Abstract
MicroRNAs are a kind of small non-coding RNAs that play important roles in various biological processes such as cell proliferation, differentiation, and apoptosis. Cellular responses to UV-induced apoptosis have been suggested to be regulated by microRNAs at the posttranscriptional level, while the detailed mechanisms underlying this process remain unclear. Our aim in this study was to investigate the effects of miR-1246 in UVB-induced apoptosis and to identify the functional targets of miR-1246 in keratinocyte HaCaT cells. The expression of miR-1246 and apoptotic genes in HaCaT cells experiencing UVB stress was determined using quantitative real-time PCR. miR-1246 functions in UVB-induced apoptosis were quantified via fluorescence-activated cell sorter analysis of miR-1246 mimic or inhibitor-transfected cells. Additionally, the regulatory relationship between RTKN2 and miR-1246 was identified by Western blot and luciferase reporter assays. miR-1246 was upregulated accompanying with UVB-irradiated apoptosis in HaCaT cells. Overexpression of miR-1246 promoted UVB-induced apoptosis, while knockdown of miR-1246, using a specific inhibitor, resulted in a significant reduction in UVB-elicited apoptosis. We further demonstrate that miR-1246 negatively regulated the expression of RTKN2 through binding to the 3′-untranslated region of RTKN2 at the posttranscriptional level. Moreover, RTKN2 was observed to be resistant to UVB-induced apoptosis and RTKN2 antagonized the pro-apoptotic effects of miR-1246 during UVB-induced apoptosis in HaCaT cells. These findings suggested that miR-1246 promotes UVB-induced apoptosis by downregulating RTKN2 expression and that UVB-upregulated miR-1246 released RTKN2-dependent resistance to UVB-induced apoptosis by targeting RTKN2 post-transcriptionally in keratinocyte cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
UVB radiation (290–320 nm) represents one of the most prevalent environmental causes of skin aging, sunburn, and skin cancer [1–3]. The induction of apoptotic cell death of keratinocytes is a hallmark event in UV exposure [4], and the insufficiency of UV-induced DNA damage repair may initiate the apoptosis of sunburn cells [5]. It has been proposed that nuclear and cell membrane effects contribute independently to the induction of apoptosis exposed to UVB radiation [6]. UVB induces the activation and suppression of a variety of genes [7, 8], and the tumor-suppressor gene p53 is known to be involved in UVB-induced apoptosis [5, 9]. Elucidation of the underlying mechanism of UVB-elicited apoptosis is pivotal for understanding how UVB acts as a pathogen to damage cells.
MicroRNAs are a kind of small non-coding RNAs that exhibit aberrant expression patterns in response to environmental stimulation and negatively regulate gene expression through binding to the 3′-untranslated region (3′-UTR) of target mRNAs [10, 11]. The Dicer and Ago2 knockdown study revealed that UV-induced apoptosis was modulated by microRNA-mediated gene silencing [12]. We predominantly focused on investigating microRNA functions in response to UV irradiation, and in our previous work, miR-23a and miR-141 have been found to be involved in the apoptotic pathways activated by UVB irradiation [13–15]. microRNA microarrays have been employed to elucidate the molecular mechanisms underlying photodamage and skin carcinogenesis by UVB, and many miRNAs show expression changes under UVB irradiation [12, 16, 17]. These findings indicate that multiple miRNAs are potentially involved in the pathogenesis of UVB-induced photodamage.
Recently, it has been reported that miR-1246 acts as a target of p53 to downregulate Down syndrome-associated DYRK1A and induce apoptosis [18, 19]. Additionally, miR-1246 is associated with nucleosomes in breast cancer [20] and miR-1246 expression was upregulated in colorectal cancer as well as esophageal carcinoma [21, 22]. These data demonstrate that miR-1246 might be functionally involved in UVB-induced photodamage or apoptosis in keratinocyte cells, which will be explored in this study.
Here, we assessed the effect of UVB irradiation on miR-1246 expression changes in HaCaT keratinocytes, and found that UVB irradiation stimulated the upregulation of miR-1246 and induced apoptosis in HaCaT cells. The activity of miR-1246 was found to promote UVB-induced apoptosis. Additionally, we demonstrated that RTKN2, a Rho-GTPase effector of the NF-kappa B activation pathway [23, 24], is a target of miR-1246 involved in the inhibition of UVB-induced apoptosis. Moreover, miR-1246 was shown to act through downregulating RTKN2 expression in HaCaT cells.
Materials and methods
Cell culture and UVB irradiation
The keratinocyte HaCaT cell line was obtained from Dr. Gu, Department of Dermatology, Changhai Hospital, Shanghai, China. Cells were cultured in RPMI-1640 medium with 10 % FBS and 100 units/mL penicillin/streptomycin at 37 °C in 5 % CO2. UVB irradiation was performed as previously described [14].
RNA isolation and real-time RT-PCR
Total RNA was isolated using TRI Reagent Kit (Ambion, USA) according to the manufacturer’s instructions. Expression of miR-1246 was analyzed using the TaqMan MicroRNA RT Kit and Taqman Universal PCR Master Mix (Applied Biosystems, CA, USA). The relative expression level of miR-1246 was normalized to an endogenous control (U6). For gene expression analysis, reverse transcription was performed using random primers with SuperScript III (Invitrogen, USA), and Taqman Universal PCR Master Mix was applied for real-time PCR analysis. The relative expression level of genes was normalized to GAPDH. The 2−ΔΔCt method was used to quantify the relative expression level of miRNAs and genes. The primer sequences used were as follows:
-
p53 forward: 5′-ACTTGTCGCTCTTGAAGCTAC-3′,
-
p53 reverse: 5′-GATGCGGAGAATCTTTGGAACA-3′;
-
Bax forward: 5′-CCCGAGAGGTCTTTTTCCGAG-3′,
-
Bax reverse: 5′-CCAGCCCATGATGGTTCTGAT-3′;
-
Bcl-2 forward: 5′-GGTGGGGTCATGTGTGTGG-3′,
-
Bcl-2 reverse: 5′-CGGTTCAGGTACTCAGTCATCC-3′;
-
RTKN2 forward: 5′-ATGCTCGACTAATGGCCTATACA-3′,
-
RTKN2 reverse: 5′-CGTCGTGATCGTTCTTTATTGCT-3′;
-
miR-1246 forward: 5′- ACACTCCAGCTGGGAATGGATTTTTGG-3′,
-
miR-1246 reverse: 5′- ACTGACTGATGCAATCTCAACTGGTGTCGTGGA-3′;
-
U6 forward: 5′-TGCGGGTGCTCGCTTCGGCAGC-3′,
-
U6 reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′;
-
GAPDH forward: 5′- GATTCCACCCATGGCAAATTC-3′,
-
GAPDH reverse: 5′- AGCATCGCCCCACTTGATT-3′.
Annexin V/fluorescein isothiocyanate staining
The apoptosis levels of treated cells were detected after UVB irradiation at indicated time points. Treated cells were isolated, washed, and stained using annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI). The apoptotic rates of HaCaT cells were determined by flow-cytometric analysis using the ELITE ESP flow cytometer (Beckman-Coulter, USA) according to our previous described methods [14].
Western blot analysis
Cell lysate was prepared using radioimmunoprecipitation assay (RIPA) buffer [150 mM 370 NaCl, 0.5 % sodium deoxycholate, 50 mM Tris–HCl (pH 8.0), 0.1 % SDS, 5 mM EDTA, 0.25 mM phenylmethanesulfonyl fluoride, 1 % NP-40, and protease inhibitors]. Proteins were quantified using the bicinchoninic acid (BCA) protein assay (Pierce, IL, USA). Equal amounts of protein were separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, IN, USA). Membranes were then blocked with PBST (PBS with 0.05 % Tween 20) containing 5 % non-fat dry milk for 1 h and washed with PBST, then incubated at 4 °C overnight with anti-RTKN2 (1:500, Sigma) or anti-β-actin (1:5,000, Sigma) antibody in fresh blocking buffer. Membranes were then washed with PBS for 3 times and incubated with horseradish peroxidase-conjugated secondary antibody (1:7,000, Sigma) for 1 h at room temperature. The blots were developed using an enhanced chemiluminescence (ECL) kit (Pierce, USA). Protein levels were normalized against β-actin.
Luciferase reporter assay
The 3′-UTRs of RTKN2 (NM_145307), CREBL2 (NM_001310), SCN3A (NM_001081677), FAM3C (NM_001040020), and BRWD1 (NM_001103179) were amplified from human genomic DNA and cloned into a miRNA luciferase reporter vector that was modified from pGL3-control plasmid (Promega, WI, USA). The 3′-UTRs fragments were inserted into the reporter plasmid using EcoR1 and Pst1 at the restriction enzyme cutting sites. Each luciferase reporter construct was co-transfected with pRL-TK and RNA oligonucleotides or vectors into HaCaT cells. After transfection for 48 h, cells were harvested for the luciferase reporter assay (Promega, WI).
Cell transfection
HaCaT cells were transfected with miR-1246 inhibitor (50 nM) or miR-1246 mimic RNA (50 or 100 nM) using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s protocol. The cells were treated and analyzed at indicated time points after transfection. The sequences used were 5′-CCUGCUCCAAAAAUCCAUU-3′ (miR-1246 inhibitor oligonucleotide); 5′-AAUGGAUUUUUGGAGCAGG-3′ (miR-1246 mimic oligonucleotide); 5′-CCAUGACCUUCGGUAACCC-3′ (control oligonucleotide). Validated shRNAs against human RTKN2 in GV118 vector were purchased from Genechem (Shanghai, China), and the sequences were KD1, GCAGGACTGCAACATTCAAGA; KD2 sequence, GCTCGACTAATGGCCTATACA. RTKN2 was cloned from human cDNA library and constructed into pcDNA3 vector. All plasmids used in this study were extracted with Plasmid Miniprep kit (QIAGEN, Canada).
Statistical analysis
Data were analyzed using the SPSS software package (Version 10.0, IL), and data were presented as the mean ± SD. All results shown in this study were repeated at least for three times. All data were analyzed statistically with the Student’s t test. Differences were considered statistically significant at *p < 0.05.
Results
miR-1246 expression was upregulated during UVB-induced apoptosis in HaCaT cells
Previously, several miRNAs including miR-141 and miR-23a have been suggested to be involved in UVB radiation-induced apoptosis in HaCaT cells [14, 15], suggesting that miRNA-dependent regulatory mechanisms play essential roles in cellular response to UVB. It has already been revealed that miR-1246 may induce p53-dependent apoptosis in response to DNA damage [18], whereas it remains unknown whether miR-1246 participates in UVB-trigged photodamage in keratinocyte cells.
HaCaT cells were used to assess miR-1246 response to UVB irradiation. As UVB has a dose-dependent cytotoxic effect on cells [14], cultured HaCaT cells were treated with 30 mJ/cm2 UVB to induce appropriate percentage of apoptotic cells. Consistent with our previous report [14], UVB irradiation resulted in a significant apoptosis rate in comparison with the control, non-treated cells, and the effects of UVB occurred in a time-dependent manner within 48 h (Fig. 1a). Then, we investigated miR-1246 levels in UVB-irradiated cells. The real-time RT-PCR results indicated that miR-1246 expression was significantly upregulated at all examined time intervals (2, 6, 12, 24 and 48 h; Fig. 1b). p53 was sensitive to UV irradiation in human skin cells and showed high expression level in UV-type DNA damage [25–27]. Consequently, p53 expression was examined as a biomarker in UVB-induced apoptosis. As illustrated in Fig. 1c, p53 expression increased gradually alongside the increasing UVB-treatment time. These data suggest that miR-1246 upregulation is a molecular event that occurs in parallel with UVB-induced apoptosis in HaCaT cells.
miR-1246 was upregulated during UVB-induced apoptosis in HaCaT cells. HaCaT cells were subjected to UVB treatment (30 mJ/cm2) for 0, 2, 6, 12, 24, and 48 h. a An annexin V-fluorescein isothiocyanate and propidium iodide staining assay was performed in order to analyze apoptotic rates. The expression of miR-1246 (b) and p53 (c) were determined by real-time PCR analysis
miR-1246 promotes UVB-induced apoptosis
Based on the miR-1246 expression response to UVB, we postulated that miR-1246 might play a role in UVB-induced apoptosis. To test our hypothesis, miR-1246 was overexpressed by miR-1246 mimic transfection in HaCaT cells. The annexin V/PI staining assay revealed that miR-1246 increased UVB-induced apoptosis significantly at 24 h; however, its pro-apoptotic effects were limited in naturally cultured cells without UVB treatment (Fig. 2a). Moreover, Western blot and real-time PCR analysis showed that the expression of p53 and pro-apoptotic Bax were upregulated, while the expression of anti-apoptotic Bcl-2 expression level was reduced in miR-1246 overexpressing cells (Fig. 2b). When miR-1246 expression was decreased by the introduction of a miR-1246 inhibitor (Fig. 2c), UVB-elicited apoptosis was obviously impaired in anti-miR-1246 group at 48 h (Fig. 2d). Furthermore, the apoptotic rates were maintained at basal apoptotic levels without significant difference in control and miR-1246-knockdown cells (Fig. 2d). It demonstrates that anti-miR-1246 protects HaCaT cells to undergo UV-induced apoptotic pathway. Correspondingly, p53 and Bax were downregulated, while Bcl-2 was upregulated by the miR-1246 inhibitor (Fig. 2e). These findings demonstrate that miR-1246 promotes UVB-induced apoptosis, mainly in a condition-dependent manner in HaCaT cells.
miR-1246 promotes UVB-induced apoptosis. a HaCaT cells were transfected with either control RNA or a miR-1246 mimic; the level of apoptosis of these cells under UVB irradiation (30 mJ/cm2) for 24 h (+UVB) or without treatment (−UVB) was examined using flow-cytometric analysis following annexin V/PI staining. Concurrently, the relative level of miR-1246 expression was determined using real-time PCR analysis. b Real-time PCR and Western blot were performed in order to detect the expression of p53, Bax, and Bcl-2 in control- or miR-1246 mimic-transfected HaCaT cells during UVB irradiation. c Control treatment (Ctrl) or a miR-1246 inhibitor was introduced into HaCaT cells by Lipofectamine 2000, and the expression of miR-1246 was determined via real-time PCR. d Control- or miR-1246 inhibitor-transfected HaCaT cells were treated with or without UVB irradiation (30 mJ/cm2) for 48 h, and the percentages of apoptotic cells was detected by flow-cytometric analysis. e The level of expression of p53, Bax, and Bcl-2 was examined in control- or inhibitor-transfected HaCaT cells irradiated with UVB for 48 h by real-time PCR and Western blot analysis
RTKN2 is a miR-1246 target
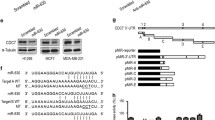
In a variety of biological processes in many different organisms, miRNAs act as posttranscriptional regulators, targeting gene expression [28]. Consequently, it is important to identify miRNA targets in order to elucidate the molecular mechanism of miRNA functions. TargetScan and Gostat-analysis were employed to predict the targets of miR-1246, and five candidate targets (RTKN2, CREBL2, SCN3A, FAM3C, BRWD1) were identified. The 3′-UTR of these candidate genes was cloned into luciferase reporter vectors for luciferase assay. The result in Fig. 3a showed that the 3′-UTR of RTKN2 and CREBL2 luciferase activity was significantly suppressed by miR-1246 mimic and that RTKN2 3′UTR activity was repressed more obviously than that of CREBL2.
RTKN2 is a miR-1246 target. a The 3′-untranslated regions (UTRs) of computationally predicted miR-1246 targets (RTKN2, CREBL2, SCN3A, FAM3C, and BRWD1) were cloned into a miRNA luciferase reporter vector. The reporter plasmids containing these 3′-UTRs were co-transfected with pRL-TK and a control (Ctrl), or a miR-1246 mimic, into HaCaT cells. After transfection for 48 h, the cells were collected for dual luciferase reporter assay. b Control or miR-1246 mimic (50 or 100 nM) were transfected into HaCaT cells and protein samples were prepared for the detection of RTKN2 and β-actin protein levels by Western blot analysis. c The 3′-UTR of the RTKN2 gene was paired to the miR-1246 seed region. The nucleotides in the cloned RTKN2 3′-UTR that were paired with the miR-1246 seed region were found to be mutated in the reporter plasmid. d The luciferase activity of wild-type or mutant 3′-UTRs of RTKN2 genes in a miRNA luciferase reporter vector was investigated following co-transfection with a control (Ctrl) or a miR-1246 mimic in HaCaT cells. The luciferase assay was performed after a transfection period of 48 h
A previous study has demonstrated that RTKN2, a Rho-GTPase effector, activates the NF-kappa B pathway to inhibit the intrinsic apoptotic pathway in HEK cells and regulates Bcl-2 gene expression [29]. Therefore, RTKN2 was selected for further study. To confirm that RTKN2 was indeed a target of miR-1246, 50 and 100 nM of miR-1246 mimic were transfected into HaCaT cells, respectively, and the protein level of RTKN2 was determined by Western blot analysis. In comparison with control RNA-transfected cells, the expression of RTKN2 was significantly downregulated by the miR-1246 mimic (Fig. 3b). Furthermore, the miR-1246 seed region was paired with the 3′-UTR of the RTKN2 gene (Fig. 3c). When the paired nucleotides were mutated in the luciferase reporter, the inhibitory effects of miR-1246 on 3′-UTR luciferase activity decreased significantly (Fig. 3d). Together, these results show that RTKN2 is targeted by miR-1246 in HaCaT cells.
RTKN2 antagonizes the promotion effect of miR-1246 on UVB-induced apoptosis
Given that RTKN2 is targeted by miR-1246 (Fig. 3), the functional correlation between miR-1246 and RTKN2 was investigated in UVB-induced apoptosis in HaCaT cells. At first, the RTKN2 expression response to UVB irradiation was examined, and we found that RTKN2 transcriptional and translational levels were downregulated dependent on treatment time of UVB (30 mJ/cm2) (Fig. 4a), which was opposite to miR-1246 expression change under UVB stimulation (Fig. 1b). In view of the situation that RTKN2 functions remain unknown in UVB-induced apoptosis, RTKN2 was knocked down efficiently by RTKN2 shRNAs (KD1 and KD2) in HaCaT cells (Fig. 4b). Under normal culture condition, the apoptotic rates of control or knockdown cells showed no significant difference (data not shown). While under the UVB irradiation for 24 h, the apoptotic rate of RTKN2-knockdown cells was much higher in comparison with the control or mock cells (Fig. 4c). Conversely, overexpression of RTKN2 by transfection of pcDNA3-RTKN2 was found to suppress cell apoptosis significantly (Fig. 4d). These data suggest that RTKN2 confers resistance to UVB-induced apoptosis in HaCaT cells.
miR-1246 promotes UVB-induced apoptosis through targeting RTKN2. a The expression of miR-1246 response to UVB (30 mJ/cm2) irradiation was determined using real-time PCR and Western blot at 0, 12, 24, and 48 h. b HaCaT cells were transfected with control shRNA (Ctrl) or RTKN2 shRNA1 or 2 (KD1 or KD2). Western blot was performed to analyze RTKN2 and β-actin protein levels (upper panel). The relative RTKN2 protein level normalized to β-actin was semi-quantified by densitometry. Intact HaCaT cells (Mock) served as control. c The apoptosis of mock, control shRNA (Ctrl)- or KD1/2-expressing HaCaT cells was examined by UVB (30 mJ/cm2) for 24 h. d pcDNA3 (vector) or pcDNA3-RTKN2 (RTKN2 OV) was forcedly expressed in HaCaT cells and the percentages of cell apoptosis were detected. e The apoptosis of three transfected groups (a Control + vector (control RNA and pcDNA3); b miR-1246 mimic + vector (miR-1246 mimic and pcDNA3); c miR-1246 mimic + RTKN2 OV (miR-1246 mimic and pcDN3-RTKN2)) under UVB (30 mJ/cm2) irradiation for 24 or 48 h was examined by flow-cytometric analysis. f The protein levels of RTKN2 and β-actin were determined by Western blot in the a, b, and c groups of transfected cells under UVB irradiation for 24 h. g Following UVB irradiation for 24 h, the expression levels of p53, Bax, and Bcl-2 mRNA in HaCaT cells with the a, b, and c transfections was analyzed with real-time PCR and Western blot analysis. h A model of the RTKN2-targeting effects of miR-1246 during UVB-induced apoptosis
To investigate whether miR-1246 promotes UVB-induced apoptosis through downregulating RTKN2 protein levels, a miR-1246 mimic and RTKN2 were co-transfected into HaCaT cells. The result in Fig. 4e showed that the promotion effect of miR-1246 on UVB-induced apoptosis was impaired by RTKN2 overexpression (Fig. 4e, f). Consistently, gene expression analysis also showed that miR-1246-upregulated p53 and Bax and -downregulated Bcl-2 mRNA as well as protein levels were reversed by forced expression of RTKN2 (Fig. 4g). Taken together, these results indicate that UVB-induced miR-1246 enhanced UVB-irradiated apoptosis mainly through targeting the Rho-GTPase effector RTKN2 to relieve its anti-apoptotic effect in keratinocyte HaCaT cells.
Discussion
Repeated UVB exposure results in damage to skin cells and induction of carcinogenesis [30, 31]. It requires the rapid removal of cells damaged irreparably after exposure to UV irradiation and condemned to cell apoptosis [32]. It is important to maintain the balance of proliferation, stratification, differentiation, and apoptosis of skin cells for epidermal homeostasis [33], and DNA damage repair and induction of apoptosis are main cellular mechanisms for this balance. As an important epigenetic regulation mechanism of gene expression under environmental stress, miRNAs has been found to play important roles in environmental response. Previously, microarray techniques have been applied to study the molecular mechanisms underlying UVB-induced photodamage and skin carcinogenesis, and multiple miRNAs have been shown to be involved in this process [12, 14–16]. The aim of this study was to identify novel miRNAs involved in UVB-induced apoptosis.
We previously found that miR-1246 expression was significantly changed in UVA-induced photoaging [13]. It reveals that miR-1246 acts downstream of p53 to induce cell apoptosis through downregulation of the expression of Down syndrome-associated DYRK1A [18, 19]. Moreover, miR-1246 might be related to malignant transformation in epithelial cells [34, 35]. In this study, we found that miR-1246 and p53 expression was increased in UVB-irradiated keratinocyte HaCaT cells undergoing apoptosis (Fig. 1). Combining these findings, it is reasonable to assume that miR-1246 may be involved in UVB-induced photodamage. As we expected, miR-1246 promoted UVB-induced apoptosis and inhibition of miR-1246 suppressed this process (Fig. 2), suggesting that miR-1246 plays a promotion role in UVB-mediated apoptosis. This is consistent with miR-1246 functions in Down syndrome [18]. We also found that the expression of p53 is synchronously upregulated with miR-1246 expression during UVB-induced apoptosis. Consequently, the possibility that miR-1246 is upregulated directly by p53 cannot be excluded. Additionally, the upregulation of p53, occurring in tandem with the overexpression of miR-1246 during UVB treatment, may be the result of miR-1246 regulatory feedback during cell apoptosis. Interestingly, the promotion effect of miR-1246 overexpression on cell apoptosis is limited without UVB stimulation, demonstrating that miR-1246 acts dependent on other regulatory machinery that is activated by UVB irradiation.
To explore the mechanism of miR-1246 functions in UVB-induced apoptosis, miR-1246 targets were screened by luciferase reporter assay and RTKN2 was identified for the first time as a mR-1246 target (Fig. 3). Functional analysis revealed that RTKN2 has anti-apoptotic effects in UVB-irradiated HaCaT cells (Fig. 4), which is consistent with a previous report that RTKN2 induces NF-kappa B-dependent resistance to intrinsic apoptosis in HEK cells [29]. RTKN2 has been identified as a Rho-GTPase effector [23], and the Rho-GTPase signaling pathway is associated with cell apoptosis and carcinogenesis [36, 37]. This suggests that Rho-GTPase signaling may be involved in UVB-induced apoptosis, with RTKN2 acting as an effector. Several groups have reported that RTKN2 activates the NF-kappa B pathway [24, 29], which is known to be involved in the inhibition of cell apoptosis [38–40]. We postulate that RTKN2 might inhibit UVB-induced apoptosis through the activation of NF-kappa B signaling, which will be investigated in the future studies.
Finally, the functional relationship between miR-1246 and RTKN2 was established. RTKN2 overexpression impaired miR-1246 promotion effect on UVB-elicited apoptosis (Fig. 4), suggesting that miR-1246 acts by targeting RTKN2. As reported previously [29], miR-1246-RTKN2 axis might regulate the expression of the anti-apoptotic Bcl-2 and the pro-apoptotic Bax, resulting in the promotion of UVB-induced apoptosis in keratinocyte cells. As summarized in Fig. 4g, UVB-induced upregulation of miR-1246 reduced the expression level of RTKN2 through pairing to the 3′-UTR of RTKN2. Then, the anti-apoptotic effects of RTKN2 were reduced by miR1246, which increased the probability of UVB-irradiated cells entering the apoptotic pathway. Consequently, miR-1246 is a potential gene therapy target for the treatment of UVB-induced photodamage in skin cells.
References
Marrot L, Meunier JR (2008) Skin DNA photodamage and its biological consequences. J Am Acad Dermatol 58:S139–S148. doi:10.1016/j.jaad.2007.12.007
Timares L, Katiyar SK, Elmets CA (2008) DNA damage, apoptosis and langerhans cells—activators of UV-induced immune tolerance. Photochem Photobiol 84:422–436. doi:10.1111/j.1751-1097.2007.00284.x
Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ (1996) Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379:335–339. doi:10.1038/379335a0
Young AR (1987) The sunburn cell. Photodermatology 4:127–134
Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ (1996) Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc 1:136–142
Kulms D, Poppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T (1999) Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA 96:7974–7979
Herrlich P, Rahmsdorf HJ (1994) Transcriptional and post-transcriptional responses to DNA-damaging agents. Curr Opin Cell Biol 6:425–431
Heck DE, Gerecke DR, Vetrano AM, Laskin JD (2004) Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol Appl Pharmacol 195:288–297. doi:10.1016/j.taap.2003.09.028
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010–1014. doi:10.1126/science.1092734
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524. doi:10.1101/gad.1399806
Hobert O (2008) Gene regulation by transcription factors and microRNAs. Science 319:1785–1786. doi:10.1126/science.1151651
Pothof J, Verkaik NS, van IW, Wiemer EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers JH, Persengiev SP (2009) MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J 28:2090–2099. doi:10.1038/emboj.2009.156
Li W, Zhou BR, Hua LJ, Guo Z, Luo D (2013) Differential miRNA profile on photoaged primary human fibroblasts irradiated with ultraviolet A. Tumour Biol. doi:10.1007/s13277-013-0927-4
Li W, Di W, Hua L, Zhou B, Guo Z, Luo D (2011) UVB suppresses PTEN expression by upregulating miR-141 in HaCaT cells. J Biomed Res 25:135–140. doi:10.1016/S1674-8301(11)60017-1
Guo Z, Zhou B, Liu W, Xu Y, Wu D, Yin Z, Permatasari F, Luo D (2013) MiR-23a regulates DNA damage repair and apoptosis in UVB-irradiated HaCaT cells. J Dermatol Sci 69:68–76. doi:10.1016/j.jdermsci.2012.10.014
Guo L, Huang ZX, Chen XW, Deng QK, Yan W, Zhou MJ, Ou CS, Ding ZH (2009) Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol 85:765–773. doi:10.1111/j.1751-1097.2008.00482.x
Pothof J, Verkaik NS, Hoeijmakers JH, van Gent DC (2009) MicroRNA responses and stress granule formation modulate the DNA damage response. Cell Cycle 8:3462–3468
Zhang Y, Liao JM, Zeng SX, Lu H (2011) p53 downregulates Down syndrome-associated DYRK1A through miR-1246. EMBO Rep 12:811–817. doi:10.1038/embor.2011.98
Liao JM, Zhou X, Zhang Y, Lu H (2012) MiR-1246: a new link of the p53 family with cancer and Down syndrome. Cell Cycle 11:2624–2630. doi:10.4161/cc.20809
Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML, Duelli DM (2012) MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res 40:9125–9138. doi:10.1093/nar/gks656
Piepoli A, Tavano F, Copetti M, Mazza T, Palumbo O, Panza A, di Mola FF, Pazienza V, Mazzoccoli G, Biscaglia G, Gentile A, Mastrodonato N, Carella M, Pellegrini F, di Sebastiano P, Andriulli A (2012) Mirna expression profiles identify drivers in colorectal and pancreatic cancers. PLoS One 7:e33663. doi:10.1371/journal.pone.0033663
Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, Ikeda N, Isozaki Y, Maruyama T, Akanuma N, Komatsu A, Jitsukawa M, Matsubara H (2013) Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer 108:644–652. doi:10.1038/bjc.2013.8
Collier FM, Gregorio-King CC, Gough TJ, Talbot CD, Walder K, Kirkland MA (2004) Identification and characterization of a lymphocytic Rho-GTPase effector: rhotekin-2. Biochem Biophys Res Commun 324:1360–1369. doi:10.1016/j.bbrc.2004.09.205
Myouzen K, Kochi Y, Okada Y, Terao C, Suzuki A, Ikari K, Tsunoda T, Takahashi A, Kubo M, Taniguchi A, Matsuda F, Ohmura K, Momohara S, Mimori T, Yamanaka H, Kamatani N, Yamada R, Nakamura Y, Yamamoto K (2012) Functional variants in NFKBIE and RTKN2 involved in activation of the NF-kappaB pathway are associated with rheumatoid arthritis in Japanese. PLoS Genet 8:e1002949. doi:10.1371/journal.pgen.1002949
Hall PA, McKee PH, Menage HD, Dover R, Lane DP (1993) High levels of p53 protein in UV-irradiated normal human skin. Oncogene 8:203–207
Maltzman W, Czyzyk L (1984) UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 4:1689–1694
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J (1991) A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 88:10124–10128
Pager CT, Wehner KA, Fuchs G, Sarnow P (2009) MicroRNA-mediated gene silencing. Prog Mol Biol Transl Sci 90:187–210. doi:10.1016/S1877-1173(09)90005-9
Collier FM, Loving A, Baker AJ, Mcleod J, Walder K, Kirkland M (2009) RTKN2 induces NF-KappaB dependent resistance to intrinsic apoptosis in HEK cells and regulates BCL-2 genes in human CD4 + lymphocytes. J Cell Death 2:9–23
Soehnge H, Ouhtit A, Ananthaswamy ON (1997) Mechanisms of induction of skin cancer by UV radiation. Front Biosci 2:d538–d551
Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW (1990) Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol 95:530–536
Metcalfe A, Streuli C (1997) Epithelial apoptosis. BioEssays 19:711–720. doi:10.1002/bies.950190812
Bernerd F, Sarasin A, Magnaldo T (1999) Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc Natl Acad Sci USA 96:11329–11334
Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM (2010) Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One 5:e13515. doi:10.1371/journal.pone.0013515
Wang Q, Li DC, Li ZF, Liu CX, Xiao YM, Zhang B, Li XD, Zhao J, Chen LP, Xing XM, Tang SF, Lin YC, Lai YD, Yang P, Zeng JL, Xiao Q, Zeng XW, Lin ZN, Zhuang ZX, Zhuang SM, Chen W (2011) Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene 30:3875–3886. doi:10.1038/onc.2011.103
Coleman ML, Olson MF (2002) Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ 9:493–504. doi:10.1038/sj/cdd/4400987
Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nat Rev Cancer 2:133–142. doi:10.1038/nrc725
Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC (2002) Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene 21:5673–5683. doi:10.1038/sj.onc.1205664
Liu H, Lo CR, Czaja MJ (2002) NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35:772–778. doi:10.1053/jhep.2002.32534
Van Antwerp DJ, Martin SJ, Verma IM, Green DR (1998) Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol 8:107–111
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81301380), and the Natural Science Foundation of Jiangsu Province (No. BK2012168).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Wu, YF., Xu, RH. et al. miR-1246 releases RTKN2-dependent resistance to UVB-induced apoptosis in HaCaT cells. Mol Cell Biochem 394, 299–306 (2014). https://doi.org/10.1007/s11010-014-2108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2108-1