Abstract
Objectives
Diabetes frequently results in cognitive impairment, but it is less clear if brain health is adversely affected during the prediabetic stage. Our aim is to identify possible changes in brain volume as measured by magnetic resonance imaging (MRI) in a large elderly population stratified according to level of “dysglycemia.”
Methods
This is a cross-sectional study of 2144 participants (median age 69 years, 60.9% female) who underwent 3-T brain MRI. Participants were divided into 4 dysglycemia groups based on HbA1c levels (%): normal glucose metabolism (NGM) (< 5.7%), prediabetes (5.7 to < 6.5%), undiagnosed diabetes (6.5% or higher), and known diabetes (defined by self-report).
Results
Of the 2144 participants, 982 had NGM, 845 prediabetes, 61 undiagnosed diabetes, and 256 known diabetes. After adjustment for age, sex, education, body weight, cognitive status, smoking, drinking, and disease history, total gray matter volume was significantly lower among participants with prediabetes (0.41% lower, standardized β = − 0.0021 [95% CI − 0.0039, − 0.00039], p = 0.016), undiagnosed diabetes (1.4% lower, standardized β = − 0.0069 [95% CI − 0.012, − 0.002], p = 0.005), and known diabetes (1.1% lower, standardized β = − 0.0055 [95% CI − 0.0081, − 0.0029], p < 0.001) compared to the NGM group. After adjustment, total white matter volume and hippocampal volume did not differ significantly between the NGM group and either the prediabetes group or the diabetes group.
Conclusion
Sustained hyperglycemia may have deleterious effects on gray matter integrity even prior to the onset of clinical diabetes.
Key Points
• Sustained hyperglycemia has deleterious effects on gray matter integrity even prior to the onset of clinical diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment in aging and elderly populations is a growing concern around the world. There is thus an urgent need to identify risk factors and protective factors for the development of Alzheimer’s disease (AD). Especially, it is critical to reduce the incidence and severity of modifiable risk factors because there are no established treatments for delaying the associated cognitive impairments. Type 2 diabetes mellitus (T2D) can result in progressive neural damage and ensuing neurological impairments, including cognitive deficits. Several studies have reported that T2D is associated with an increased risk of AD [1,2,3,4]. Furthermore, the previous meta-analysis also showed that prediabetes (prodromal state of T2D) was a significant predictor on risk of progression from amnestic mild cognitive impairment to AD [5]. However, it is still uncertain whether prediabetes can have measurable deleterious effects on brain. The impact of prediabetes on brain health is of great public health significance considering that half of adults aged 60 years and older may have this condition [6]. Therefore, there is great interest in identifying brain changes in prediabetes condition.
Imaging can help us to detect brain abnormalities and to identify etiological factors. Several studies of patients with T2D have also reported reduced brain volume and accelerated rates of brain atrophy in sites prone to AD pathology, including the hippocampus and parahippocampus [7,8,9]. However, few studies have specifically examined changes in brain volume during prediabetes. Dong et al. found a significant difference in left hippocampal volume (HV) between a relatively small cohort of subjects with prediabetes (n = 17) and matched subjects with normal glucose metabolism (NGM) [10]. Studies with larger populations have also reported global brain volume loss in T2D, but adjusted analyses revealed no significant differences between subjects with NGM and prediabetes [8, 9]. Thus, the underlying brain structural correlates in the prediabetes condition remain unclear, although the prediabetes showed a risk factor for cognitive decline [5].
Several glycemic measures are used in clinical practice to diagnose and gage the severity of dysglycemia. Among these, blood hemoglobin A1c (HbA1c) level provides a more stable measure of long-term hyperglycemia than blood glucose, thereby facilitating the detection of undiagnosed T2D or glucose intolerance [11]. In this study, we reviewed data from a population-based prospective study of cerebro- and cardiovascular diseases and dementia in a large population of older Japanese people (the Iki-Iki study) to assess associations between dysglycemia severity (including prediabetes) as measured by HbA1c and changes in brain volume.
Methods
This study was conducted following the ethical guidelines of the Declaration of Helsinki, and the use of data from the Iki-Iki Health Promotion Project (Iki-Iki study) was approved by the Ethics Committee of Hirosaki University School of Medicine (authorization number 2019–064-1). Written informed consent was obtained from all participants.
Study population and study design
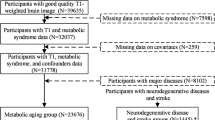
The Iki-Iki Health Promotion Project was established in 2016 as a population-based prospective study of cerebro- and cardiovascular diseases and dementia in an older Japanese population from the Iwaki area of Hirosaki City, located in western Aomori Prefecture, Japan. In 2016 and 2017, 2390 residents aged > 64 years participated in the screening survey. Of the 2390 residents, 2226 (93.1%) underwent brain MRI. We excluded 22 participants with image distortions (4 with metal artifacts, 7 with excessive motion artifacts, 11 for whom HV could not be measured accurately for other reasons), 32 participants without available MRI data (32 without T1-weighted images), and one participant without available blood HbA1c measurements. We also excluded 27 participants with diabetes mellitus receiving insulin therapy. Thus, 2144 participants (1305 females and 839 males) were enrolled in the present study.
Blood samples were analyzed for HbA1c using the Roche modular 7600 automatic biochemistry analyzer, and participants were divided into the following four “dysglycemia” categories based on HbA1c levels as defined by American Diabetes Association guidelines [12]: (1) NGM (< 5.7%), (2) prediabetes (from 5.7 to < 6.5%), (3) undiagnosed diabetes (6.5% or higher), and (4) known diabetes (confirmed by self-reported use of disease-specific medications). The known diabetes group was further divided into two groups, with and without diabetic retinopathy. In this study, all participants with diabetes were diagnosed as T2D.
Image processing for brain volume
All brain MRI data were obtained using the same protocol on a single 3-T MRI scanner (Signa EXCITE 3 T; GE Healthcare) with an 8-channel brain phased-array coil. Original T1-weighted images were acquired in the steady state using a three-dimensional (3D) fast-spoiled gradient recalled sequence with the following parameters: repetition time, 10 ms; echo time, 4.1 ms; inversion time, 700 ms; flip angle, 10; field of view, 26 cm; section thickness, 1.2 mm; and resolution, 1.0 × 1.0 × 1.2 mm. Analysis was conducted using the CAT12 toolbox (C. Gaser, Structural Brain Mapping Group, Jena University Hospital; http://dbm.neuro.uni-jena.de/cat/) implemented in Statistical Parametric Mapping 12 (SPM12) software (Wellcome Trust Center for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) [13, 14]. The 3D T1-weighted images in native space were spatially normalized; segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF); and modulated using the diffeomorphic anatomical registration through exponential Lie algebra toolbox [13, 15]. To preserve the GM volumes within each voxel, we modulated the images using the Jacobian determinants derived from the spatial normalization. The resulting modulated GM images were smoothed using an 8-mm full-width at half-maximum Gaussian kernel. We calculated total intracranial volume (ICV), total gray matter volume (GMV), and total white matter volume (WMV). The GMV and WMV included both supratentorial and infratentorial regions. Bilateral hippocampal volume (HV) was then calculated the latter using the 40-brain LPBA40 atlas [16]. We also calculated the GMV/ICV ratio as an indicator of global GM atrophy, WMV/ICV ratio as an indicator of global WM atrophy, and HV/ICV ratio as an indicator of hippocampal atrophy.
Statistical analyses
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University) [17]. Nominal variables are expressed as percentages and continuous variables as mean ± standard deviation, median (interquartile range), or range based on distribution. Multivariable linear regression was used to investigate the associations of dysglycemia status (NGM, prediabetes, undiagnosed diabetes, and known diabetes) with brain volume measures (GMV/ICV ratio, WMV/ICV ratio, HV/ICV ratio). Regression coefficients within groups were determined using dummy variables for prediabetes, undiagnosed diabetes, and known diabetes, with NGM as the reference group. In the known diabetes group, by the multivariable linear regression, we investigated the associations of diabetic retinopathy with brain volume measures (GMV/ICV ratio, WMV/ICV ratio, and HV/ICV ratio). Analyses were adjusted for age, sex, education level (less than high school, high school or equivalent, college or graduate, or professional school), body mass index (BMI), mini-mental state examination (MMSE) score, smoking status, alcohol consumption, and self-reported medical history (hypertension, hyperlipidemia, and depression). We classified smoking and alcohol drinking habits as currently habitual or not. For visualization purposes of the analysis, GMV/ICV ratio, WMV/ICV ratio, and HV/ICV ratio for prediabetes, undiagnosed diabetes, and known diabetes groups were expressed as percent change relative to the NGM group median and plotted as median percent change with bootstrapped 95% confidence intervals for each group [18].
Results
The general characteristics of the study population stratified by dysglycemia severity (NGM, prediabetes, undiagnosed diabetes, and known diabetes) are summarized in Table 1. Of the 2144 participants, 982, 845, 61, and 256 had NGM, prediabetes, undiagnosed diabetes, and known diabetes, respectively. Of the 256 participants with known diabetes, we found 8 participants with and 248 without diabetic retinopathy. Median age was 69 years, and 60.9% were women.
After adjustment for potential confounders (age, sex, education level, BMI, MMSE score, smoking status, alcohol frequency, and medical history), the GMV/ICV ratio was significantly lower among the prediabetes group (0.41% lower, standardized β = − 0.0021 [95% CI − 0.0039, − 0.00039], p = 0.016), undiagnosed diabetes group (1.4% lower, standardized β = − 0.0069 [95% CI − 0.012, − 0.002], p = 0.005), or known diabetes group (1.1% lower, standardized β = − 0.0055 [95% CI − 0.0081, − 0.0029], p < 0.001) compared to the NGM group (Table 2 and Fig. 1). Furthermore, compared to prediabetes group, the GMV/ICV ratio was lower among the undiagnosed diabetes group (1.0% lower, standardized β = − 0.0047 [95% CI − 0.0097, 0.00022], p = 0.06), or known diabetes group (0.7% lower, standardized β = − 0.0033 [95% CI − 0.006, − 0.0006], p = 0.017) after adjustment for the potential confounders. In contrast, total WM/ICV ratio and HV/ICV ratio did not differ significantly between the prediabetes and NGM groups or between the total diabetes (undiagnosed and known) and NGM groups after the adjustment. However, HV/ICV ratio was lower in participants with diabetes (undiagnosed and known) than in those without diabetes (NGM and prediabetes).
Associations between dysglycemia categories and gray matter volume, white matter volume, and hippocampal volume/intracranial volume ratio compared to normal glucose metabolism. Boxes represent the 95% confidence interval for the relative rate of change of gray matter volume/intracranial volume (GMV/ICV), white matter volume/intracranial volume (WMV/ICV), and hippocampal volume/intracranial volume (HV/ICV) compared to normal glucose metabolism (NGM). After adjustment, the GMV/ICV ratio is significantly lower in prediabetes and total diabetes (undiagnosed and known) than in NGM groups, although for the WMV/ICV and HV/ICV ratios, there are no significant differences between prediabetes and NGM groups or between total diabetes and NGM groups
After adjustment for potential confounders, the GMV/ICV ratio was significantly lower among the known diabetes group with diabetic retinopathy than without diabetic retinopathy (0.03% lower, standardized β = − 0.017 [95% CI − 0.030, − 0.0032], p = 0.016), whereas total WM/ICV ratio and HV/ICV ratio did not differ significantly between the known diabetes groups with and without diabetic retinopathy.
Discussion
The aim of the present study was to identify possible changes in total and regional brain volume among elderly individuals with prediabetes by taking advantage of the large Iki-Iki Health Promotion Project cohort. Total GMV was significantly reduced in the prediabetes group compared to the elderly with NGM. Thus, the GMV atrophy frequently observed in older subjects with T2D [19,20,21] appears to begin during the preclinical period of relatively poor blood glucose control. Conversely, we found no significant difference in normalized bilateral HV/ICV ratio between the prediabetes and NGM groups, suggesting that total GM atrophy precedes hippocampal atrophy during the early trajectory of T2D.
Only a few studies have specifically examined associations between prediabetes as defined by HbA1c and/or FG and changes in brain volume. Reitz et al. reported that prediabetes based on HbA1c levels was associated with decreased GMV, WMV, and HV as measured by MRI at regular field strength (1.5 T); however, no significant differences remained between the NGM (n = 115) and prediabetes (n = 224) groups after adjustment for confounders [9]. In a large population-based study by Schneider et al., subjects with T2D and high HbA1c (≥ 7.0%) exhibited reduced total and regional brain volumes, but subjects with prediabetes (n = 514) according to the same HbA1c criterion used in the current study (from 5.7 to < 6.5%) showed no significant differences compared to nondiabetic subjects (n = 597) [8]. This discrepancy may be explained by our segmentation of images into GM and WM, as no difference in WM/ICV ratio was found between the prediabetes and NGM groups in the current study population.
Multiple pathophysiological mechanisms may contribute to the progressive GM atrophy observed in elderly individuals with dysglycemia. Chronically elevated insulin (hyperinsulinemia) in the brain due to insulin resistance may stimulate amyloid β secretion and inhibit the extracellular degradation of amyloid β by competing for insulin-degrading enzymes [22], resulting in localized tissue degeneration. Furthermore, regional atrophy in the cortex and hippocampus may be explained by the higher expression of insulin receptors in these structures (GM) [23]. We speculate that these processes begin during the preclinical phase (prediabetes) due to chronic hyperinsulinemia. In addition, compared to the prediabetes group, the GMV/ICV ratio was 1.0% lower in the undiagnosed diabetes group (p = 0.06), and 0.7% lower in the known diabetes group (p = 0.017), possibly suggesting that a long exposure of dysglycemia worsens GM atrophy. Moreover, we found the GMV/ICV ratio was significantly lower in the known diabetes group with diabetic retinopathy than in the group without diabetic retinopathy (p = 0.016). The longer a patient has had diabetes, the higher the risk of developing diabetic retinopathy [24]. Therefore, the result also supports our speculation.
Although many previous studies have reported reduced HV in patients with T2D [7,8,9], we found no significant differences in prediabetes compared to matched subjects with NGM, in accord with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [25]. Similarly, Schneider et al. reported that reduced HV was associated with severe T2D, but not with prediabetes or less severe T2D [8]. We also found that HV/ICV ratio was lower in participants with diabetes (undiagnosed and known) than in those without diabetes (NGM and prediabetes), suggesting that the effects of hyperglycemia on HV do not manifest until a later disease stage. Thus, HV may be preserved in the prediabetic state, providing a therapeutic window of opportunity for preventing or delaying the onset and progression of cognitive decline. In the previous study, the hippocampus showed the highest level of insulin resistance [26], which suggests that possible reasons should be considered to explain why we found the reduced volume in GM, but not in the hippocampus. One possible technical explanation is that pathological changes in the prediabetes condition may be too subtle to be detected with our MRI analysis. For pathophysiological mechanisms, T2D could also lead to vascular abnormality, including macrovascular and microvascular changes [22]. In prediabetes condition, the GM may be more vulnerable to vascular changes compared to the hippocampus.
Brain reserve is a “passive” cognitive capacity that is thought to depend on individual brain structure and circuit complexity [27], and patients with less brain reserve capacity are thought to have a lower threshold for functional impairment, following the onset of brain pathology. Conversely, subjects with greater premorbid brain reserve are expected to express symptoms gradually and of lesser severity. Intracranial volume (ICV) is the most widely used proxy measure of brain reserve as it is easily derived from structural MRI [28]. However, a recent review article emphasized the need for measures more strongly predictive of current and future cognitive function, and the authors proposed whole-brain volume and bilateral HV as alternatives. Therefore, our finding of reduced GMV/ICV in prediabetes suggests that brain reserve may be compromised even in the earliest stages of the disorder, underscoring the importance of interventions for effective glucose control among nondiabetic elderly people.
Our study has several strengths. First, the community-based design and large sample size reduced sampling bias and the effects of individual variability, and permitted detailed evaluation of confounding factors. Furthermore, all brain MRI data were obtained using the same protocol and MRI scanner, and previous investigations have suggested that volume measurements across platforms (vendor, MRI sequence, and scanner upgrade) introduce difference bias [29, 30]. Second, dysglycemia diagnosis was based on HbA1c level, a more reliable marker of long-term hyperglycemia than fasting blood glucose and also allowing identification of undiagnosed diabetes and glucose intolerance. Third, Asian participants are significantly under-represented in MRI studies of diabetes-associated brain changes.
Several limitations should also be noted. First, as the present findings were derived from cross-sectional data, it is not possible to draw conclusions on the causal associations between glycemic measures and brain atrophy. Therefore, future longitudinal studies are needed to address if hyperglycemia proceeds cognitive decline and if the severity of hyperglycemia is correlated with the magnitude of cognitive dysfunction. Second, we also used only one HbA1c measurement and, thus, have no estimate of measurement reproducibility or accuracy. In addition, we could not account for different glycemia trajectories. Therefore, measurement error could have resulted in underestimation of the associations between dysglycemia and changes in brain volume. Furthermore, for understanding the pathophysiological mechanisms (hyperinsulinemia and higher expression of insulin receptors) for the GM atrophy, it is important to analyze the blood insulin/glucose concentrations. However, in our many participants, the glucose and insulin levels were not measured under overnight fasting conditions. Therefore, further studies will be needed to clarify relationship between insulin/glucose concentrations and brain volumes in prediabetes condition. Third, the diagnosis of “known diabetes” was based on self-report, and reliable data on duration of diabetes were not available. In addition, although the prediabetes and undiagnosed diabetes groups were consistent with treatment-naïve populations, the diabetes group was consisted of different grades of disease severity; short and long disease duration patients or compensated and decompensated patients. The high inhomogeneity with the diabetes group could affect the results for the comparison among the groups. Fourth, our sample was restricted to relatively well-educated ethnic Japanese, which limits the generalizability of our findings to other racial/ethnic groups, particularly in less developed countries. Finally, some relevant confounders may have been excluded from the multivariable linear regression analysis.
Our findings suggest that sustained dysglycemia, even before the clinical diagnosis of diabetes, may have deleterious effects on GM integrity in the elderly. Therefore, careful monitoring and early intervention during the prediabetes stage may delay the onset or progression of cognitive decline.
Abbreviations
- AD:
-
Alzheimer’s disease
- GMV:
-
Gray matter volume
- HV:
-
Hippocampal volume
- ICV:
-
Intracranial volume
- NGM:
-
Normal glucose metabolism
- WMV:
-
White matter volume
References
Bangen KJ, Beiser A, Delano-Wood L et al (2013) APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 22:1361–1369
Cheng G, Huang C, Deng H, Wang H (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42:484–491
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53:1937–1942
Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging S (2002) Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 51:1256–1262
Cooper C, Sommerlad A, Lyketsos CG, Livingston G (2015) Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 172:323–334
Luchsinger JA (2010) Diabetes, related conditions, and dementia. J Neurol Sci 299:35–38
Moran C, Phan TG, Chen J et al (2013) Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36:4036–4042
Schneider ALC, Selvin E, Sharrett AR et al (2017) Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Diabetes Care 40:1514–1521
Reitz C, Guzman VA, Narkhede A, DeCarli C, Brickman AM, Luchsinger JA (2017) Relation of dysglycemia to structural brain changes in a multiethnic elderly cohort. J Am Geriatr Soc 65:277–285
Dong S, Dongwei L, Zhang J, Liang J, Sun Z, Fang J (2019) Individuals in the prediabetes stage exhibit reduced hippocampal tail volume and executive dysfunction. Brain Behav 9:e01351
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK (2016) Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomarker Insights 11:95–104
Association AD (2019) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 42:S13–S28
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800
Ashburner J (2009) Computational anatomy with the SPM software. Magn Reson Imaging 27:1163–1174
Shattuck DW, Mirza M, Adisetiyo V et al (2008) Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39:1064–1080
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’for medical statistics. Bone Marrow Transplant 48:452–458
Vogt NM, Hunt JF, Adluru N et al (2020) Cortical microstructural alterations in mild cognitive impairment and Alzheimer’s disease dementia. Cereb Cortex 30:2948–2960
Espeland MA, Bryan RN, Goveas JS et al (2013) Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women’s Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 36:90–97
Falvey CM, Rosano C, Simonsick EM et al (2013) Macro-and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care 36:677–682
Saczynski JS, Siggurdsson S, Jonsson PV et al (2009) Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care 32:1608–1613
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74
Cholerton B, Baker LD, Craft S (2011) Insulin resistance and pathological brain ageing. Diabet Med 28:1463–1475
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R (2016) Diabetic Retinopathy Nat Rev Dis Primers 2:16012
Sundermann EE, Thomas KR, Bangen KJ et al (2021) Prediabetes is associated with brain hypometabolism and cognitive decline in a sex-dependent manner: a longitudinal study of nondemented older adults. Front Neurol 12:144
Talbot K, Wang HY, Kazi H et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig 122:1316–1338
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012
Van Loenhoud AC, Groot C, Vogel JW, Van Der Flier WM, Ossenkoppele R (2018) Is intracranial volume a suitable proxy for brain reserve? Alzheimer’s Res Ther 10:1–12
Jovicich J, Czanner S, Han X et al (2009) MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46:177–192
Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P et al (2009) Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage 44:1324–1333
Acknowledgements
The authors thank all the investigators and participants of this study.
Funding
This study has received funding by AMED (Japan Agency for Medical Research and Development) under Grant Number JP16dk0207025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shingo Kakeda.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study/observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tatsuo, S., Watanabe, K., Ide, S. et al. Association of prediabetes with reduced brain volume in a general elderly Japanese population. Eur Radiol 33, 5378–5384 (2023). https://doi.org/10.1007/s00330-023-09509-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09509-z