Abstract
The purpose of the study was to assess the effect of the internal tandem duplication in FMS-like tyrosine kinase 3 (FLT3-ITD) on the outcome in pediatric acute myeloid leukemia (AML) patients. We identified eligible studies from several databases including PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) (from January 1995 to July 2015). Ten studies of 1661 pediatric patients with AML were included in exploring the relationship between the FLT3-ITD and overall survival (OS)/event free survival (EFS). Pediatric patients with AML with FLT3-ITD had worse OS [HR = 2.19 (1.60–3.01)]/EFS [HR = 1.70 (1.37–2.11)] than those patients without FLT3-ITD. Furthermore, FLT3-ITD had unfavorable effect on OS/EFS in the subgroups of NOS, uni/multivariate model, number of patients, the length of following-up, and patient source. The findings of this meta-analysis indicated that FLT3-ITD had negative impact on pediatric patients with AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a group of heterogeneous diseases with respect to biological and clinical outcomes, which have been considered to be related to cytogenetic and gene lesions in hematopoietic stem or progenitor cells [1]. The internal tandem duplication in FMS-like tyrosine kinase 3 (FLT3-ITD) was one of the most common mutations in AML [2]. FLT3-ITD was postulated to cause constitutive phosphorylation of the FLT3 protein thereby impairing normal haematopoiesis and contributing to leukemogenesis [3].
In adult AML, patients with FLT3-ITD have aggressive disease characterized by early relapse and decreased survival [4–6] compared to those with FLT3 wild type (WT). However, the prognosis of FLT3-ITD in pediatric AML was still controversial. Some studies showed that FLT3-ITD had a worse prognostic implication [7–9], whereas others reported no additional prognostic value of FLT3-ITD [10–12]. Thus, it is necessary to perform a meta-analysis to further clarify the relationship between FLT3-ITD and prognosis in pediatric patients with AML.
Materials and methods
Eligibility criteria
Studies were eligible for inclusion in the meta-analysis if they met all the following criteria: (1) Cohort studies were published from January 1995 to July 2015. (2) The patients with AML in the study were diagnosed according to FAB classification [13] or WHO2008 criteria [14]. (3) The age of patients included in the study was under 20. (4) The number of patients with FLT3-ITD and without WT was available in the study. (5) They offered the data of overall survival (OS) and/or event free survival (EFS). Studies were excluded if the number of patients with AML in the study was under 50. Multiple reports of a single study were considered as one publication, and only the most complete or recent article was examined.
Information sources and search
Studies were identified by searching electronic databases and scanning reference lists of articles. No limits were applied for language, and foreign papers were translated. This search was applied to PubMed (1995–2015), Embase (1995–2015), and the Cochrane Central Register of Controlled Trials (CENTRAL) (1995–2015). We used the following strategy to search the databases: (“FLT3*” OR “FMS-like tyrosine kinase 3”) AND (“Acute Myeloid Leukemia” OR“AML”) AND (“pediatric*” OR “paediatric*” OR “child*”).
Study collection
Eligibility assessment was performed independently by two investigators. Disagreements between two investigators were resolved by asking the opinion of the third investigator. Endnote 6.0 was used for the managing the articles and removing most of the duplicates. Unrelated articles and remaining duplicates were excluded by reading the abstracts carefully. Then the remaining full-text articles were retrieved and reviewed to identify the eligible studies. The selection process was documented in a flow chart recommended in the PRISMA statement [15] (Fig. 1).
Data collection process
Two reviewers reviewed all of the articles that met the including criteria independently. We abstracted and tabulated the following information for each eligible study: first author, journal, year, location of publication, the number of pediatric patients with AML, and Kaplan–Meier curves. Primary outcome was the OS and secondary outcome was EFS.
Risk of bias in individual studies
Two authors independently assessed the methodological quality of each individual study using the Newcastle-Ottawa quality assessment Scale (NOS) for cohort studies [16]. Any discrepancies were resolved by another reviewer. This scale has nine items classified into three major categories: selection (four items), comparability (two items) and outcome for cohort design (three items). According to the NOS for cohort studies, the qualities of these studies were classified into three groups: high (7–9 points), intermediate (4–6 points), and low (1–3 points).
Summary measures
Hazard ratio (HR) was used to assess the OS or EFS (OS/EFS) between FLT3-ITD and WT in pediatric AML. The natural logarithm of a crude HR and its variance within the study was calculated by using the abstracted survival probabilities at each time point with the methods proposed by Tierney et al. [17]. We pooled the HRs for OS/EFS by using fixed and random effect models simultaneously [18]. However, only the estimates from random effect model were selected as the basis of our conclusion, because this approach could provide a more conservative assessment of the average effect size.
Assessment of heterogeneity and subgroup analysis
We assessed statistical heterogeneity of the pooled HRs for OS/EFS by visual inspection of the forests plots and by a formal statistical test using Chi-square test with a significance level at P < 0.1. I 2 statistics was also used to quantitatively assess the possible heterogeneity (I 2 = 0–30 %: not be important; I 2 = 30–50 %: moderate heterogeneity; I 2 = 50–75 %: substantial heterogeneity; I 2 = 75–100 %: considerable heterogeneity) [19]. We explored the possible causes of heterogeneity for OS/EFS by five subgroups: NOS; uni/multivariate model; number of patients; the length of following-up; patient source.
Assessment of reporting biases
The risk for OS/EFS in pediatric patients with AML was selected to form contour enhanced funnel plots [20]. Contour-enhanced funnel plots display areas of statistical significance on a funnel plot by contour lines representing different levels of statistical significance. If studies seemed to be “missing” in areas of non-significance (P < 10 %), the asymmetry of the funnel plot might be due to publication bias, although other explanations should still be considered. In addition, we tried to figure out missing studies and assessed the robustness of pooled HR for OS/EFS in childhood patients with AML by the trim and fill adjustment method [21].
A P < 0.05 was considered significant. All calculations were conducted with Stata version 12.0 software (Stata Corp., College Station, TX, USA).
Results
Study selection
As shown in flow diagram (Fig. 1), the initial search yielded a total of 1020 studies and 184 studies were excluded because of the duplication. Then, 836 studies were reviewed the titles and abstracts and 53 were considered potentially eligible and were retrieved in full text, 10 studies met the inclusion criteria and were included in the meta-analyses.
Study characteristics
As shown in Table 1, 10 studies of 1661 pediatric patients with AML (250 FLT3-ITD and 1411 WT) were included in the meta-analysis. Among these studies, five studies [9, 11, 12, 22, 23] from Asia, three studies [10, 24, 25] form European and two studies from USA [7, 8].
Risk of bias in within studies
The NOS for cohort studies was used for assessing the quality of all 10 studies in this meta-analysis (Table 2). The qualities of seven studies were high (7–9) and the remaining three studies were moderate (4–6). The median overall score was 7, which indicated that the methodological quality was high.
The impact of FLT3-ITD on the OS and EFS in total population
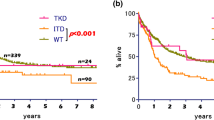
As shown in Fig. 2, data were extracted from 10 studies, with a total of 1661 pediatric patients with AML, including 250 patients with FLT3-ITD mutation, to analyze the OS. In this population, patients with FLT3-ITD mutation had inferior OS [HR = 2.19 (1.60–3.01), P < 0.001] as compared to WT patients. There was substantial heterogeneity by I 2 testing [I 2 = 40 %, P = 0.091].
As shown in Fig. 3, data were extracted from 8 studies, with a total of 1428 pediatric patients with AML, including 224 patients with FLT3-ITD mutation, to analyze the EFS. In this population, patients with FLT3-ITD mutation had worse EFS [HR = 1.70 (1.37–2.11), P < 0.001] as compared to WT patients. The heterogeneity among the studies was not important [I = 0 %, P = 0.472].
Prognostic impact of FLT3-ITD in different subgroups
We pooled the HRs for OS/EFS in different groups by fixed and random effect models simultaneously, which are shown in Table 3. In the majority of subgroups, FLT3-ITD had an unfavorable impact on the OS/EFS in pediatric patients with AML. Due to the limited studies included, FLT3-ITD might not be associated with worse EFS [HR = 1.63 (0.91–2.86), P < 0.091] in the NOS subgroup (middle quality). In pediatric patients with AML from Europe, FLT3-ITD was prone to have adverse effect on the OS [HR = 1.58 (0.97–2.59)] and EFS [HR = 1.92 (0.98–3.75)].
Publication bias
Due to the limited studies included for the EFS, only the data about the OS of total population were utilized to search publication bias. Supplementary Figure 1 demonstrated the distribution of 10 real studies and 3 filled studies in the aspect of OS. The trim and fill method imputed a total of 3 filled studies in a random effects model, which located in the region of statistical non-significance (P < 10 %). Thus, the asymmetry of the funnel plot might be due to publication bias, although other explanations should still be considered. After adding the studies to the funnel plot, the pooled fixed effects HR was 1.70 (1.38–2.11), and the pooled random effects HR was 1.73 (1.20–2.51), which implied that FLT3-ITD was associated with shorter OS in pediatric patients with AML.
Discussion
We have performed a meta-analysis to figure out the association between FLT3-ITD and survival in pediatric patients with AML. Ten studies with 1661 patients with AML were included in our meta-analysis. According to the NOS scale, 7 studies belonged to high quality and the others had moderate quality.
The primary outcome was OS. FLT3-ITD conferred shorter OS in total population. Moreover, FLT3-ITD had an adverse effect on the OS in subgroups of NOS, uni/multivariate model, number of patients and the length of following-up. The secondary outcome was EFS. FLT3-ITD was associated with poor EFS in the total population. This conclusion was also suitable for the subgroups of uni/multivariate model, number of patients, and the length of following-up.
According to the results described above, we found that FLT3-ITD suggested a significantly negative prognostic effect in pediatric patients with AML, which might help us to justify the risk-adapted management decisions. Furthermore, given the relatively high frequency of FLT3-ITD in pediatric patients with AML, much interest has been generated in whether FLT3 may be potential therapeutic targets. These findings will hopefully yield critical information for further research on the FLT3 inhibitor [26, 27], which may highlight the need to identify FLT3-ITD in the clinical management of patients with AML.
Although our study is the first meta-analysis relating to the controversial prognostic impact of FLT3-ITD in pediatric AML, the result must be viewed cautiously due to its own limitations. First, the analyses were based on cohort studies rather than random controlled trials. Second, we used abstracted data only from publications, because individual patient data could not be obtained, when a meta-analysis based on individual patient data would provide a more robust estimate of the association. Third, we did not assess the potential effects of other factors, such as therapeutic regimens [28, 29], chromosomal aberration [30], and gene lesions. Fourth, we did not consider the impact of FLT3-ITD allelic ratio and their association of outcome. Fifth, due to the limited data, we did not assess the achievement of CR and disease post-induction. A study of children with de novo AML determined that an FLT3-ITD allelic ratio ≥0.4 identified the highest risk group with the worse prognosis, whereas children with allelic ratios <0.4 had similar outcomes as children with FLT3-WT.
In conclusion, the findings of this study indicated that FLT3-ITD mutation had negative impact on OS and EFS in pediatric patients with AML and related subgroups. These findings may help to justify risk-adapted therapeutic strategies for pediatric AML based on FLT3-ITD. However, combining other important genetic biomarkers, such as NPM1 [31] and CEBPA [32], FLT3-ITD would contribute to a more precise clinical risk stratification and decision of treatment. More cohort studies concerning FLT3-ITD are needed in an effort to further verify or modify the pooled estimates to a certain extent.
References
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474
Network CGAR (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368:2059
Tse K, Mukherjee G, Small D (2000) Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia 14:1766–1776
Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Döhner H, Döhner K (2002) Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 100:4372–4380
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T (2002) Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 100:59–66
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, Wermke M, Bornhäuser M, Ritter M, Neubauer A (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99:4326–4335
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, Bernstein ID, Radich JP (2001) Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood 97:89–94
Brown P, McIntyre E, Rau R, Meshinchi S, Lacayo N, Dahl G, Alonzo TA, Chang M, Arceci RJ, Small D (2007) The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood 110:979–985
Kinoshita A, Miyachi H, Matsushita H, Yabe M, Taki T, Watanabe T, Saito AM, Tomizawa D, Taga T, Takahashi H (2014) Acute myeloid leukaemia with myelodysplastic features in children: a report of Japanese Paediatric Leukaemia/Lymphoma Study Group. Br J Haematol 167:80–86
Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, von Bergh AR, Cloos J, Kaspers GJ, de Haas V (2011) Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica 96:1478–1487
Leow S, Kham SKY, Ariffin H, Quah TC, Yeoh AEJ (2011) FLT3 mutation and expression did not adversely affect clinical outcome of childhood acute leukaemia—a study of 531 Southeast Asian children by the Ma-Spore study group. Hematol Oncol 29:211–219
Liu Y, Tang J, Wakamatsu P, Xue H, Chen J, Gaynon PS, Shen S, Sun W (2014) High-resolution melting curve analysis, a rapid and affordable method for mutation analysis in childhood acute myeloid leukemia. Front Pediatr 2:96
Neame PB, Soamboonsrup P, Browman GP, Meyer RM, Benger A, Wilson W, Walker IR, Saeed N, McBride JA (1986) Classifying acute leukemia by immunophenotyping: a combined FAB-immunologic classification of AML. Blood 68:1355–1362
Sabattini E, Bacci F, Sagramoso C, Pileri S (2010) WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 102:83–87
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux P, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Internal Med 151:W-65–W-94
Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Deeks J, Higgins J, Altman D (2011) Chapter 9—analysing data and undertaking meta-analyses: Cochrane handbook for systematic reviews of interventions, Version 5.1.0 [updated March 2011]
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61:991–996
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Ruan M, Wang Y, Zhang L, Liu T, Liu F, Liu X, Zhang J, Zou Y, Chen Y, Zhu X (2011) FLT3 mutations in children with acute myeloid leukemia: a single center study. Zhongguo dang dai er ke za zhi (Chin J Contemp Pediatr) 13:863–866
Sano H, Shimada A, Tabuchi K, Taki T, Murata C, M-j Park, Ohki K, Sotomatsu M, Adachi S, Tawa A (2013) WT1 mutation in pediatric patients with acute myeloid leukemia: a report from the Japanese Childhood AML Cooperative Study Group. Int J Hematol 98:437–445
Staffas A, Kanduri M, Hovland R, Rosenquist R, Ommen HB, Abrahamsson J, Forestier E, Jahnukainen K, Jónsson ÓG, Zeller B (2011) Presence of FLT3-ITD and high BAALC expression are independent prognostic markers in childhood acute myeloid leukemia. Blood 118:5905–5913
Yatsenko Y, Kalennik O, Maschan M, Kalinina I, Maschan A, Nasedkina T (2013) NPM1, FLT3, and c-KIT mutations in pediatric acute myeloid leukemia in Russian population. J Pediatr Hematol Oncol 35:e100–e108
Tam WF, Gilliland DG (2008) Can FLT3 inhibitors overcome resistance in AML? Best Pract Res Clin Haematol 21:13–20
Sudhindra A, Smith CC (2014) FLT3 inhibitors in AML: are we there yet? Curr Hematol Malig Rep 9:174–185
Klusmann J-H, Reinhardt D, Zimmermann M, Kremens B, Vormoor J, Dworzak M, Creutzig U, Klingebiel T (2012) The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica 97:21–29
Creutzig U, Zimmermann M, Bourquin J-P, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C (2013) Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood 122:37–43
von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, Zemanova Z, Stary J, Bourquin J-P, Haas OA (2010) Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol 2009(25):6321
Hollink I, Zwaan C, Zimmermann M, Arentsen-Peters T, Pieters R, Cloos J, Kaspers G, de Graaf S, Harbott J, Creutzig U (2009) Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia 23:262–270
Mizushima Y, Taki T, Shimada A, Yui Y, Hiraumi Y, Matsubara H, Watanabe M, K-i Watanabe, Kamitsuji Y, Hayashi Y (2010) Prognostic significance of the BAALC isoform pattern and CEBPA mutations in pediatric acute myeloid leukemia with normal karyotype: a study by the Japanese Childhood AML Cooperative Study Group. Int J Hematol 91:831–837
Acknowledgments
This study is funded by project supported by Science and Technology Department of Hebei Province (No. 152777206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2775_MOESM1_ESM.docx
Supplementary material 1 (DOCX 278 kb). Contour enhanced funnel about the association between FLT3-ITD and OS with filled studies from meta-trim in a random effects model
Rights and permissions
About this article
Cite this article
Wu, X., Feng, X., Zhao, X. et al. Prognostic significance of FLT3-ITD in pediatric acute myeloid leukemia: a meta-analysis of cohort studies. Mol Cell Biochem 420, 121–128 (2016). https://doi.org/10.1007/s11010-016-2775-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2775-1