showed that 23-HTA at a concentration of 25 μM does not cause normal human dermal fibroblast cytotoxicity, and 15 μM is the IC50 (Youn et al. 2017). Animal experiments have shown that 10 mg/kg 23-HTA pretreated significantly reduced cisplatin-induced nephrotoxicity (Sohn et al. 2011). Our preliminary experiments show that the protective effect of 23-HTA on brain injury caused by I/R has a dose-dependent manner, and the highest concentration of 160 mg/kg has the best effect (Supplemental Figure 1). It indicates that high concentrations of 23-HTA may directly act in brain tissue through the blood-brain barrier (BBB). However, this setting of the highest concentration requires further experiments to verify.
During I/R, brain cells produce ROS, and vascular endothelial cells produce excessive amounts of NO (Dubois-Deruy et al. 2020; Ravingerova et al. 2020). They react with superoxide anions to convert them into more active peroxynitrous anions, which increase oxidative stress injury in I/R neuronal (Chai et al. 2019). In addition, ROS stimulates the expression of cytokines and adhesion molecules, mediates inflammation and immune response, and aggravates brain tissue reperfusion injury (Crack and Wong 2008). 23-HTA is a hexacyclic triterpenoid compound extracted. Asiatic acid, another triterpene compound extracted from Chinese herbal medicine, pretreatment protected cardiomyocytes from ROS-mediated autophagy in MIRI (Yi et al. 2020). In our study, we evaluated the effect of 23-HTA on I/R-caused oxidative stress. We found that 23-HTA significantly reduced relative hydroperoxide and iNOS levels to the I/R group. The Western blotting results also revealed that the expression levels of Sod1, Sod2, catalase, and iNOS were greatly increased under the administration of 23-HTA, compared with that in the I/R group. To the best of our knowledge, this study was the first to report that 23-HTA reduced I/R-caused oxidative stress. These results confirmed our hypothesis. 23-HTA and other monomers extracted from Rubiaceae, such as jasminoidin, have protective effects on brain injury after I/R (Li et al. 2016; Li et al. 2018; Zhang et al. 2019). This anti-oxidation effect is in agreement with previous studies.
Microvascular dysfunction caused by inflammatory response is one of the most important causes of secondary injury of cerebral I/R (Chen et al. 2009). The inflammatory response of brain tissue is out of control, which increases the production of a variety of inflammatory mediators, causing aggravation in the damage of the I/R zone (Crack and Wong 2008; He et al. 2020). We found that the expression levels of pro-inflammation cytokines IL-1β and TNF-α were greatly reduced by 23-HTA treatment. Consistent with previous studies, we again established the anti-inflammation effect of 23-HTA on cerebral I/R damage.
The mechanism of cerebral I/R injury is complex. It mainly includes events such as ROS, inflammatory response, and cell apoptosis, forming a complex network that promotes each other (Patel et al. 2020; Xing et al. 2008). During these processes, many toxic factors are released, which triggers the caspase cascade and eventually leads to apoptosis in brain cells during reperfusion (Yin et al. 2013). Our results also revealed that cell apoptosis was elevated greatly in the infarcted part of the I/R rats. However, 23-HTA could help reduce cell mortality. The expression of Bcl-2 anti-apoptotic protein was enhanced, and the expressions of Bax pro-apoptotic protein and caspase-3 were suppressed under the treatment of 23-HTA. In addition, we also found that 23-HTA induced Akt activation. Our results further support the protective effect of 23-HTA on cerebral I/R damage through inhibition of cell apoptosis.
Conclusion
Taken together, our results indicated that 23-HTA has a neuroprotective effect due to its anti-apoptotic, antioxidant, and anti-inflammation effect.
References
Arslan EA, Arslan E, Yaman SÖ, Karahan SC (2019) The effects of edaravone on experimental brain ischemia/reperfusion injury in rats
Cao C-x, Yang Q-w, Lv F-l, Cui J, Fu H-b, Wang J-z (2007) Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun 353:509–514
Chai S et al (2019) Activation of G protein–coupled estrogen receptor protects intestine from ischemia/reperfusion injury in mice by protecting the crypt cell proliferation. Clin Sci 133:449–464
Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu CA, Liu XY, Li CL, Zhao SM, Campbell W (2009) Adiponectin protects against cerebral ischemia–reperfusion injury through anti-inflammatory action. Brain Res 1273:129–137
Chen C, Li T, Zhao Y, Qian Y, Li X, Dai X, Huang D, Pan T, Zhou L (2018) Platelet glycoprotein receptor Ib blockade ameliorates experimental cerebral ischemia–reperfusion injury by strengthening the blood–brain barrier function and anti-thrombo-inflammatory property. Brain Behav Immun 69:255–263
Chumboatong W, Thummayot S, Govitrapong P, Tocharus C, Jittiwat J, Tocharus J (2017) Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem Int 102:114–122
Crack PJ, Wong CH (2008) Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem 15:1–14
Della A, Paraskeva-Hadjichambi D, Hadjichambis AC (2018) Rubiaceae, Psychotria poeppigiana, hot lips, labios de puta Adansonia
Dubois-Deruy E, Peugnet V, Turkieh A, Pinet F (2020) Oxidative Stress in Cardiovascular Diseases Antioxidants (Basel) 9 https://doi.org/10.3390/antiox9090864
Faris P, Negri S, Perna A, Rosti V, Guerra G, Moccia F (2020) Therapeutic potential of endothelial colony-forming cells in ischemic disease: strategies to improve their regenerative efficacy. Int J Mol Sci:21. https://doi.org/10.3390/ijms21197406
Gao L, Gu W (2020) Efficacy of Bunao Fuyuan decoction on cerebral ischemia and reperfusion injury in vitro. J Tradit Chin Med 40:758–765. https://doi.org/10.19852/j.cnki.jtcm.2020.05.005
Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, Mehrjerdi FZ, Gheibi A (2014) Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci 54:264–270
Hao Y, Xin M, Feng L, Wang X, Wang X, Ma D, Feng J (2020) Review cerebral ischemic tolerance and preconditioning: methods, Mechanisms, Clinical Applications, and Challenges. Front Neurol 11:812. https://doi.org/10.3389/fneur.2020.00812
He Y, Wan H, Du Y, Bie X, Zhao T, Fu W, Xing P (2012) Protective effect of Danhong injection on cerebral ischemia–reperfusion injury in rats. J Ethnopharmacol 144:387–394
He Q, Li Z, Wang Y, Hou Y, Li L, Zhao J (2017) Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int Immunopharmacol 50:208–215
He J, Wu H, Zhou Y, Zheng C (2020) Tomentosin inhibit cerebral ischemia/reperfusion induced inflammatory response via TLR4/ NLRP3 signalling pathway - in vivo and in vitro studies. Biomed Pharmacother 131:110697. https://doi.org/10.1016/j.biopha.2020.110697
Henderson L (2006) Comparisons of invasive plants in southern Africa originating from southern temperate, northern temperate and tropical regions. Bothalia 36:201–222
Kim Y-H et al (2011a) 23-Hydroxytormentic acid and niga-ichgoside F1 isolated from Rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK1 cells. Biol Pharm Bull 34:906–911
Kim YH et al (2011b) 23-Hydroxytormentic acid and niga-ichgoside f(1) isolated from Rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK(1) cells. Biol Pharm Bull 34:906–911. https://doi.org/10.1248/bpb.34.906
LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J (2019) A new approach for the prevention and treatment of cardiovascular disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 24. https://doi.org/10.3390/molecules24112076
Li B et al (2016) Vertical and horizontal convergences of targeting pathways in combination therapy with Baicalin and Jasminoidin for cerebral ischemia. CNS Neurol Disord Drug Targets 15:740–750. https://doi.org/10.2174/1871527315666160321111053
Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, Zhang C (2017) An antagomir to MicroRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol 54:2901–2921. https://doi.org/10.1007/s12035-016-9842-1
Li H, Wang J, Wang P, Zhang Y, Liu J, Yu Y, Li B, Wang Z (2018) Gene expression profiling confirms the dosage-dependent additive neuroprotective effects of Jasminoidin in a mouse model of ischemia-reperfusion injury. Biomed Res Int 2018:2785636. https://doi.org/10.1155/2018/2785636
Li YQ, Hui ZR, Tao T, Shao KY, Liu Z, Li M, Gu LL (2020) Protective effect of hypoxia inducible factor-1alpha gene therapy using recombinant adenovirus in cerebral ischaemia-reperfusion injuries in rats. Pharm Biol 58:438–446. https://doi.org/10.1080/13880209.2020.1762667
Liao S, Apaijai N, Chattipakorn N, Chattipakorn SC (2020) The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury. Arch Biochem Biophys 695:108629. https://doi.org/10.1016/j.abb.2020.108629
Ling C, Lei C, Zou M, Cai X, Xiang Y, Xie Y, Li X, Huang D, Wang Y (2020) Neuroprotective effect of apigenin against cerebral ischemia/reperfusion injury. J Int Med Res 48:300060520945859. https://doi.org/10.1177/0300060520945859
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Mayor D, Tymianski M (2018) Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 134:178–188
Murphy SJ, Werring DJ (2020) Stroke: causes and clinical features. Medicine (Abingdon) 48:561–566. https://doi.org/10.1016/j.mpmed.2020.06.002
Nam J-H, Jung H-J, Choi J, Lee K-T, Park H-J (2006) The anti-gastropathic and anti-rheumatic effect of niga-ichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus coreanus in a rat model. Biol Pharm Bull 29:967–970
Palencia G, Núñez-Medrano JÁ, Ortiz-Plata A, Farfán DJ, Sotelo J, Sánchez A, Trejo-Solís C (2015) Anti-apoptotic, anti-oxidant, and anti-inflammatory effects of thalidomide on cerebral ischemia/reperfusion injury in rats. J Neurol Sci 351:78–87
Patel AMR, Apaiajai N, Chattipakorn N, Chattipakorn S (2020) The protective and reparative role of colony stimulating factors in the brain with cerebral ischemia / reperfusion injury neuroendocrinology https://doi.org/10.1159/000512367
Ravingerova T et al (2020) The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int J Mol Sci:21. https://doi.org/10.3390/ijms21217889
Sarkar S, Chakraborty D, Bhowmik A, Ghosh MK (2019) Cerebral ischemic stroke: cellular fate and therapeutic opportunities. Front Biosci (Landmark Ed) 24:435–450
Shukla V, Shakya AK, Perez-Pinzon MA, Dave KR (2017) Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflamm 14:21
Sohn SI et al (2011) The ameliorative effect of 23-hydroxytormentic acid isolated from Rubus coreanus on cisplatin-induced nephrotoxicity in rats. Biol Pharm Bull 34:1508–1513. https://doi.org/10.1248/bpb.34.1508
Sun J, Ling Z, Wang F, Chen W, Li H, Jin J, Zhang H, Pang M, Yu J, Liu J (2016) Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett 613:30–35
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293. https://doi.org/10.1038/jcbfm.1990.47
Wang P, Sui HJ, Li XJ, Bai LN, Bi J, Lai H (2021) Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer’s disease. Neural Regen Res 16:757–764. https://doi.org/10.4103/1673-5374.295349
Wen Z et al (2018) 6'-O-Galloylpaeoniflorin attenuates cerebral ischemia reperfusion-induced neuroinflammation and oxidative stress via PI3K/Akt/Nrf2 activation. Oxidative Med Cell Longev 2018:8678267. https://doi.org/10.1155/2018/8678267
Xiao T, Palida K, Dan Y (2017) Luteolin protects brain injury and improves endogenous neural stem cells proliferation on cerebral ischemia-reperfusion injury in rat. Chin J Biochem Pharm 37:37–40, 43
Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S (2008) Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke 39:2362–2369
Xu M, Wu R, Liang Y, Fu K, Zhou Y, Li X, Wu L, Wang Z (2020) Protective effect and mechanism of Qishiwei Zhenzhu pills on cerebral ischemia-reperfusion injury via blood-brain barrier and metabonomics. Biomed Pharmacother 131:110723. https://doi.org/10.1016/j.biopha.2020.110723
Yang Q, Huang Q, Hu Z, Tang X (2019) Potential neuroprotective treatment of stroke: targeting excitotoxicity, oxidative stress, and inflammation. Front Neurosci 13:1036. https://doi.org/10.3389/fnins.2019.01036
Yang T, Feng C, Wang D, Qu Y, Yang Y, Wang Y, Sun Z (2020a) Neuroprotective and anti-inflammatory effect of tangeretin against cerebral ischemia-reperfusion injury in Rats. Inflammation 43:2332–2343. https://doi.org/10.1007/s10753-020-01303-z
Yang Z et al (2020b) Synthesis and identification of a novel derivative of salidroside as a selective, competitive inhibitor of monoamine oxidase B with enhanced neuroprotective properties. Eur J Med Chem:112935. https://doi.org/10.1016/j.ejmech.2020.112935
Yi C, Si L, Xu J, Yang J, Wang Q, Wang X (2020) Effect and mechanism of asiatic acid on autophagy in myocardial ischemia-reperfusion injury in vivo and in vitro. Exp Ther Med 20:54. https://doi.org/10.3892/etm.2020.9182
Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, Xiao X (2013) Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res 1491:188–196
Youn HJ, Kim KB, Han HS, An IS, Ahn KJ (2017) 23-Hydroxytormentic acid protects human dermal fibroblasts by attenuating UVA-induced oxidative stress. Photodermatol Photoimmunol Photomed 33:92–100. https://doi.org/10.1111/phpp.12294
Zhang Y, Li H, Guo H, Li B, Zhao Z, Wang P, Wu H, Liu J, Chen Y, Zhang X, Wu P, Wang Z, Wang J (2019) Genome analysis reveals a synergistic mechanism of ursodeoxycholic acid and jasminoidin in mice brain repair after ischemia/reperfusion: crosstalk among muti-pathways. Front Pharmacol 10:1383. https://doi.org/10.3389/fphar.2019.01383
Zhu J, Wang L, Zhang J (2020) Galuteolin inhibited autophagy for neuroprotection against transient focal cerebral ischemia in rats. NeuroMolecular Med. https://doi.org/10.1007/s12017-020-08606-2
Author information
Authors and Affiliations
Contributions
YMW, FRL, and PL: conceived and designed research, manuscript writing, animals’ experiments, literature search, data analysis, and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal Care and Use Committee of the 80th Army Hospital of the Chinese People’s Liberation Army approved all our experiments. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental figure 1
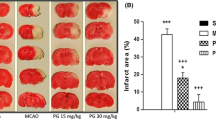
23-hydroxytormentic acid reduces cerebral I/R damage in rats by dose-dependent effects. (A) Neurological scores, (B) The infarct volume were evaluated by TTC staining. N = 6. Compared with sham operation group, ##P < 0.01; compared with I/R group, *P < 0.05, **P < 0.01. (TIF 8036 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, F. & Liu, P. 23-Hydroxytormentic acid reduces cerebral ischemia/reperfusion damage in rats through anti-apoptotic, antioxidant, and anti-inflammatory mechanisms. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1045–1054 (2021). https://doi.org/10.1007/s00210-020-02038-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-02038-2