Abstract
Osteoarthritis (OA) is a degenerative disease characterized by the destruction of cartilage. The greatest risk factors for the development of OA include age and obesity. Recent studies suggest the role of inflammation in the pathogenesis of OA. The two most common locations for OA to occur are in the knee and hip joints. The knee joint experiences more mechanical stress, cartilage degeneration, and inflammation than the hip joint. This could contribute to the increased incidence of OA in the knee joint. Damage-associated molecular patterns (DAMPs), including high-mobility group box-1, receptor for advanced glycation end products, and alarmins (S100A8 and S100A9), are released in the joint in response to stress-mediated chondrocyte and cartilage damage. This facilitates increased cartilage degradation and inflammation in the joint. Studies have documented the role of DAMPs in the pathogenesis of OA; however, the comparison of DAMPs and its influence on OA has not been discussed. In this study, we compared the DAMPs between OA knee and hip joints and found a significant difference in the levels of DAMPs expressed in the knee joint compared to the hip joint. The increased levels of DAMPs suggest a difference in the underlying pathogenesis of OA in the knee and the hip and highlights DAMPs as potential therapeutic targets for OA in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
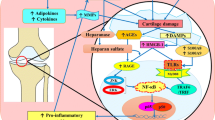
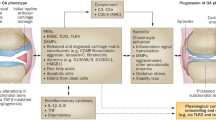
Osteoarthritis (OA) is a degenerative joint disease of the articular cartilage characterized by a limited range of motion, joint pain, tenderness, stiffness, crepitus, effusion, and inflammation without systemic effects [1]. The ultimate course of OA is an imbalance between degradative and repair process that result in loss of cartilage, joint space narrowing, and subchondral bone thickening [2]. The knee and hip joints are the most commonly affected joints by OA [2]. The most common risk factors for OA include age, obesity, biomechanics, trauma, and inflammation [3]. The progression of OA depends upon continued cartilage destruction due to increased mechanical loads and inflammation [4]. The damage to chondrocytes within the cartilage allows the release of damage-associated molecular patterns (DAMPs), including high-mobility group box-1 (HMGB-1), advanced glycation end products (AGEs), and alarmins (S100A8, and S100A9). The overexpression of DAMPs in joints has been linked to the pathogenesis of OA [3]. The DAMPs secreted as result of chondrocyte and cartilage damage induce cellular expression of inflammatory genes through receptor for advanced glycation end products (RAGE), toll-like receptors (TLRs), nuclear factor-kappa B (NF-κB), and MAPK signaling cascades. Thus, increased DAMPs in the joint space could act in an autocrine or paracrine manner to release pro-inflammatory cytokines [e.g., interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6], matrix metalloproteinases (MMPs), and more DAMPs. This leads to increased MMPs and activated macrophages in the joint, resulting in extracellular and cartilage matrix damage and chondrocyte apoptosis [3, 5,6,7,8,9,10,11,12,13,14,15]. Apoptosis of chondrocytes results in the secretion of HMGB-1, while chondrocyte senescence leads to the accumulation of advanced glycation end products (AGEs) [16,17,18].
HMGB-1 is ubiquitous in eukaryotic cells as a nuclear protein, and its release from the nucleus is associated with apoptosis or necrosis of the cells or inflammatory stimuli [9, 19]. HMGB-1 has been documented as a significant contributor to the pathogenesis of chronic inflammatory diseases, including OA and rheumatoid arthritis [9, 20]. Terada et al. [21] have shown increased levels of HMGB-1 in knee cartilage of patients with progressively higher grades of OA, relative to non-arthritic knee joints. Further, overexpressed extracellular HMGB-1 in OA synovium and amplification of inflammation in synovium in cooperation with IL-1β leading to the increased production of cytokines, chemokines, and MMPs suggest the crucial role of HMGB-1 in OA pathogenesis [9]. HMGB-1 activates the cell through RAGE and TLRs and increases the secretion of pro-inflammatory cytokines, MMPs leading to cartilage degeneration [5,6,7,8,9,10,11,12,13,14,15, 22]. RAGE is a member of the immunoglobulin family and is expressed in a variety of cells to include chondrocytes and macrophages. An increase in RAGE is noted in OA and can result in the production of MMPs suggesting that RAGE and its ligands play a direct role in the degradation of a joint [16]. Further, increased AGEs with aging and its association with RAGE play a role in OA pathogenesis [23]. Decreased RAGE activity in healthy joints and increased RAGE activity in arthritic joints suggests the potential role of RAGE in the pathogenesis of OA [6, 15]. In addition to HMGB-1, RAGE binds to S100 proteins and significant upregulation of RAGE, HMGB-1, and S100 has been associated with OA [16, 21]. Increased levels of the calcium binding proteins called alarmins, S100A8 and S100A9 found in granulocytes, macrophages, and chondrocytes, have been associated with inflammatory arthritis, cartilage destruction, macrophage recruitment, and induction of MMPs [24, 25]. S100A8 and S100A9 potentiate signals through TLR-4 and RAGE in a manner similar to HMGB-1 [24, 25].
Increased expression of DAMPs has been correlated with increased catabolism of articular cartilage and pathogenesis and progression of OA of knee and hip joints [3, 16, 26,27,28,29]. However, a comparative study of the levels of DAMPs to analyze the possible differential biomechanics involved in OA pathogenesis has not been reported. In this study, we hypothesized that due to the differences in stress levels and biomechanics of the knee and hip joints, there should be different DAMPs levels in the knee and hip joints. The findings of this study would provide an insight into the potential role of DAMPs in the pathogenesis of OA by comparing the levels of DAMPs in knee and hip joints via histological and molecular evidence.
Materials and methods
Patient selection
The Institutional Review Board of Creighton University approved the research protocol of this study as exempted since all knee and hip tissues were collected anonymously without any potential identification of the patient. Tissues from a total of ten patients undergoing total knee or hip replacement for severe osteoarthritis at Creighton University Medical Center (CUMC) and Immanuel Medical Center were collected and placed in a jar containing the University of Wisconsin (UW) solution. The age, gender, and the BMI of individual patient were anonymously provided by a nurse who collected the knee and hip and not involved with the study (Table 1).
Tissue acquisition and processing
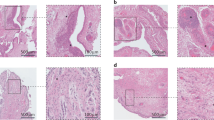
Post knee and hip joint replacement tissues were obtained and transported to the lab in the University of Wisconsin (UW) solution and maintained at 4 °C. Articular cartilage tissues from the medial and lateral tibial condyles and femoral head near the area of cartilage loss, and fat tissue present around the joint was collected with the scalpel and fixed in 4% formalin (Fig. 1). Cartilage tissues were also used to prepare the RNA and cDNA.
Post-surgical tissue, hematoxylin and eosin staining, and immunohistochemistry of knee and hip joint cartilage and fat. Hematoxylin and eosin staining showed greater number of chondrocytes in the hip cartilage compared to the knee cartilage suggesting more cartilage degeneration in the knee joint. Immunohistochemistry of the tissues showed greater staining for HMGB-1, RAGE, and S100 proteins in the knee compared to the hip joint. Post-surgical cartilage tissues (I-a–I-c source of cartilage and fat tissues), hematoxylin and eosin staining in cartilage (II-a, II-b) and fat (II-c), and immunohistochemistry for HMGB-1 (III-a, III-e), RAGE (III-b, III-f), S100A8 (III-c, III-g), and S100A9 (III-d, III-h) in knee and hip joint cartilage. The images are representative of all the study subjects. Stained slides were scanned at 40× with a scale bar of 200 μm with an inverted Olympus microscope
Preparation and staining of specimen
Tissue specimens fixed in 4% formalin for 24 h were transversely sectioned at 2 mm, processed in Sakura Tissue Tek VIP Tissue Processor, and embedded in paraffin. Thin sections (5 μm) were cut using a microtome (Leica, Germany) and placed on glass slides for staining. Hematoxylin and eosin (H&E) following manufacturer’s standard protocol (Newcomer/supply), immunohistochemistry, and immunofluorescence studies were performed on the tissue sections to analyze the tissue structure and expression of various proteins of interest (HMGB-1, RAGE, S100A8, and S100A9). All the images to examine the cartilage tissue were scanned at 20× using an Olympus inverted microscope (Olympus BX51) with the scale bar of 200 μm (Fig. 1). All slides were reviewed by two different observers.
Immunofluorescence (IF) study
Prior to immunostaining, the sectioned tissue (cartilage and fat) slides underwent deparaffinization, rehydration, and antigen retrieval as per the standard protocol in our laboratory. Rabbit anti-HMGB-1 (ab191583), rabbit anti-RAGE (ab3611), rabbit anti-S100A8 (ab196680), rabbit anti-S100A9 (ab63818), mouse anti-CD14 (ab182032), and rabbit anti-Heparan sulfate (ab23418) at 1:200 dilution primary antibodies, and Alexa Fluor 594 (red) conjugated secondary antibodies (Invitrogen, Grand Island, NY, USA) at 1:500 dilution were used for immunostaining. The slides were counterstained with DAPI (4,6-diamidino-2-phenylindole) to stain nuclei. Isotypes for each fluorochrome were run as negative controls. All slides were scanned at 20× with an Olympus inverted fluorescent microscope (Olympus BX51). Fluorescence intensity for HMGB-1, RAGE, S100A8, and S100A9 was measured in the stained slides using Image-J software and mean fluorescence intensity (MFI) was calculated. Three separate images of each tissue were used to measure the MFI.
RNA isolation, cDNA synthesis, and real-time PCR
The articular cartilage tissue obtained from the surgical specimens was used to isolate the total RNA using TRI reagent (Trizol reagent, Sigma, St Louis, MO, USA) as per the manufacturer’s instructions. The RNA was quantified using NanoDrop (Thermo Scientific, Rockford, IL, USA). The cDNA was synthesized using ImProm II reverse transcription kit (Promega, Madison, WI, USA). Subsequently, the real-time PCR (RT-PCR) was performed in triplicate using SYBR Green Master Mix and a Real-time PCR system (CFX96, BioRad Laboratories, and Hercules, CA, USA). The primers for different genes (Table 2) were obtained from Integrated DNA Technologies (Coralville, IA, USA). The PCR cycling conditions were 5 min at 95 °C for initial denaturation, 40 cycles of 30 s at 95 °C, 30 s at 55–60 °C (per the primer annealing temperatures), and 30 s at 72 °C followed by melting curve analysis. Fold expression of mRNA transcripts relative to controls was determined after normalizing to GAPDH.
Cell culture and immunofluorescence
Normal Human Articular Chondrocyte (NHAC) cells (HC1824, Lonza, Walkersville, MD) and Osteoarthritic Human Chondrocytes (HCOA, 402OA-05a, Cell Applications Inc. San Diego, CA) were cultured in the complete chondrocyte basal media (CC-3217, 10% FBS + 1% penicillin–streptomycin) and chondrocyte growth medium (411–500, Cell Application), respectively, in the T25 flask. After 80% confluence, about 30,000 cells were plated in each chamber of the chamber slides and immunofluorescence staining was performed for HMGB-1 (ab191583), RAGE (ab3611), S100A8 (ab196680), and S100A9 (ab63818) as per the standard protocol in our laboratory using Alexa Fluor 594 secondary antibody and DAPI to counterstain the nuclei. All slides were scanned at 20× with an Olympus inverted fluorescent microscope (Olympus BX51). Fluorescence intensity in ten randomly selected cells was quantified with Image-J software for each gene and mean fluorescence intensity (MFI) was calculated.
Statistical analysis
Data are presented as mean ± SD (N = 5 in each experimental group). Data were analyzed by Mann–Whitney test (SPSS) and Student’s t test for significance. A value of p < 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001) was considered statistically significant.
Results
Increased immunoreactivity of HMGB-1, RAGE, and alarmins in the knee joint
Immunofluorescence staining showed increased immunoreactivity for HMGB-1 (Fig. 2a, d), RAGE (Fig. 2g, j), S100A8 (Fig. 3a, d), and S100A9 (Fig. 3g, j) in the knee joint cartilage compared to the hip joint cartilage. The immunoreactivity of RAGE was higher compared to HMGB-1 (Fig. 2m) and the immunoreactivity for S100A8 was found higher compared to S100A9 (Fig. 3m) in both knee and hip joints. Heparan sulfate did not show any immunoreactivity either in the knee or in the hip joint cartilage (data not shown). There was minimal or no immunoreactivity for HMGB-1, RAGE, (Supplementary Fig. 1), S100A8, and S100A9 (Supplementary Fig. 2) in the fat tissues of the knee and hip joints.
Immunofluorescence and mRNA analysis (RT-PCR) for HMGB-1 and RAGE in knee and hip joint osteoarthritic cartilage. HMGB-1 (a, d), RAGE (g, j), DAPI (b, e, h, k), merged images (c, f, i, l), MFI for HMGB-1 and RAGE (m), and fold change in mRNA expression of HMGB-1 and RAGE (n). These are the representative figures from five knee and five hip joints in the study. All data have been represented as mean ± SD (N = 5). A p < 0.05 was considered as significant (*p < 0.05, **p < 0.01, and ***p < 0.001). DAPI 4,6-diamidino-2-phenylindole, HMGB-1 High-mobility group box-1, MFI mean fluorescence intensity, RAGE receptor for advanced glycation end products
Immunofluorescence and mRNA analysis (RT-PCR) for S100A8 and S100A9 in knee and hip joint osteoarthritic cartilage. S100A8 (a, d), S100A9 (g, j), DAPI (b, e, h, k), merged images (c, f, i, l), MFI for S100A8 and S100A9 (m), and fold change in mRNA expression of S100A8 and S100A9 (n). These are the representative figures from five knee and five hip joints in the study. All data have been represented as mean ± SD (N = 5). A p < 0.05 was considered as significant (**p < 0.01 and ***p < 0.001). DAPI 4,6-diamidino-2-phenylindole, MFI mean fluorescence intensity
Increased expression and mean fluorescence intensity of DAMPs in osteoarthritic chondrocytes cells
Immunostaining of the normal (NHAC) and osteoarthritic chondrocytes (HCOA) showed increased immunoreactivity for HMGB-1 (Fig. 4a, d), RAGE (Fig. 4g, j), S100A8 (Fig. 5a, d), and S100A9 (Fig. 5g, j) in the osteoarthritic chondrocytes (HCOA) compared to normal human chondrocytes (NHAC). The immunoreactivity for HMGB-1 was significantly higher than that for S100A8, S100A9, and RAGE (HMGB-1 > RAGE > S100A8 > S100A9) (Figs. 4, 5). Immunofluorescence revealed the nuclear expression of HMGB-1, and nuclear as well as the cytoplasmic expression of RAGE, S100A8, and S100A9 in NHAC and HCOA chondrocytes.
Immunofluorescence and mRNA analysis (RT-PCR) for HMGB-1 and RAGE in normal and osteoarthritic chondrocytes. HMGB-1 (a, d), RAGE (g, j), DAPI (b, e, h, k), merged images (c, f, i, l), MFI for HMGB-1 and RAGE (m), and fold change in mRNA expression of HMGB-1 and RAGE (n). All data have been represented as mean ± SD (N = 3). A p < 0.05 was considered as significant (***p < 0.001 and ****p < 0.0001). DAPI 4,6-diamidino-2-phenylindole, HCOA human chondrocyte osteoarthritic, HMGB-1 High-mobility group box-1, MFI mean fluorescence intensity, NHAC normal human articular chondrocytes, RAGE receptor for advanced glycation end products
Immunofluorescence and mRNA analysis (RT-PCR) for S100A8 and S100A9 in normal and osteoarthritic chondrocytes. S100A8 (a, d), S100A9 (g, j), DAPI (b, e, h, k), merged images (c, f, i, l), MFI for S100A8 and S100A9 (m), and fold change in mRNA expression of S100A8 and S100A9 (n). All data have been represented as mean ± SD (N = 3). A p < 0.05 was considered as significant (**p < 0.01, ***p < 0.001 and ****p < 0.0001). DAPI 4,6-diamidino-2-phenylindole, HCOA human chondrocyte osteoarthritic, HMGB-1 High-mobility group box-1, MFI mean fluorescence intensity, NHAC normal human articular chondrocytes, RAGE receptor for advanced glycation end products
Increased mRNA expression and of HMGB-1, RAGE, S100A8, and S100A9 in knee joint
Mean fluorescence intensity (MFI) evaluation with Image-J software showed significantly increased MFI in knee joint cartilage compared to hip joint cartilage (Figs. 2m, 3m), and in HCOA compared to NHAC (Figs. 4m, 5m). The RT-PCR analysis revealed significantly increased mRNA expression of the HMGB-1, RAGE, and S100A8 in knee joint cartilage compared to hip joint cartilage (Figs. 2n, 3n) and in HCOA compared to NHAC (Figs. 4n, 5n).
Increased expression of macrophages (CD14+ cells) in knee joint cartilage
Immunofluorescence staining of the knee and hip joint cartilage showed increased expression for CD14+ macrophages in knee joint cartilage (Fig. 6a) compared to the hip joint cartilage (Fig. 6d).
Immunofluorescence for macrophages (CD14+ cells) in knee and hip joint cartilage. CD14 (a, d), DAPI (b, e), and merged images (c, g). These are the representative images from five osteoarthritic knee and five osteoarthritic hip joints included in this study. CD14- cluster differentiation 14 (macrophage marker), DAPI 4,6-diamidino-2-phenylindole
Discussion
In this study, we found significantly increased protein and gene expression of HMGB-1, RAGE, S100A8, and S100A9 in the articular cartilage of the osteoarthritic human knee joint compared to the hip joint. Further, significantly higher protein and gene expression of HMGB-1, RAGE, S100A8, and S100A9 was found in human osteoarthritic chondrocytes compared to normal human chondrocytes. There was minimal to no immunoreactivity for HMGB-1, RAGE, S100A8, and S100A9 in the fat tissue of the knee joint. The higher prevalence of OA in the knee compared to the hip suggests the role of different or amplified factors contributing to the progression of the disease. The increased expression of HMGB-1, RAGE, S100A8, and S100A9 observed in the knee joint compared to the hip joint suggests that these DAMPs may be a significant factor facilitating the increased cartilage loss and rapid progression of OA in the knee joint compared to hip joint [2]. Further, the significant differential expression of DAMPs in the knee and hip joints may suggest that the underlying molecular pathogenesis of OA in the knee and hip could involve different molecules and downstream signals and, thus, may differentially affect the development and progression of arthritis [11, 12, 16, 20, 24, 25, 30,31,32,33,34].
HMGB-1, RAGE, S100A8, and S100A9 are released in response to chondrocyte damage or inflammatory processes and have the greatest catabolic effect on cartilage in the joint mediating the cartilage degeneration and osteoarthritis [20, 24, 25, 34]. Increased cytoplasmic expression of HMGB-1 along with nuclear expression in HCOA cells compared to only nuclear expression of HMGB-1 in NHAC cell on immunofluorescence in our study suggests the subcellular location change in the expression of HMGB-1 in osteoarthritic chondrocytes due to the disease pathology [9, 19]. AGEs and free HMGB-1 bind to RAGE and enhance the secretion of pro-inflammatory cytokines. This continuous and chronic inflammatory environment in the joint may act as a trigger for arthritis [6]. The binding of the free HMGB-1 to RAGE on the cellular surface activates RAGE and the downstream signaling. Increased cytoplasmic expression of RAGE protein in HCOA compared to NHAC on immunofluorescence in our study indicates the role of HMGB-1 released from degenerating cartilage and chondrocytes in activating the RAGE and its increased expression [16, 23]. The significant differential expression of HMGB-1 and RAGE in the knee and hip joints in our study suggests that the increased amount of inflammation in the knee joint could be a facilitating factor for rapid progression and higher prevalence of knee OA [35]. Increased expression of HMGB-1 and RAGE in osteoarthritis knee and hip joints and the relevance of increased HMGB-1 and RAGE with inflammation collaborate with the previous studies suggesting the role of HMGB-1 and RAGE in chronic inflammation mediating the development of OA [4, 6, 15, 35, 36]. Furthermore, significantly increased protein and gene expression of HMGB-1 and RAGE in HCOA compared to NHAC in our study also support the role of HMGB-1 and RAGE in the pathogenesis of OA. Increased expression of RAGE in the OA cartilage tissues in this study may also be due to the old age (average age for patients with hip replacement = 70 years and knee replacement = 65 years) of the study subjects [37]. The prevalence of OA increases with age and increasing age is the most important risk factor for the development of OA [38]. It is noteworthy that the three patients who were not obese were the oldest, suggesting the crucial role of aging in the development of OA in those joints. The normal aging process increases AGEs, and their accumulation may have an implication in the pathogenesis of OA. Accumulation of AGEs and their association with their receptor RAGE increase the inflammation through increased secretion of pro-inflammatory cytokines that may contribute to cartilage degeneration [39].
RAGE is a receptor not only for AGEs and HMGB-1 but also for S100 proteins, and RAGE-S100 binding contributes to chronic inflammation [22, 40, 41]. S100 calgranulins (calcium binding proteins) have a known role in inflammation and inflammatory arthritis [42, 43]. The expression of S100 proteins in osteoarthritic knee and hip joints in our study suggests the role of alarmins in the pathogenesis of OA and the presence of inflammation in the cartilage tissue. Higher expression of S100A8 and S100A9 in the knee joint compared to hip joint could also suggest greater inflammation in the knee joint. The role of S100A8 and S100A9 in OA is further supported by the significantly increased protein and gene expression of S100A8 and S100A9 in HCOA compared to NHAC. Interestingly, increased cytoplasmic expression of S100 proteins in HCOA compared to NHAC in our study suggests the change in the subcellular location of S100A8 and S100A9, and this may be due to the binding of activated RAGE to S100 proteins and activating their translocation. The released S100 proteins will further promote inflammation [16, 21, 22, 40,41,42,43]. Further, increased levels of extracellular alarmins are associated with the release of pro-inflammatory cytokines including IL-6, IL-8, and IL-1β, which recruit macrophages to the local area, thereby increasing the inflammation [25]. Attenuation of artificially induced OA in mice after removing the activated macrophages suggests the therapeutic potential of removal of inflammatory stimuli in attenuating the progression of OA and indicative of the role of inflammation in OA pathogenesis [25].
Since S100 proteins recruit macrophage and increase inflammation, the presence of macrophage in degenerating cartilage in our previous study [4] and in osteoarthritic cartilage in this study correlates with the higher levels of S100A8 and S100A9 in the knee joint compared to the hip joint. Higher expression of macrophages and S100 proteins in the knee joint cartilage is indicative of higher inflammation in knee compared to hip joint. Further, obesity is a risk factor for OA, the association of mechanical stress with the increased release of alarmins rather than systemic environment or factors, and high BMI of patients in our study (average BMI = 35.2, average BMI in patients with hip replacement = 33.9, and average BMI in patients with total knee replacement = 36.5) links the release of alarmins, inflammation, and OA [25, 44]. The significantly increased immunoreactivity of S100A8 and S100A9, gene expression of S100A8, and increased gene expression of S100A9 in OA knee cartilage compared to OA hip joint cartilage suggest that mechanical stress could have the differential effect on the release of these alarmins and the pathogenesis of OA. Higher immunopositivity and gene expression of S100A8 and S100A9 in the knee and hip joints compared to the fat tissue observed in this study suggest the role of mechanical stress and cellular damage in the secretion of these alarmins [45].
Minimal or no expression of DAMPs in the fat tissue from the OA knee and hip joints in our study and increased levels of macrophage and other mediators of inflammation documented in previous studies suggest that fat tissue around the knee and hip joints may play a systemic role in increasing the inflammation [4]. Since macrophages play a significant role in the pathogenesis of OA, activated macrophages signify inflammation, and fat is not load-bearing, these findings support the theory that DAMP expression increases more from mechanical stress than systemic inflammation [4, 25, 46, 47]. The association between the increased mechanical stress, increased level of DAMPs, and cartilage loss has been documented [48, 49]. Obesity, a risk factor for OA, increases compression forces due to increased body mass and increases shear forces by affecting gait. The hip joint, when affected by an increase in body weight, is more resistant to cartilage degradation than the knee joint [28]. Thus, obesity may affect the pathogenesis of OA through increasing the inflammation and shear stress. The different weight-bearing capacity of the knee and hip joints could also affect the pathogenesis of OA by regulating the secretion of mediators due to cartilage damage and inflammation (HMGB-1, RAGE, S100A8, and S100A9). The hip is more effective at bearing weight than the knee, so compression forces are less of a contributing factor to cartilage degradation and release of DAMPs. The greater relationship between obese patients with OA of the knee than the OA of the hip and increased stress on the knee joint than hip joint may lead to more mechanical damage in knee joint compared the hip joint [28]. The significantly increased expression of HMGB-1, RAGE, S100A8, and S100A9 in OA knee joint compared to OA hip joint observed in this study supports the association between differential weight-bearing capacity and release of DAMPs.
Although inflammation as a direct causative factor in the pathogenesis of OA has not been proved, increased local inflammation acts in accordance with increased DAMPs to contribute to the disease process [18, 21, 27]. The significantly increased expression of DAMPs in OA knee and hip joints in this study appears to be due to an increased mechanical load on the knee and hip joints. Interestingly, the patient with the greatest BMI had osteoarthritis of the knee and was the youngest. Further, higher BMI of the patients and increased expression of DAMPs signify the presence of local and systemic inflammation and its role in the pathogenesis of OA. The majority of joints examined in this study were knee and hip joints from females and only one hip and one knee joint were from the male patient. This suggests that the sex of the patient may be a factor affecting the development of the OA, with a higher prevalence for female sex [50].
Conclusion
The findings of our study signify that DAMPs play a significant role and make the difference in the pathogenesis of knee and hip joint OA. Further, inflammation, obesity, mechanical and shear stress, and aging collectively affect the joint homeostasis. Since DAMPs (HMGB-1, RAGE, S100 proteins) play a crucial role in OA pathogenesis, they may act as potential novel targets for decreasing the inflammation in the joint, attenuating the progression, and treatment of OA [51, 52]. HMGB-1 has been suggested as a target to modulate the immunity in chronic inflammation [20, 53]. Similarly, the reduction of the severity of AGE-induced arthritis with pioglitazone by decreasing the AGEs, and antioxidant action of polyphenols via anti-glycation and MMP inhibition to decrease inflammation has been discussed [39].
Limitations of the study
The limited number of patients is a major limitation of this study. The confounding factors such as the distribution of body fat, current medications, current medical status, and history of trauma were unknown for all patients and could have impacted the results of the study. Despite these limitations, our study strongly highlights the potential role of DAMPs in OA pathogenesis and the differential pathogenesis in knee and hip joints.
References
Yan CH, Chan WL, Yuen WH, Yung PS, Ip KY, Fan JC, Chiu KY (2015) Efficacy and safety of hylan G-F 20 injection in treatment of knee osteoarthritis in Chinese patients: results of a prospective, multicentre, longitudinal study. Hong Kong Med J 21(4):327–332
Turkiewicz A, Petersson IF, Bjork J, Hawker G, Dahlberg LE, Lohmander LS, Englund M (2014) Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr Cartil 22(11):1826–1832
Sokolove J, Lepus CM (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5(2):77–94
Rai V, Dietz NE, Dilisio MF, Radwan MM, Agrawal DK (2016) Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res Ther 18(1):203
Attur M, Belitskaya-Lévy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, Smiles S, Lee S, Patel J, Al-Mussawir H, McDaniel G, Kraus VB, Abramson SB (2011) Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheumatol 63(7):1908–1917
Chen YJ, Chan DC, Chiang CK, Wang CC, Yang TH, Lan KC, Chao SC, Tsai KS, Yang RS, Liu SH (2016) Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-kappaB pathway activation. J Orthop Res 34(5):791–800
Dreier R, Grassel S, Fuchs S, Schaumburger J, Bruckner P (2004) Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp Cell Res 297(2):303–312
Dreier R, Wallace S, Fuchs S, Bruckner P, Grassel S (2001) Paracrine interactions of chondrocytes and macrophages in cartilage degradation: articular chondrocytes provide factors that activate macrophage-derived pro-gelatinase B (pro-MMP-9). J Cell Sci 114(Pt 21):3813–3822
Garcia-Arnandis I, Guillen MI, Gomar F, Pelletier JP, Martel-Pelletier J, Alcaraz MJ (2010) High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes. Arthritis Res Ther 12(4):R165
Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, Arbiser JL, Apperley JF (1994) Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest 94(6):2307–2316
Liu-Bryan R (2013) Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep 15(5):323
Rasheed Z, Akhtar N, Haqqi TM (2011) Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-kappaB in human osteoarthritis chondrocytes. Rheumatology 50(5):838–851
Sillat T, Barreto G, Clarijs P, Soininen A, Ainola M, Pajarinen J, Korhonen M, Konttinen YT, Sakalyte R, Hukkanen M, Ylinen P, Nordström DC (2013) Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop 84(6):585–592
Zeng GQ, Chen AB, Li W, Song JH, Gao CY (2015) High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res 14(4):14811–14822
Sun XH, Liu Y, Han Y, Wang J (2016) Expression and significance of high-mobility group protein B1 (HMGB1) and the receptor for advanced glycation end-product (RAGE) in knee osteoarthritis. Med Sci Monit 22:2105–2112
Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD (2005) Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheumatol 52(8):2376–2385
Loeser RF (2010) Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med 26(3):371–386
Heinola T, Kouri VP, Clarijs P, Ciferska H, Sukura A, Salo J, Konttinen YT (2010) High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin Exp Rheumatol 28(4):511–518
Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, Fukuda T, Yamada S, Nagayama S, Hashiguchi K, Sunahara N, Fukuzaki K, Nagata R, Komiya S, Maruyama I, Fukuda T, Abeyama K (2008) Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheumatol 58(9):2675–2685
Ulloa L, Batliwalla FM, Andersson U, Gregersen PK, Tracey KJ (2003) High mobility group box chromosomal protein 1 as a nuclear protein, cytokine, and potential therapeutic target in arthritis. Arthritis Rheumatol 48(4):876–881
Terada C, Yoshida A, Nasu Y, Mori S, Tomono Y, Tanaka M, Takahashi HK, Nishibori M, Ozaki T, Nishida K (2011) Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1)in human osteoarthritic cartilage. Acta Med Okayama 65(6):369–377
Ingels C, Derese I, Wouters PJ, Van den Berghe G, Vanhorebeek I (2015) Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: impact of glycemic control with insulin and relation with clinical outcome. Shock 43(2):109–116
Gkogkolou P, Bohm M (2012) Advanced glycation end products: key players in skin aging? Dermatoendocrinology 4(3):259–270
Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H (2006) The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther 8(3):R69
Zreiqat H, Belluoccio D, Smith MM, Wilson R, Rowley LA, Jones K, Ramaswamy Y, Vogl T, Roth J, Bateman JF, Little CB (2010) S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res Ther 12(1):R16
Das SK, Farooqi A (2008) Osteoarthritis. Best Pract Res Clin Rheumatol 22(4):657–675
Goldring MB (2012) Articular cartilage degradation in osteoarthritis. HSS J 8(1):7–9
Harris EC, Coggon D (2015) HIP osteoarthritis and work. Best Pract Res Clin Rheumatol 29(3):462–482
van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, Cats H, Vogl T, Roth J, van den Berg WB (2012) Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheumatol 64(5):1466–1476
Liu-Bryan R, Terkeltaub R (2012) The growing array of innate inflammatory ignition switches in osteoarthritis. Arthritis Rheumatol 64(7):2055–2058
Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatol 64(6):1697–1707
Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, Sculco TP, Crow MK (2007) Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartil 15(5):516–523
Rosenthal AK (2011) Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol 23(2):170–173
Schelbergen RF, Blom AB, van den Bosch MH, Slöetjes A, Abdollahi-Roodsaz S, Schreurs BW, Mort JS, Vogl T, Roth J, van den Berg WB, van Lent PL (2012) Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheumatol 64(5):1477–1487
Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil 21(1):16–21
Thankam FG, Dilisio MF, Dietz NE, Agrawal DK (2016) TREM-1, HMGB1 and RAGE in the shoulder tendon: dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS ONE 11(10):e0165492
Chayanupatkul M, Honsawek S (2010) Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin Biochem 43(13–14):1133–1137
Li Y, Wei X, Zhou J, Wei L (2013) The age-related changes in cartilage and osteoarthritis. Biomed Res Int 2013:916530
Li Y, Zhang Y, Chen C, Zhang H, Ma C, Xia Y (2016) Establishment of a rabbit model to study the influence of advanced glycation end products accumulation on osteoarthritis and the protective effect of pioglitazone. Osteoarthr Cartil 24(2):307–314
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL (2013) Functions of S100 proteins. Curr Mol Med 13(1):24–57
Leclerc E, Fritz G, Vetter SW, Heizmann CW (2009) Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta 1793(6):993–1007
Goyette J, Geczy CL (2011) Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 41(4):821–842
Perera C, McNeil HP, Geczy CL (2010) S100 calgranulins in inflammatory arthritis. Immunol Cell Biol 88(1):41–49
Subramanian S, Pallati PK, Rai V, Sharma P, Agrawal DK, Nandipati KC (2017) Increased expression of triggering receptor expressed on myeloid cells-1 in the population with obesity and insulin resistance. Obesity 25(3):527–538
Srikrishna G, Freeze HH (2009) Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 11(7):615–628
Sellam J, Berenbaum F (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6(11):625–635
Scanzello CR, Goldring SR (2012) The role of synovitis in osteoarthritis pathogenesis. Bone 51(2):249–257
Favre J, Erhart-Hledik JC, Chehab EF, Andriacchi TP (2016) Baseline ambulatory knee kinematics are associated with changes in cartilage thickness in osteoarthritic patients over 5 years. J Biomech 49(9):1859–1864
Varady NH, Grodzinsky AJ (2016) Osteoarthritis year in review 2015: mechanics. Osteoarthr Cartil 24(1):27–35
Hubertsson J, Turkiewicz A, Petersson IF, Englund M (2017) Understanding occupation, sick leave and disability pension due to knee and hip osteoarthritis from a gender perspective. Arthritis Care Res 69(2):226–233
Goldring MB, Berenbaum F (2015) Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol 22:51–63
Sun MM, Beier F, Pest MA (2017) Recent developments in emerging therapeutic targets of osteoarthritis. Curr Opin Rheumatol 29(1):96–102
Yang H, Tracey KJ (2010) Targeting HMGB1 in inflammation. Biochim Biophys Acta 1799(1):149–156
Funding
This work was supported by research Grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception and design: VR, MFD, and DKA; contributed reagents/materials/analysis tool: MFD, TDS, and DKA; analysis and interpretation of the data: JHR and VR; drafting of the manuscript: JHR, VR, MFD, TDS, and DKA; critical revision of the article for important intellectual content: VR, MFD, and DKA; final approval of the article: JHR, VR, MFD, TDS, and DKA.
Corresponding author
Ethics declarations
Conflict of interest
As the corresponding author, I declare that this manuscript is original, that the article does not infringe upon any copyright or other proprietary rights of any third party, and that neither the text nor the data have been reported or published previously. All the authors have no conflict of interest and have read the journal’s authorship statement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rosenberg, J.H., Rai, V., Dilisio, M.F. et al. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol Cell Biochem 436, 59–69 (2017). https://doi.org/10.1007/s11010-017-3078-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3078-x