Abstract
Grossamide, a representative lignanamide in hemp seed, has been reported to possess potential anti-inflammatory effects. However, the potential anti-neuroinflammatory effects and underlying mechanisms of action of grossamide are still unclear. Therefore, the present study investigated the possible effects and underlying mechanisms of grossamide against lipopolysaccharide (LPS)-induced inflammatory response in BV2 microglia cells. BV2 microglia cells were pre-treated with various concentrations of grossamide before being stimulated with LPS to induce inflammation. The levels of pro-inflammatory cytokines were determined using the enzyme-linked immunoassay (ELISA) and mRNA expression levels were measured by real-time PCR. The translocation of nuclear factor-kappa B (NF-κB) and contribution of TLR4-mediated NF-κB activation on inflammatory effects were evaluated by immunostaining and Western blot analysis. This study demonstrated that grossamide significantly inhibited the secretion of pro-inflammatory mediators such as interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α), and decreased the level of LPS-mediated IL-6 and TNF-α mRNA. In addition, it significantly reduced the phosphorylation levels of NF-κB subunit p65 in a concentration-dependent manner and suppressed translocation of NF-κB p65 into the nucleus. Furthermore, grossamide markedly attenuated the LPS-induced expression of Toll-like receptor 4 (TLR4) and myeloid differentiation factor 88 (MyD88). Taken together, these data suggest that grossamide could be a potential therapeutic candidate for inhibiting neuroinflammation in neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia cells are the immune cells of the central nervous system and consequently play important roles in brain infections and inflammation [1] by phagocytosing and killing pathogens and regulating immune responses. However, in response to abnormal stimulation, such as endogenous proteins or chemical toxins, they become overactive and release inflammatory molecules that cause neuronal damage and neurodegenerative diseases (i.e., Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and other nervous system pathologies) [1–3]. Thus, inhibiting the overactivation of microglia cells might be a promising approach for the treatment of neurodegenerative diseases [4].
Lipopolysaccharide (LPS), a stimulator of microglia, is recognized by Toll-like receptor 4 (TLR4) on the surface of microglia and induces TLR4 activation, which subsequently recruits the adapter protein MyD88 resulting in activation of NF-κB and MAPK signaling pathways. Nuclear factor-kappa B (NF-κB), a major transcription factor in the inflammatory response, can enhance the expression of pro-inflammatory mediators and enzymes [5]. Previous studies have shown that the NF-κB signaling pathway can be activated via phosphorylation and degradation of IκBα. Consequently, p65 translocates to the nucleus to regulate the expression of related genes. Overactivation of the NF-κB signaling pathway causes the excessive production of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [3, 6]. Consequently, an array of microglia responses is triggered leading to the release of inflammatory mediators [7, 8].
Grossamide is a representative lignanamide that has been isolated from the herbal plant heep seed (fructus cannabis, the dried fruit of Cannabis sativa L.), an important medicinal plant that has been used in traditional Chinese medicine for thousands of years. Recent research has found that hemp seed has a broad pharmacological effect on cardiovascular system [9], central nervous system [10], and the immune system. Crude extracts of hemp seed or hemp seed meal showed antioxidant [11, 12], anti-aging [13, 14], anti-inflammatory [15, 16], and learning and memory improving activities [17, 18]. Previous research has shown that hemp seed is rich in lignanamides [19–23]. Some reports showed that lignanamides were good antioxidant and anti-inflammatory agents [24]. In our previous chemical study of hemp seed [23], we have found that grossamide is one of the main lignanamides of hemp seed. The anti-inflammatory activity of grossamide related to NO production had been reported [24, 25].
In this study, we evaluated the anti-inflammatory effects of grossamide in the LPS-stimulated BV2 microglia cells. Our results show that grossamide exhibits anti-neuroinflammatory effects in BV2 microglia cells through suppression of TLR4-mediated NF-κB pathway.
Materials and methods
Cell line and reagents
Murine BV2 microglia cells were obtained from the China Infrastructure of Cell Line Resources (Beijing, China). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Macgene (Beijing, China). LPS (E. coli 0111:B4), DAPI, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, MO, USA).The microscope cover glasses were purchased from NEST (Wuxi, China). The antibodies against NF-κB p65 were obtained from Santa Cruz (Santa Cruz, CA, USA); the antibodies against IκBα, phospho-IκBα, and TLR4 were from Abcam (Cambridge, UK). Antibodies against MyD88, phospho-p65, and β-actin were obtained from cell signaling (Danvers, MA, USA). TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Boster (Wuhan, China). Trizol reagent was purchased from Takara (Takara; Shiga, Japan); RT-PCR primers were obtained from Sangon Biotech (Shanghai, China); and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from ZSGB-BIO (Beijing, China). Immobilon Western Chemiluminescent Substrate (ECL) was purchased from Millipore (Billerica, MA, USA), and other materials for Western blot analysis were purchased from Bio-Rad (Hercules, CA, USA).
Grossamide was isolated from hemp seed as previously described by us [23], with the purity of more than 98% according to the analysis using high-performance liquid chromatography (HPLC). Briefly, the air-dried hemp seed (5.7 kg) were crushed and defatted with petroleum ether before percolating with 75% EtOH. The 75% EtOH extract was successively partitioned with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate extract was subjected to reverse-phase column liquid chromatography (methanol/H2O as eluent), MCI gel column chromatography (methanol/H2O as eluent), and silica gel column chromatography (dichlormethan/methanol as eluent) successively, and then grossamide (22.94 mg) was crystallized from some fractions.
Cell culture
The murine BV-2 microglia cell line was maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin antibiotics, and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability
Cell viability was measured using the MTT assay according to the manufacturer’s instructions [26]. Briefly, 100 μL cells (8 × 104 cells/mL) were seeded into 96-well plates and incubated overnight. Then the cells were treated with different concentrations of grossamide (0, 10, 15, and 20 μM) for 1 h and co-cultured in the absence or presence of 100 ng/mL LPS for 24 h. Next, cells were incubated with 10 μL MTT solution (5 mg/mL) for 4 h. The formazan crystals were dissolved with 100 μL DMSO, and the absorbance was measured at 570 nm using a microplate reader (Bio-rad). Results are expressed as a percentage of the control.
ELISA
The production of TNF-α and IL-6 in the culture supernatants was evaluated using ELISA kits, as previously described [27]. Briefly, BV2 cells (8 × 104 cells/mL) were seeded in a 96-well plate and incubated overnight. Then, the cells were treated with or without different concentrations of grossamide for 1 h, and co-cultured with LPS (100 ng/mL) for another 24 h. Cell-free supernatants were collected and stored at −20 °C before analysis. TNF-α and IL-6 levels were measured with ELISA kits according to the manufacturer’s instructions (Boster), and the absorbance was read at 450 nm on a microplate spectrophotometer (Bio-rad). For TNF-α, the cell-free supernatants were diluted ten times before measurement, and for IL-6, the cell-free supernatants were diluted three times.
Real-time PCR
BV2 microglia cells (2 × 106 cells/mL) were plated in 6-well culture plates overnight and pre-treated with various concentrations of grossamide for 1 h, after which they were co-cultured with LPS (100 ng/mL) for another 6 h. Total RNA was extracted using Trizol (Takara) and evaluated at 260 and 280 nm. RNA (1 μg) was reverse-transcribed using the PrimeScript TMRT reagent kit (Takara) according to the manufacturer’s instructions. cDNA was used for real-time PCR with SYBR Primix Ex Taq™ (Takara). The real-time PCR reactions were performed for 40 cycles in 10 μL reaction volumes. Samples were incubated at 95 °C for 15 s, 53 °C for 15 s, and at 72 °C for 20 s. The relative amounts of mRNA were calculated with the comparative CT method. GAPDH was used as the internal control. The primer sequences were: 5′-ATGAGCACAGAAAGCATGATC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′ forward and reverse primers for TNF-α, 5′-CCACTTCACAAGTCGGAGGC-3′ and 5′-CCAGCTTATCTGTTAGGAGA-3′ forward and reverse primers for IL-6, 5′-GCA GTG GCA AAG TGG AGA TTG-3′ and 5′-TGC AGG ATG CAT TGC TGA CA-3′ forward and reverse primers for GAPDH.
Western blot analysis
The BV2 cells were harvested and washed with PBS two times. Then, the cells were lysed on ice for 30 min using RIPA buffer (Beyotime Biotechnology, China), followed by centrifugation at 14,000×g for 10 min at 4 °C. The protein concentrations were determined using the BCA Protein Assay Kit according to the manufacturer’s instructions (Beyotime Biotechnology, China). Protein extracts were mixed with the sample loading dye and heated at 95 °C for 5 min. Samples were separated on 8–12% sodium dodecyl sulfate polyacrylamide gels and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Germany). Then, the PVDF membranes were blocked in blocking buffer (1X TBS, 0.1% Tween-20, 5% nonfat dry milk) for 4 h and incubated at 4 °C overnight with primary antibodies against IκBα, p-IκBα (1:1000, Abcam), p65 (1:1000, Santa Cruz), phospho-p65 (1:1000, Cell Signaling), TLR4 (1:500, Abcam), MyD44 (1:500, Cell Signaling), and β-actin (1:2000, Cell Signaling). After washing, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:5000, ZSGB-BIO) for another 1 h at room temperature. Then, the blots were washed in TBST (Tris-buffered saline, 0.1% Tween 20) 4 times and developed using an enhanced chemiluminescence detection kit (Millipore). Optical density analysis of signals was performed using Image Lab software.
Immunofluorescence assay
To determine the intracellular localization of the p65 subunit of NF-κB, BV2 cells (5 × 105 cells/well) were cultured overnight in 24-well plates containing sterilized microscope cover glasses. Then, the cells were pre-treated with grossamide for 1 h and treated with LPS for 1 h, fixed in 4% paraformaldehyde for 10 min, and washed with PBS for 5 min. Next, cells were permeabilized with 1% Triton X-100 in PBS for 10 min and then incubated with 5% BSA for 1 h at room temperature. Treated cells were stained with NF-κB p65 primary antibody (1:200, Santa Cruz) overnight at 4 °C. The cells were washed with PBS for 5 min, incubated with an Alexa Fluor 594-labeled antibody (1:100, ZSGB-BIO) for 1 h at room temperature, and washed again with PBS containing 0.02% Tween-20 for 5 min. The nuclei were stained with 2.5 μg/mL DAPI (Sigma) for 10 min at room temperature and then washed. Finally, all of the images were captured using a confocal fluorescence microscope (Carl Zeiss, Göttingen, Germany), and all images were observed at ×63 Oil Mirror.
Statistical analysis
Experiments were repeated 3–5 times. All of the data are expressed as the mean ± standard deviation (SD). The difference between treated and control cells was analyzed by one-way analysis of variance (ANOVA) using Graphpad Prism v5.0 software (GraphPad, La Jolla, CA, USA). p values less than 0.05 were considered statistically significant.
Results
Effects of grossamide and LPS on viability of BV2 microglia cells
MTT assay showed that grossamide itself had no obvious cytotoxicity at 10, 15, and 25 µM in the BV2 microglial cells after 24 or 48 h treatments (data not shown). To evaluate the cytotoxic effects of grossamide and LPS on BV2 microglia, the cells were treated with grossamide with or without LPS (100 ng/mL) for 24 h. We have found that grossamide had no significant effects on cell viability at concentrations up to 20 μM, and 100 ng/mL LPS had no obvious cytotoxic effects (Fig. 1). Thus, 100 ng/mL LPS and concentrations of 10, 15, and 20 μM grossamide were used for the subsequent experiments.
Effects of grossamide (0, 10, 15, and 20 μM) on viability of BV2 microglia cells. Cell viability was determined using the MTT assay. Cells were treated with grossamide with or without LPS (100 ng/mL) for 24 h. The results are presented as mean ± SD from three independent experiments. *p < 0.05 and **p < 0.01 versus cells without treatment
Effects of grossamide on the release of pro-inflammatory cytokines from LPS-stimulated BV2 microglia cells
TNF-α and IL-6 are representative pro-inflammatory cytokines produced by microglia cells [28]. As shown in Fig. 2, LPS significantly increased TNF-α and IL-6 release in the absence of grossamide. However, pre-treatment of cells with grossamide followed by stimulation with LPS led to a significant and dose-dependent inhibition of TNF-α and IL-6 release. These results suggest that grossamide downregulates LPS-mediated production of inflammatory molecules.
Effects of grossamide on TNF-α and IL-6 release from LPS-stimulated BV2 microglia cells. BV2 microglia cells were pre-treated with grossamide for 1 h prior to incubation with LPS (100 ng/mL) for another 24 h. The culture supernatants were harvested, and secretion of TNF-α (a) and IL-6 (b) was measured by ELISA. The data are presented as mean ± SD from at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus cells treated with LPS
Effects of grossamide on TNF-α and IL-6 mRNA expression in BV2 microglia cells
To determine if treatment with grossamide suppressed the LPS-mediated pro-inflammatory process, RT-PCR was used to quantify the mRNA levels of TNF-α and IL-6. As shown in Fig. 3, grossamide inhibited the mRNA levels of TNF-α and IL-6 in a dose-dependent manner.
Effects of grossamide on TNF-α and IL-6 mRNA levels in LPS-stimulated BV2 microglia cells. BV2 microglia cells were pre-treated with grossamide for 1 h prior to incubation with LPS (100 ng/mL) for 6 h. The mRNA expression levels of TNF-α (a) and IL-6 (b) were measured with RT-PCR. The data are presented as mean ± SD from at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus cells treated with LPS
Effects of grossamide on NF-κB activation in BV2 microglia cells
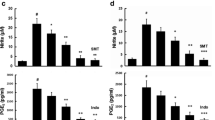
NF-κB plays a major role in controlling a number of genes involved in inflammatory responses [29]. In the active state, NF-κB is released from IκBα through phosphorylation and degradation of IκBα, followed by translocation into the nucleus and regulation of pro-inflammatory gene expression [30]. To determine the anti-inflammatory mechanism underlying the effects of grossamide in BV2 microglia cells, LPS-stimulated activation of the NF-κB pathway was evaluated by Western blot analysis. Upon LPS stimulation, the phosphorylation and degradation of IκBα resulted in activation of NF-κB and subsequent translocation into the nucleus. As shown in Fig. 4a, pre-treatment of cells with grossamide inhibited LPS-induced phosphorylation of IκBα and significantly reduced phosphorylation of NF-κB p65 levels. To further confirm the effects of grossamide on NF-κB, we explored whether grossamide prevented LPS-induced NF-κB p65 nuclear localization. Immunofluorescence images of NF-κB p65 showed that grossamide markedly blocked the nuclear localization of NF-κB p65 (Fig. 4d).
Effects of grossamide on the NF-κB pathway in LPS-stimulated BV2 microglia cells. BV2 microglia cells were pre-treated with grossamide for 1 h followed by LPS (100 ng/mL) stimulation for 1 h. a Protein samples were evaluated by Western blot analysis. Phospho-IκBα and phospho-p65 proteins were analyzed. β-actin was used as the internal control for normalization. The bar chart shows the quantitative evaluation of phospho-p65 (b) and phospho-IκBα (c) bands by densitometry. d Confocal microscopic images of immunofluorescent staining of NF-κB p65 in BV2 microglia cells (magnification = ×63, scale bar = 20 μm, red arrows labeled co-localization of the representative cell). The data are presented as mean ± SD from at least three independent experiments. *p < 0.05 and **p < 0.01 versus cells treated with LPS
Effects of grossamide on TLR4 and MyD88 expression in BV2 microglia cells stimulated with LPS
TLR4 regulates the expression of LPS-induced inflammatory mediators through the activation of NF-κB [31]. Therefore, the effects of grossamide on LPS-induced TLR4 expression were examined. As shown in Fig. 5a, in LPS-stimulated microglia cells, pre-treatment with grossamide dose-dependently decreased the expression of TLR4. The interaction of MyD88 and TLR4 is critical for TLR4 to stimulate downstream signaling pathways and activate inflammatory responses. Therefore, the effects of grossamide with MyD88 were also evaluated. As shown in Fig. 5a, after treatment of BV2 microglia cells with LPS (100 ng/mL), MyD88 protein levels gradually increased, but were suppressed upon treatment with grossamide. These data suggest that TLR4 and MyD88 proteins are involved in the anti-neuroinflammatory effects of grossamide in LPS-stimulated BV2 microglia cells.
Effects of grossamide on TLR4 and MyD88 protein expression in LPS-stimulated BV2 microglia cells. Cells were pre-incubated with grossamide for 1 h and/or incubated with LPS (100 ng/mL) for 24 h. a Total protein lysates were evaluated by Western blot analysis for TLR4 and MyD88 protein expression. β-actin was used as the internal control for normalization. The bar chart shows the quantitative evaluation of TLR4 (b) and MyD88 (c) bands by densitometry. The data are presented as mean ± SD from at least three independent experiments. *p < 0.05 and **p < 0.01 versus cells treated with LPS
Discussion
This study showed that pre-treatment with grossamide decreased the release of the pro-inflammatory cytokines, including IL-6 and TNF-α, and significantly inhibited inflammatory processes and associated signaling molecules including phospho-IκBα and phospho-NF-κB p65 proteins. In addition, grossamide decreased TLR4 and MyD88 protein expression in LPS-stimulated BV2 microglia cells. These data suggest that grossamide exhibits anti-neuroinflammatory effects in LPS-stimulated BV2 microglia cells.
Previous studies have reported that hemp seed has been used as a medicine in China for at least 3000 years due to its anti-aging and inflammatory effects [32–34]. Recent phytochemical studies isolated active components, including cannabinoids and lignanamides, which are attractive candidates for the treatment of neurodegenerative diseases [21, 35–38]. Grossamide is a lignanamide isolated from hemp seed, which has been shown to inhibit nitric oxide production in LPS-stimulated RAW 264.7 macrophages [25]. However, its anti-neuroinflammatory effects are not fully explored. Hence, as an example of lignanamides in hemp seed, we investigated the anti-inflammatory effects and underlying molecular mechanisms of grossamide in LPS-stimulated BV2 microglia cells.
LPS-activated microglia cells secrete pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, all of which play major roles in the inflammation-associated diseases [39]. Microglia cells release pro-inflammatory cytokines to promote neuronal cell damage. TNF-α, a crucial mediator of the inflammatory response, activates the expression of chemokines, thereby affecting biological functions [40]. IL-6 is also an important pro-inflammatory cytokine in the immune response [41]. In this study, we found that LPS highly stimulated the release of both TNF-α and IL-6, and this release was inhibited by grossamide in a dose-dependent manner. In addition, grossamide suppressed the mRNA levels of TNF-α and IL-6 in a dose-dependent manner. These results demonstrate that grossamide decreases LPS-induced gene expression and protein production and release of IL-6 and TNF-α in BV2 microglia cells in a concentration-dependent manner.
A number of reports have indicated that NF-κB activity can control the immune and inflammatory responses, and regulate the expression of inflammation-associated genes such as iNOS, COX-2, TNF-α, IL-6, and IL-1β [42]. Under inactive conditions, NF-κB is located in the cytoplasm and is associated with the inhibitory protein IκBα. In response to LPS stimuli, IκBα is phosphorylated and degraded, and activated NF-κB then translocates into the nucleus [43–45]. This study found that grossamide inhibited the phosphorylation of IκBα and NF-κB p65. In addition, grossamide suppressed NF-κB p65 protein translocation to the nucleus. Thus, the decreased production of pro-inflammatory molecules by grossamide is probably due to the suppressed activation of the NF-κB signaling pathway. LPS binds to TLR4 on the surface of microglia cells, resulting in the overexpression of pro-inflammatory genes and oversecretion of pro-inflammatory molecules by NF-κB activation [31, 46]. Here, grossamide suppressed the protein expression of TLR4 and MyD88, which probably resulted in the decreased production of pro-inflammatory molecules. Thus, it appears that grossamide suppressed the NF-κB signaling pathway by downregulating the protein expression of TLR4 and MyD88.
Conclusions
The present study showed that grossamide significantly reduced the production of pro-inflammatory mediators, and these effects were due to inhibition of the TLR4-mediated NF-κB signaling pathway. These results suggest that grossamide may be a potent natural anti-neuroinflammatory agent. Based on the anti-neuroinflammatory effects of grossamide, future studies should determine if other chemical compounds from hemp seed also have anti-inflammatory effects.
References
Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. FEBS Lett 585:3798–3805
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87:10–20
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
O’Neill LA, Kaltschmidt C (1997) NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20:252–258
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362
Kaminska B, Mota M, Pizzi M (2016) Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta 1862:339–351
Tan Y, Kagan JC (2014) A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell 54:212–223
Richard MN, Ganguly R, Steigerwald SN, Al-Khalifa A, Pierce GN (2007) Dietary hempseed reduces platelet aggregation. J Thromb Haemost 5:424–425
Lee MJ, Park SH, Han JH, Hong YK, Hwang S, Lee S, Kim D, Han SY, Kim ES, Cho KS (2011) The effects of hempseed meal intake and linoleic acid on Drosophila models of neurodegenerative diseases and hypercholesterolemia. Mol Cells 31:337–342
Hong S, Sowndhararajan K, Joo T, Lim C, Cho H, Kim S, Kim G, Jhoo J (2015) Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac J Reprod 4:147–152
Chen T, He J, Zhang J, Li X, Zhang H, Hao J, Li L (2012) The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem 134:1030–1037
Cao J, Li Z, Chen J, Li H (2005) Influence of semen cannabis iol on NO, SOD, GSH-Px, MDA in D-galactose-induced Aging Mice Serum. J Sichuan Tradit Chin Med 23(3):29–30
Wu SH, Guo YY, Zhang ST, Yang Y, Wang R, Zhang XY, Li HB (2015) The anti-aging effect of semen cannabis oil on the sex difference of Bombyx mori L. Pharmacol Clin Chin Mater Med 31:100–103
Zhang M, Shen Y, Zhu Z, Wang H (1999) Reseach on anti-inflammation, anti-analgesic and anti-thrombus function of semen cannabis. Res Pract Chin Med 13:13–15
Li G, Cao Y, Wu S, Zhang Z, Zhang Y, Yang Y, Li H (2015) Influence of semen cannabis iol on liqid levels, inflammmatory cytokines and anti-oxidant of aging model mice. Pharmacol Clin Chin Mater Med 31:109–111
Su J, He H, Shi M, Xiong X, Chen L (2011) Protective effect of semen cannabis iol on learning and memory impairment mice induced by D-galactose. Chin J Clin Pharmacol Ther 16:1332–1339
Luo J, Zheng T, Mo Z, Wei Q (2003) Effects of extracts of fructus cannabis on learning and memory capacity and related substances of senile mice induced by D-galactose. J Beijing Normal Univ (Nat Sci) 39(3):386–389
Flores-Sanchez IJ, Verpoorte R (2008) Secondary metabolism in cannabis. Phytochem Rev 7:615–639
Sakakibara I, Ikeya Y, Hayashi K, Okada M, Maruno M (1995) Three acyclic bis-phenylpropane lignanamides from fruits of Cannabis sativa. Phytochemistry 38:1003–1007
Sakakibara I, Katsuhara T, Ikeya Y, Hayashi K, Mitsuhashi H (1991) Cannabisin A, an arylnaphthalene lignanamide from fruits of Cannabis sativa. Phytochemistry 30:3013–3016
Harborne JB, Smith TA, Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H (1992) Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 31:3219–3223
Yan X, Tang J, dos Santos Passos C, Nurisso A, Simoes-Pires CA, Ji M, Lou H, Fan P (2015) Characterization of Lignanamides from Hemp seed (Cannabis sativa L.) and their antioxidant and acetylcholinesterase inhibitory activities. J Agric Food Chem 63:10611–10619
Sun J, Gu YF, Su XQ, Li MM, Huo HX, Zhang J, Zeng KW, Zhang Q, Zhao YF, Li J, Tu PF (2014) Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 98:110–116
Girgih AT, Alashi A, He R, Malomo S, Aluko RE (2014) Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur J Nutr 53:1237–1246
Liou CJ, Len WB, Wu SJ, Lin CF, Wu XL, Huang WC (2014) Casticin inhibits COX-2 and iNOS expression via suppression of NF-kappaB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J Ethnopharmacol 158:310–316
Wun ZY, Lin CF, Huang WC, Huang YL, Xu PY, Chang WT, Wu SJ, Liou CJ (2013) Anti-inflammatory effect of sophoraflavanone G isolated from Sophora flavescens in lipopolysaccharide-stimulated mouse macrophages. Food Chem Toxicol 62:255–261
Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH (2011) Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol 49:1745–1752
Mancino A, Lawrence T (2010) Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 16:784–789
Kim BW, Koppula S, Kumar H, Park JY, Kim IW, More SV, Kim IS, Han SD, Kim SK, Yoon SH, Choi DK (2015) alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 97:46–57
Li N, Zhang X, Dong H, Zhang S, Sun J, Qian Y (2016) Lithium ameliorates LPS-induced astrocytes activation partly via inhibition of Toll-Like Receptor 4 expression. Cell Physiol Biochem 38:714–725
Bindukumar B, Mahajan S, Reynolds J, Hu Z, Sykes D, Aalinkeel R, Schwartz S (2008) Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res 1191:1–11
Cai P, Fu X, Deng AG, Zhan XJ, Cai GM, Li SX (2010) Anti-aging effect of hemp seed oil, protein and lignanamide of bama on old mice. Cent S Pharm 3:003
Wang X, Yang X, Tang C (2007) Nutritional assessment of Hemp (Cannabis sativa L.) proteins. Mod Food Sci Technol 7:002
Brenneisen R (2007) Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In: Marijuana and the Cannabinoids, Springer, New York, pp 17–49
Chen H, Olatunji OJ, Zhou Y (2016) Anti-oxidative, anti-secretory and anti-inflammatory activities of the extract from the root bark of Lycium chinense (Cortex Lycii) against gastric ulcer in mice. J Nat Med 70:610–619
Girgih AT, Udenigwe CC, Aluko RE (2011) In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc 88:381–389
Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H (1992) Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 31:3219–3223
Kim BW, More SV, Yun YS, Ko HM, Kwak JH, Lee H, Suk K, Kim IS, Choi DK (2016) A novel synthetic compound MCAP suppresses LPS-induced murine microglial activation in vitro via inhibiting NF-κB and p38 MAPK pathways. Acta Pharmacol Sin 37:334–343
Zhai XT, Zhang ZY, Jiang CH, Chen JQ, Ye JQ, Jia XB, Yang Y, Ni Q, Wang SX, Song J, Zhu FX (2016) Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-kappaB signaling pathway. J Ethnopharmacol 183:159–165
Lee KC, Chang HH, Chung YH, Lee TY (2011) Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-kappaB pathway. J Ethnopharmacol 135:678–684
Lee JW, Choi YJ, Kim SI, Lee SY, Kang SS, Kim NH, Kwon YS, Lee HJ, Chun WJ, Kim SS (2013) Betulinic acid inhibits LPS-induced MMP-9 expression by suppressing NF-κB activation in BV2 microglial cells. Biomol Ther (Seoul) 19:431–437
Markus RP, Cecon E, Pires-Lapa MA (2013) Immune-pineal axis: nuclear factor κB (NF-κB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int J Mol Sci 14:10979–10997
Sung HC, Liang CJ, Lee CW, Yen FL, Hsiao CY, Wang SH, Jiang-Shieh YF, Tsai JS, Chen YL (2015) The protective effect of eupafolin against TNF-alpha-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J Ethnopharmacol 170:136–147
Cho KH, Kim DC, Yoon CS, Ko WM, Lee SJ, Sohn JH, Jang JH, Ahn JS, Kim YC, Oh H (2016) Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-κB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem Int 95:55–62
Jiang L, Xu F, He W, Chen L, Zhong H, Wu Y, Zeng S, Li L, Li M (2016) CD200Fc reduces TLR4-mediated inflammatory responses in LPS-induced rat primary microglial cells via inhibition of the NF-kappa B pathway. Inflamm Res 65:521–532
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81473323); Key R&D program in Shandong Province (No. 2015GSF119025) and the China-Australia Centre for Health Science Research (Grant No. 2015GJ04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Luo, Q., Yan, X., Bobrovskaya, L. et al. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem 428, 129–137 (2017). https://doi.org/10.1007/s11010-016-2923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2923-7