Abstract
Acute hyperglycaemia impairs cognitive function. It is however not known, whether different brain regions are equally exposed to glucose during acute hyperglycemia or whether the brain is able to adjust its glucose uptake or metabolism in response to blood glucose fluctuation. We studied the effect of acute hyperglycaemia on the brain glucose concentration in seven men with type 1 diabetes with daily glucose fluctuations of 11 ± 3 mmol/l, and in eleven age-matched non-diabetic men. Glucose was quantified with proton magnetic resonance spectroscopy in three different brain regions at baseline (fasting glycaemia) and twice during a 2 h hyperglycaemic clamp with plasma glucose increase of 12 mmol/l. The increase in brain glucose during acute hyperglycaemia in the non-diabetic group was: cortex (2.7 ± 0.9 mmol/l) > thalamus (2.3 ± 0.7 mmol/l) > white matter (1.7 ± 0.7 mmol/l, P = 0.021 vs. cortex) and in the diabetic group: cortex (2.0 ± 0.7 mmol/l) > white matter (1.3 ± 0.7 mmol/l) > thalamus (1.1 ± 0.4 mmol/l, P = 0.010 vs. cortex). In the diabetic group, the glucose increase in the thalamus was attenuated compared to the non-diabetic participants (P = 0.011). In conclusion, the increase of glucose during acute hyperglycaemia seems to be dependent on the brain tissue type. The high exposure of cortex to excess glucose and the altered glucose uptake or metabolism in the thalamus may thus contribute to hyperglycaemia related cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes increases the risk of brain structure abnormalities such as atrophy, lacunar infarcts, and white matter lesions (van Harten et al. 2006) as well as decline in a large variety of cognitive tasks (Brands et al. 2005). Chronic hyperglycaemia is the most important risk factor for diabetic complications (The Diabetes Control and Complications Trial Research Group 1993) but as seen in patients developing peripheral neuropathy, the nervous system may possess a special sensitivity to high glucose variability (Bragd et al. 2008). On the other hand, also naturally-occurring daily hypoglycaemic and hyperglycaemic blood glucose fluctuations have been shown to cause deterioration in cognitive function in patients with type 1 diabetes (Gonder-Frederick et al. 2009).
Glucose is the principal source of energy for the brain. It has been shown that the glucose concentration in extracellular fluid parallels the plasma glucose concentration with a time lag of 30 min in healthy individuals (Abi-Saab et al. 2002). In diabetes, the blood glucose is abnormally high but may also fluctuate from low to high concentrations. Previously, only the effects of frequent episodes of hypoglycaemia and consistent chronic hyperglycaemia on the brain glucose uptake have been studied; in the entire brain by positron emission tomography (PET; Fanelli et al. 1998) and Kety-Schmidt technique (Boyle et al. 1995), and in the occipital cortex by proton magnetic resonance spectroscopy (1H MRS; Criego et al. 2005, Seaquist et al. 2005). Most studies in humans (Boyle et al. 1995; Criego et al. 2005) and rats (McCall et al. 1986; Simpson et al. 1999) agree that frequent episodes of hypoglycaemia may up-regulate the glucose uptake in the brain although also adverse findings has been reported (Segel et al. 2001). The effect of chronic hyperglycaemia is not as unambiguous. Both down-regulated (Duelli et al. 2000) and unchanged (Simpson et al. 1999) glucose uptake has been found in rats. In diabetic patients with poor glycaemic control, the glucose uptake has been observed to be unchanged both during a hypoglycaemic (Fanelli et al. 1998) and a hyperglycaemic (Seaquist et al. 2005) clamp. Indirect signs of down-regulation have, however, been found, as chronic hyperglycaemia in diabetes has been associated with decelerated blood-to-brain glucose transport during low blood glucose of 3.0 mmol/l (Boyle et al. 1995). Notably, various brain regions differ in tissue composition, blood supply, and metabolic rate (Quirce et al. 1997) as well as in their glucose uptake or metabolism.
We therefore suggested two hypotheses: 1. Different brain regions may differ in their glucose concentrations in response to acute hyperglycaemia. 2. Chronic exposure to high glucose concentrations may alter glucose uptake/metabolism. To test the hypotheses we studied the glucose content in the cortex, white matter and thalamus repeatedly during acute hyperglycaemia in type 1 diabetic patients with high daily glucose variability and in non-diabetic individuals.
Materials and methods
Participants
We studied seven men with type 1 diabetes and eleven age- and BMI-matched non-diabetic men as control subjects. The diabetic participants were recruited from the outpatient clinic at the Helsinki University Central Hospital and the non-diabetic participants by advertisements from the Helsinki metropolitan area. Exclusion criteria were smoking, alcohol or drug abuse, a family history of type 2 diabetes, a history or present signs of cerebrovascular, cardiovascular, neurological, psychiatric disease or a head trauma. All participants were required to have magnetic resonance images in the normal range. Diabetic participants reported no history of unconsciousness due to low glucose nor hypoglycaemia unawareness at a blood glucose below 3.0 mmol/l. This study focused on patients with frequent fluctuations in their blood glucose concentrations and therefore the mean amplitude of glycaemic excursions (MAGE) was assessed by monitoring the tissue glucose concentration with the Medtronic MiniMed Continuous Glucose Monitoring System (CGMS; Medtronic MiniMed, Northridge, CA, USA) during three days preceding the study visit (Gross and Mastrototaro 2000).
None of the participants had any clinical symptoms (numbness, tingling, muscle cramps, or weakness in the lower extremities) or signs (abnormal Achilles and patellar tendon reflexes, pressure and vibration perception thresholds) of peripheral neuropathy. Retinopathy was quantified in a blinded fashion from fundus photographs by an ophthalmologist using the Early Treatment Diabetic Retinopathy Study scale (Davis et al. 1998). Four of the diabetic participants had background diabetic retinopathy whereas the other had no signs of retinopathy. Seventeen participants had normal urinary albumin excretion rate (<30 mg/24 h) and took no other regular medication than insulin. One patient with diabetes was microalbuminuric (115 mg/24 h) and was treated with 10 mg ramipril and 50 mg acetyl salicylic acid daily. The Ethical committee of the Helsinki University Central Hospital approved the study and informed written consent was obtained from all participants.
All participants were studied in the morning after an overnight fast. In the morning of the study, the diabetic patients using glargine insulin (n = 4) injected their normal dose, whereas the patients using NPH insulin (n = 3) reduced their dose by 50% to avoid ypoglycaemia. Hypoglycaemic symptoms or tissue glucose below 2.9 mmol/l during the preceding 24 h led to re-scheduling of the study visit.
Study protocol
Initially, the brain was investigated with 1H MRS during steady-state baseline conditions for 40 min (MRI + MRS 0). Thereafter, the patient lying in the magnet, the blood glucose concentration was raised to the hyperglycaemic level within 30 min. The aim was to achieve a blood glucose increase of similar magnitude in the diabetic and non-diabetic participants. During hyperglycaemia blood glucose was kept stable for 50 min and two MRS data collections lasting 25 min were performed (MRS I and II; Fig. 1).
At baseline, the participants received 0.9% saline infused (50 ml/h) through a cannula inserted into their right antecubital vein. The hyperglycaemic clamp aimed to increase the plasma glucose concentration by 12 mmol/l above the baseline concentration. This was achieved by giving the participants a bolus of 50% glucose (0.5 ml/body weight [kg]) followed by an infusion of 20% glucose into their right antecubital vein. The endogenous insulin secretion was blocked by infusing a somatostatin analogue (Sandostatin; Novartis) into the left antecubital vein (bolus of 25 μg and infusion 0.75 μg/min) of the non-diabetic participants.
A retrograde venous cannula was placed in the left hand dorsum, which was kept warm with a heat pack in order to arterialize the venous blood (McGuire et al. 1976). Blood glucose samples were drawn every 10 min and analyzed bed side (HemoCue Glucose 201+; HemoCue, Ängelholm, Sweden) in order to adjust the glucose infusion. In addition, samples for the determination of plasma glucose and serum insulin concentrations were drawn before and after each of the three (MRS 0, I, and II) data collections.
Magnetic resonance imaging and spectroscopy
The 1H MRS data were collected using a 1.5 T clinical MR imager (Siemens Magnetom Sonata, Erlangen, Germany) equipped with a standard birdcage head coil. The MRS voxels were localized in the left frontal cortex (8.0 ml; 25 mm × 16 cm × 20 mm), left frontal white matter (7.7 ml; 30 mm × 16 mm × 16 mm), and in the left thalamus (7.2 ml; 20 mm × 20 mm × 18 mm) based on anatomical T1-weighted sagittal, T2-weighted coronal, and FLAIR axial images. Water-suppressed metabolite spectra were measured with the point-resolved spectroscopy (PRESS) sequence and chemical shift-selective (CHESS) water suppression scheme (echo time 30 ms, repetition time 3,000 ms, 64 acquisitions). Non-water-suppressed spectra were collected from the same voxels using four acquisitions. A glucose reference spectrum was collected from a 100 mM solution phantom with a 8.0 ml voxel (echo time 30 ms, repetition time 6,000 ms, 64 acquisitions). In all spectra, the spectral width was 1,000 Hz and the number of acquired complex data points was 1024.
The spectra were processed and analyzed using an in-house written script running on Matlab 7.2 (MathWorks, Natick, MA, USA). The free induction decay (FID) was apodized using a Gaussian function with a broadening factor of 2.5 Hz and zero-filled up to 2,048 complex points prior to Fourier transformation. Signal intensities were evaluated by integration of the characteristic spectral regions and were automatically corrected for coil loading and voxel size by the analysis program. Correction for receiver gain was not needed as it was kept constant in all MRS measurements.
The quantified resonances included NAA (N-acetylaspartate), tCr (total creatine), glucose, and tissue water (H2O; Fig. 2). The spectra were baseline corrected and the signal intensities were evaluated by integration of the spectral regions: NAA 1.98–2.06, tCr 3.00–3.08, glucose 3.40–3.46, and H2O 4.2–5.2 p.p.m. The integral data was analyzed by using tissue water as and internal reference (metabolite/H2O; Urrila et al. 2006) to minimize the effect of random fluctuations in the intensity levels originated from the instrument or the subject.
Mean thalamus spectra at baseline (MRS 0; upper solid line) and during hyperglycaemia (MRS II; upper dashed line) in participants with type 1 diabetes (a) and non-diabetic participants (b). Below the brain spectrum, a brain difference spectrum (MRS II–MRS 0; lower dashed line) and 100 mmol/l glucose fit that was used as an internal reference (lower solid line). The difference spectrum and the glucose spectrum are scaled up by a factor of five for better visualization
The increase in the brain glucose concentration during the hyperglycaemic clamp was determined from difference spectra (Fig. 2). Two difference spectra for each brain area were calculated by subtracting the baseline (MRS 0) spectrum from the second (MRS I) and the third spectrum (MRS II). The glucose concentration in the difference spectra was determined by fitting the glucose spectrum recorded from the 100 mM solution to the difference spectra. Spectral region 3.1–4.0 ppm was used in the fitting procedure. The fitting was performed using Matlab 7.2 program. The obtained concentration data from difference spectra represent the change in glucose concentration from the baseline situation.
Laboratory analyses
Plasma glucose was analyzed by the glucose oxidase method (Beckman Glucose Analyzer II; Beckman Instruments, Fullerton, CA, USA) and serum insulin by time-resolved fluoroimmunoassay (PerkinElmer, Turku, Finland). Total cholesterol was determined using an enzymatic photometric assay (Roche Diagnostics, Mannheim, Germany). HbA1c (glycated haemoglobin A1c) was analyzed by immunoturbidimetry (Bayer, Tarrytown, NY, USA). Urinary albumin excretion rate was assessed from 24-hour urine collections by immunoturbidimetry (Orion Diagnostica, Espoo, Finland).
Statistical analyses
The significance of differences between samples is reported using the non-parametric Mann Whitney U test. Analyses were also performed with the parametric Student’s t-test that gave similar results. The significance between repeated measurements was analyzed using the non-parametric Wilcoxon test. Correlations were assessed using the Spearman’s rank correlation coefficient. The data are given as means (SD) or median (range) when data are not normally distributed, and P-values <0.05 were considered statistically significant. The analyses were performed with the Sigma Stat Statistical Software (SPSS 15.0, Chicago, IL, USA).
Results
Participants
The study groups were matched for age (29.4 ± 4.5 vs. 30.8 ± 6.9 years, P = 0.930) and BMI (25.1 ± 4.0 vs. 22.4 ± 2.3 kg/m2, P = 0.056) and had similar concentrations of serum total cholesterol (4.4 ± 0.8 vs. 4.4 ± 1.1 mmol/l, P = 0.927 for diabetic vs. non-diabetic participants). Systolic blood pressures were 134 ± 6 and 125 ± 7 mmHg (P = 0.010) and diastolic blood pressures were 76 ± 9 and 75 ± 7 mmHg (P = 0.717) in the diabetic and non-diabetic participants. HbA1 was 7.6 ± 0.8% in the diabetic and 5.5 ± 0.2% (P < 0.001) in the non-diabetic group. The patients with diabetes had age at onset of diabetes of 16.9 ± 4.4 years, diabetes duration of 12.4 ± 2.8 years and daily mean amplitude of glycaemic excursions of 11.3 ± 2.9 mmol/l.
The plasma glucose and serum insulin concentrations during the hyperglycaemic clamp are presented in Fig. 1. The increase of plasma glucose during the clamp was of similar magnitude in the diabetic and non-diabetic study groups (11.8 ± 2.9 vs. 12.3 ± 1.5 mmol/l, P = 0.892).
Brain glucose and brain water
Change in brain glucose during the hyperglycaemic clamp
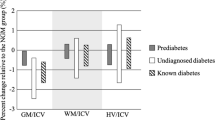
In the diabetic participants, the glucose increase was: cortex (2.0 ± 0.7 mmol/l) > white matter (1.3 ± 0.7 mmol/l) > thalamus (1.1 ± 0.4 mmol/l; Fig. 3). The increase in brain glucose was statistically significantly greater in the cortex than in the thalamus (P = 0.010). In the non-diabetic participants, the increase in brain glucose was: cortex (2.7 ± 0.9 mmol/l) > thalamus (2.3 ± 0.7 mmol/l) > white matter (1.7 ± 0.7 mmol/l). The increase in brain glucose was statistically significantly greater in the cortex than in the white matter (P = 0.021).
The increase in brain glucose did not differ between the diabetic and non-diabetic participants in the cortex (P = 0.093) or in the white matter (P = 0.306). In contrast, in the thalamus, the increase in glucose content using an absolute glucose concentration as the reference was smaller in the diabetic participants (P = 0.010 for MRS I and P = 0.011 for MRS II; Fig. 3). Also, by using water as the reference (glucose/H2O), the increase in glucose content in the thalamus was attenuated although it did not reach statistical significance (Table 1).
Brain glucose content
At baseline (MRS 0), the absolute glucose/H2O -ratios showed no difference between the diabetic and non-diabetic participants neither in the cortex nor in the thalamus, but the diabetic patients had higher glucose/H2O in the white matter (P = 0.027; Table 1). At MRS I and II, the absolute glucose/H2O -ratios were no different between the diabetic and non-diabetic participants in any of the studied brain regions.
Brain tissue water
The tissue H2O content remained stable during the hyperglycaemic clamp (P > 0.05, Table 2) in all studied brain regions and did not correlate with the brain or plasma glucose concentration (data not shown, P > 0.05). The H2O contents were similar in the diabetic and the non-diabetic participants in all brain regions during the entire study. In the non-diabetic participants, the cortex contained 8% more H2O than the white matter (P = 0.006). In the diabetic participants, there was no regional variation (P = 0.088).
Brain metabolites
The diabetic and non-diabetic groups had similar levels of NAA/H2O and tCr/H2O in the cortex, the white matter, and in the thalamus during acute hyperglycaemia. The changes in NAA/H2O and tCr/H2O during the clamp (from MRS 0 to II) varied from −3.4% to +4.7% (Table 3).
Discussion
This study showed that during acute hyperglycaemia, brain glucose concentration increased more in the frontal cortical gray matter than in the frontal white matter and that the increase in the glucose concentration in the thalamus was attenuated in the diabetic participants. Both are novel findings.
We compared the effect of a hyperglycaemic episode on brain glucose in diabetic patients with high daily blood glucose fluctuations but no history of hypoglycaemic events and in non-diabetic subjects in small but well characterized and homogenous study groups. The inclusion criteria were chosen to avoid any asymptomatic cardiovascular disease (Bonora 2006) that could have an effect on the brain. Based on the HbA1c, the glycaemic control in the participants with type 1 diabetes was slightly above the target of international guidelines and this may explain why the patients reported no severe episodes of hypoglycaemia or hypoglycaemia unawareness. This was important since hypoglycaemia may modulate brain glucose transport or metabolism (Boyle et al. 1995; Criego et al. 2005).
Our approach to quantify brain glucose repeatedly in several brain regions during acute hyperglycaemia with 1H MRS is new. PET allows the study of glucose kinetics but the differentiation of brain regions is difficult due to limited spatial resolution. 1H MRS provides an opportunity to compare brain regions and to assess the amount of excess glucose in the tissue.
Some methodological considerations have to be discussed. Two different methods that measure different aspects of the phenomenon were used to assess the increase in the glucose concentration in the studied brain regions. One is using tissue water as the reference and the other the absolute glucose concentration. In our hands both seem to perform adequately. The reproducibility of the method that estimates the difference between the brain glucose/H2O at the two time points (MRS 0 and II) can be assessed using the brain NAA/H2O and tCr/H2O -ratios because they are not expected to change during a 2-hour study (Baslow 2003; de Graaf et al. 2001). The variation of these metabolites was less than five percent and was in line with previous studies (Geurts et al. 2004). Tissue water is often chosen for the internal reference for the metabolites because also its concentration is considered to be relatively stable (Kreis et al. 1996). In the non-diabetic participants, the cortical voxel contained 8% more water than the white matter, which matches with the literature (Norton 1975). In principle, the cortical voxel may become contaminated with cerebrospinal fluid, but in our patients the water content shows that this contamination was negligible and does not therefore account for the higher glucose content in the cortex. The other method using the glucose as reference may be considered more reliable because it requires less manual manipulation.
The non-diabetic participants received somatostatin to reduce the secretion of insulin. Although somatostatin uptake into the brain is restricted (Fricker et al. 2002) some vasoconstriction cannot be ruled out (Long et al. 1992). The possible vasoconstriction may have reduced the glucose entry into the brain. In such a situation, the difference in the thalamic glucose increase between the non-diabetic and diabetic participants would be even larger than we observed. Most importantly, somatostatin enabled the study to be performed during controlled serum insulin concentrations because also insulin has an effect on the vessel diameter and in addition on cell energy metabolism in the brain (Schwartz et al. 1992). During the clamp, we studied the change in cerebral glucose concentration and therefore the aim was to achieve, instead of similar plasma glucose concentrations, similar change in plasma glucose increase in both groups.
The normoinsulinemic clamp increased plasma glucose by 12 mmol/l which corresponds well with the daily hyperglycaemic episodes observed in the diabetic participants (MAGE 11.3 ± 2.9 mmol/l). The acute hyperglycaemia led to a 1.1–2.0 mmol/l increase in the brain glucose concentration which according to previous studies is between 0.5 mmol/l and 2.5 mmol/l (Bingham et al. 2005; Schlenk et al. 2008). Therefore, daily hyperglycaemic blood glucose fluctuation in diabetic patients may even double their brain glucose content.
Although shown for the first time with 1H MRS, it may not be surprising that acute hyperglycaemia increased the glucose content more in the cortex than in the white matter. It has been well established that the gray matter has higher metabolic rate of glucose than the white matter (Phelps et al. 1979; Mason et al. 1999). However, the result also means that during acute hyperglycaemia, the cortex is more exposed to excess glucose than the white matter. Because no difference was seen between the diabetic and non-diabetic participants, the cortex might not be able to adjust its glucose uptake or metabolism. We have previously shown that in diabetic patients with chronic hyperglycaemia, accumulation of excess glucose is the greatest in the white matter (Heikkilä et al. 2009). The present study provides evidence that chronic and acute hyperglycaemias increase the glucose concentrations differently in different brain regions, chronic in the white matter and acute in the gray matter.
In the patients with type 1 diabetes, the increase in the glucose concentration during acute hyperglycaemia was attenuated in the thalamus. The thalamus consists mainly of gray matter and receives its blood flow through long non-branching perforating arteries whose endothelium dependent vasodilatation function has been found to be impaired in chronically hyperglycaemic rats (Oizumi et al. 2006). Therefore we may have observed a decreased supply of glucose. On the other hand, a PET study in young healthy adults has found increased regional blood flow in the thalamus after repeated episodes of hypoglycaemia (Arbelaez et al. 2008). In our previous study we found that in healthy men with cardiovascular risk factors, thalamic tCr (marker of oxidative metabolism) was increased and associated with both fasting plasma glucose concentration and 2 h plasma glucose concentration in an oral glucose tolerance test (Heikkilä et al. 2008). Therefore the attenuated increase in glucose concentration may indicate increased glucose metabolism in the thalamus. It also is possible that the total uptake of glucose in the thalamus was saturated, since the diabetic participants had been exposed to hyperglycemia for a longer period of time. This would mean that the glucose uptake saturates easier in the thalamus than in the cortex or in the white matter. Although we cannot provide any clear explanation for the phenomenon, evidence is emerging that the thalamus somehow reacts to glycaemic variation or participates in the regulation of glucose metabolism.
In the cortex and white matter, acute hyperglycaemia increased glucose concentrations as much in the diabetic as in the non-diabetic participants. We have previously shown that during fasting glycaemia, the glucose concentration in these brain regions is higher in the diabetic than in the non-diabetic individuals (Heikkilä et al. 2009). This suggests decreased metabolic rate in relation to the amount of available glucose in the cortex and white matter of diabetic individuals.
In conclusion, the distribution of glucose in the brain during acute hyperglycaemia seems to be dependent on the tissue type. The high exposure of cortex to excess glucose may thus contribute to hyperglycaemia related cognitive dysfunction. Chronic exposure to high glucose concentration alters the glucose uptake/metabolism in the thalamus. Thalamus has not been in the focus of brain research in patients with diabetes but the present and previous results suggest that further studies are required to determine its contribution in the regulation glucose metabolism and development of diabetic brain disease.
Abbreviations
- 1H MRS:
-
proton magnetic resonance spectroscopy
- H2O:
-
brain tissue water
- MRS 0, I, and II:
-
1H MRS data collections
- NAA:
-
N-acetylaspartate
- tCr:
-
total creatine
References
Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS (2002) Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab 22:271–279
Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE (2008) Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 57:470–475
Baslow MH (2003) N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 28:941–953
Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA (2005) Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-D-glucose PET study. Diabetologia 48:2080–2089
Bonora E (2006) The metabolic syndrome and cardiovascular disease. Ann Med 38:64–80
Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ (1995) Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 333:1726–1731
Bragd J, Adamson U, Backlund LB, Lins PE, Moberg E, Oskarsson P (2008) Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diab Metab 34:612–616
Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP (2005) The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diab Care 28:726–735
Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER (2005) Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res 79:42–47
Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL 3rd, Knatterud GL (1998) Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: early treatment diabetic retinopathy study report #18. Invest Ophthalmol Vis Sci 39:233–252
de Graaf RA, Pan JW, Telang F, Lee JH, Brown P, Novotny EJ, Hetherington HP, Rothman DL (2001) Differentiation of glucose transport in human brain gray and white matter. J Cereb Blood Flow Metab 21:483–492
Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W (2000) Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res 858:338–347
Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Cryer PE, Powers WJ (1998) Blood-to-brain glucose transport and cerebral glucose metabolism are not reduced in poorly controlled type 1 diabetes. Diabetes 47:1444–1450
Fricker G, Nobmann S, Miller DS (2002) Permeability of porcine blood brain barrier to somatostatin analogues. Br J Pharmacol 135:1308–1314
Geurts JJ, Barkhof F, Castelijns JA, Uitdehaag BM, Polman CH, Pouwels PJ (2004) Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: metabolite concentrations, quantification precision, and reproducibility. J Magn Reson Imaging 20:366–371
Gonder-Frederick LA, Zrebiec JF, Bauchowitz AU, Ritterband LM, Magee JC, Cox DJ, Clarke WL (2009) Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with type 1 diabetes: a field study. Diabetes Care
Gross TM, Mastrototaro JJ (2000) Efficacy and reliability of the continuous glucose monitoring system. Diab Technol Ther 2(Suppl 1):S19–S26
Heikkilä O, Lundbom N, Timonen M, Groop PH, Heikkinen S, Mäkimattila S (2008) Risk for metabolic syndrome predisposes to alterations in the thalamic metabolism. Metab Brain Dis 23:315–324
Heikkilä O, Lundbom N, Timonen M, Groop PH, Heikkinen S, Mäkimattila S (2009) Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain. Diabetologia 52:534–540
Kreis R, Arcinue E, Ernst T, Shonk TK, Flores R, Ross BD (1996) Hypoxic encephalopathy after near-drowning studied by quantitative 1H-magnetic resonance spectroscopy. J Clin Invest 97:1142–1154
Long JB, Rigamonti DD, Dosaka K, Kraimer JM, Martinez-Arizala A (1992) Somatostatin causes vasoconstriction, reduces blood flow and increases vascular permeability in the rat central nervous system. J Pharmacol Exp Ther 260:1425–1432
Mason GF, Pan JW, Chu WJ, Newcomer BR, Zhang Y, Orr R, Hetherington HP (1999) Measurement of the tricarboxylic acid cycle rate in human grey and white matter in vivo by 1H-[13C] magnetic resonance spectroscopy at 4.1T. J Cereb Blood Flow Metab 19:1179–1188
McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB (1986) Chronic hypoglycemia increases brain glucose transport. Am J Physiol 251:E442–E447
McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M (1976) Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41:565–573
Norton WT (1975) The nervous system in the basic neurosciences. Raven, New York
Oizumi XS, Akisaki T, Kouta Y, Song XZ, Takata T, Kondoh T, Umetani K, Hirano M, Yamasaki K, Kohmura E, Yokono K, Sakurai T (2006) Impaired response of perforating arteries to hypercapnia in chronic hyperglycemia. Kobe J Med Sci 52:27–35
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388
Quirce R, Carril JM, Jimenez-Bonilla JF, Amado JA, Gutierrez-Mendiguchia C, Banzo I, Blanco I, Uriarte I, Montero A (1997) Semi-quantitative assessment of cerebral blood flow with 99mTc-HMPAO SPET in type I diabetic patients with no clinical history of cerebrovascular disease. Eur J Nucl Med 24:1507–1513
Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS (2008) Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med 34:1200–1207
Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr (1992) Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 13:387–414
Seaquist ER, Tkac I, Damberg G, Thomas W, Gruetter R (2005) Brain glucose concentrations in poorly controlled diabetes mellitus as measured by high-field magnetic resonance spectroscopy. Metabolism 54:1008–1013
Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE (2001) Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes 50:1911–1917
Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR (1999) Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 72:238–247
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Urrila AS, Hakkarainen A, Heikkinen S, Huhdankoski O, Kuusi T, Stenberg D, Häkkinen AM, Porkka-Heiskanen T, Lundbom N (2006) Preliminary findings of proton magnetic resonance spectroscopy in occipital cortex during sleep deprivation. Psychiatry Res 147:41–46
van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ (2006) Brain imaging in patients with diabetes: a systematic review. Diab Care 29:2539–2548
Acknowledgments
Financial support was provided by grants from the Folkhälsan Research Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Diabetes Research Foundation, Waldemar von Frenckell’s Foundation, the Wilhelm and Else Stockmann Foundation, Finnish Neurological Society, and from a special governmental grant for health sciences research (no. 5103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heikkilä, O., Lundbom, N., Timonen, M. et al. Evidence for abnormal glucose uptake or metabolism in thalamus during acute hyperglycaemia in type 1 diabetes—a 1H MRS study. Metab Brain Dis 25, 227–234 (2010). https://doi.org/10.1007/s11011-010-9199-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-010-9199-5