Abstract
Sepsis is one of the most common causes of mortality in intensive care units. Although sepsis-associated encephalopathy (SAE) is reported to be a leading manifestation of sepsis, its pathogenesis remains to be elucidated. In this study, we investigated whether exogenous recombinant human erythropoietin (rhEPO) could protect brain from neuronal apoptosis in the model of SAE. We showed that application of rhEPO enhanced Bcl-2, decreased Bad in lipopolysaccharide treated neuronal cultures, and improved neuronal apoptosis in hippocampus of cecal ligation and peroration rats. We also found that rhEPO increased the expression of phosphorylated AKT, and the antiapoptotic role of rhEPO could be abolished by phosphoinositide 3-kinase (PI3K)/AKT inhibitor LY294002 or SH-5. In addition, systemic sepsis inhibited the hippocampal-phosphorylated mammalian target of rapamycin (mTOR) and p70S6K (downstream substrates of PKB/AKT signaling), which were restored by administration of exogenous rhEPO. Moreover, treatment with mTOR-signaling inhibitor rapamycin or transfection of mTOR siRNA reversed the neuronal protective effects of rhEPO. Finally, exogenous rhEPO rescued the emotional and spatial cognitive defects without any influence on locomotive activity. These results illustrated that exogenous rhEPO improves brain dysfunction by reducing neuronal apoptosis, and AKT/mTOR signaling is likely to be involved in this process. Application of rhEPO may serve as a potential therapy for the treatment of SAE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Sepsis and its consequences are the most common causes of death in intensive care units. Sepsis-associated encephalopathy (SAE), which manifests itself with symptoms such as irritability, confusion, stupor, and even outright coma, is a common complication of systemic sepsis [1]. Patients with SAE have a higher mortality rate compared to those without brain involvement, likely reflecting the severity of disease and the direct adverse effects of central nervous system (CNS) [2]. Recent study has demonstrated that cecal ligation and peroration (CLP) rats manifest dysfunctions in the creatine kinase activities and mitochondrial respiratory chain [3]. In addition, the uncoupling of oxidative phosphorylation in mitochondria also takes place in the brain of septic mice [4]. These evidences illustrated that cell apoptosis and mitochondrial dysfunction within the CNS may be involved in the SAE pathogenesis. Moreover, a recent investigation indicated that sepsis is associated with long-term neurological disorder in animal models [5].
Recombinant human erythropoietin (rhEPO) is a promising candidate for neuroprotective therapy in sepsis. Endogenous erythropoietin (EPO) is a 30.4 kDa glycoprotein that regulates red blood cell differentiation by inhibiting apoptosis of erythroid progenitors in marrow. Immunochemical staining with anti-EPOR antibody showed that erythropoietin receptor (EPOR) is expressed in rodent hippocampal areas, and also in primary-cultured hippocampal neurons [6, 7]. With the use of radioiodinated 125I-labeled rhEPO, specific EPO-binding sites were also found in some defined areas of the rodent brain including the hippocampus [8]. Administration of rhEPO has been used for many years to treat anemia resulting from various causes including cancer, prematurity, and renal insufficiency [9]. Recently, several studies have shown that exogenous rhEPO was beneficial in systemic sepsis. For example, rhEPO application improved the tissue bioenergetics and skeletal muscle microcirculation in septic model [10]. In lipopolysaccharide (LPS)-treated rats, exogenous rhEPO reduced the thymic and splenic apoptosis [11, 12]. In addition, rhEPO treatment also ameliorated the renal dysfunction during endotoxaemia [13]. The neuronal protective effect or rhEPO on sepsis-induced brain dysfunction, especially the neuronal apoptosis and cognitive disorders, remains unclear.
The serine/threonine kinase PKB/AKT has emerged as a critical signaling node within CNS and as one of the most important and versatile protein kinases at the core of neurodegenerative disease [14]. The mammalian target of rapamycin (mTOR), which is the downstream substrate of PKB/AKT signaling, was reported to regulate many major cellular processes and is implicated in an increasing number of pathological conditions, including neuronal apoptosis [15]. EPO was shown to regulate PKB/AKT pathway in several models. For example, EPO activates PKB/AKT signaling in erythroid cells [16]. In addition, EPO also protects cardiac myocytes from hypoxia-induced apoptosis through an AKT-dependent pathway [17]. The present study was designed to test the hypothesis that AKT/mTOR pathway may mediate the neuronal protective effects of EPO in septic models, using LPS-treated neuronal cultures and CLP rat.
Materials and methods
Animals and regents
All studies performed on animals were approved by the Institutional Animal Care and Use Committee (Medical College, Zhejiang University, Hangzhou, China). Sprague–Dawley rats were gained from Zhejiang University (Hangzhou, China). The rats (120 days old and each weighing 240–280 g) were housed four per cage at 21 °C under a 12:12 h light: dark regime and received standard laboratory food and tap water ad libitum. Every effort was made to minimize the extent of suffering and the number of animals.
The primary antibodies total AKT (Rabbit IgG), P-AKT-Ser-473 (Rabbit IgG), mTOR (Rabbit IgG), P-mTOR-Ser-2448 (Rabbit IgG), total p70S6K and P-p70S6K-Thr-389 were purchased from Cell Signaling Technology (Beverly, MA, USA). The primary antibodies Bcl-2 (Rabbit IgG), Bad (Rabbit IgG), Actin (Rabbit IgG), and secondary antibodies goat anti-rabbit IgG[HRP] and goat anti-Mouse IgG[HRP] were obtained from Sigma-Aldrich (St. Louis, MO, USA). The TUNEL-staining reaction solution was purchased from Roche Applied Science (Indianapolis, IN, USA). Other regents were purchased from GIBCO Invitrogen (Carlsbad, CA, USA) unless specified.
Primary neuronal cultures and cell transfection
The primary rat hippocampal neurons were derived from embryonic days of 17–18 SD rat embryos as described previously [18]. In Brief, hippocampal tissue was dissected in 1 × HBSS, minced gently, and trypsinized (trypsin 0.1 %; 37 °C, 5 % CO2 for 10 min), and the digestion was stopped by DMEM and 10 % FBS. Cells were plated on dishes or coverslips, which were coated with 100 mg/ml of poly-l-lysine (Sigma, St. Louis, Missouri, USA). Neurons were maintained at 37 °C, 5 % CO2, and fed with Neurobasal media supplemented with 2 % B27, 0.5 mM of l-glutamine (all from Invitrogen). Half of the medium was replaced once in every 2–3 days. Cells were used at 14 days in vitro. The neuronal cultures were infected with the recombinant mTOR short interfering RNA (mTOR siRNA) lentiviruses for 48 h using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions.
Cecal ligation and perforation (CLP) Surgery
Sepsis was induced by the well-established CLP model as described previously [3]. Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection. A 2.5-cm ventral midline incision was performed to allow exposure of the cecum with the adjoining intestine. The cecum was then ligated, and perforated with a 14-gauge needle. The cecum was gently compressed to extrude a small amount of cecal contents and then returned to the peritoneal cavity. Animals of control group underwent the same procedure with the exception that the cecum was neither ligated nor punctured. After surgery, the CLP rats received saline of 50 ml/kg s.c. immediately and 12 h after CLP plus ceftriaxone at 30 mg/kg and clindamycin at 25 mg/kg every 6 h over a total of three days. The sham-operated group received only saline (50 mL/kg, immediately and 12 h after CLP surgery). All animals were observed after CLP to determine the signs of infection (pyloerection, lethargy, tachypnoea and weight loss). Survival was 100 % in the sham group, and approximately 34 % in the sepsis group, which is in accordance with previous reports [19].
Minipump implantation and drug administration
An osmotic Alza minipump (Durect, Cupertino, California, USA) was implanted subcutaneously into the back of each animal, and a needle from the minipump was placed in the lateral ventricle [20]. Recombinant human erythropoietin was dissolved in 0.01 M PBS and 0.1 % BSA. rhEPO at a dose of 5 units per day was infused consecutively for 7 days into the left lateral ventricle of rat. Control animals received the same volume of vehicle infusion. After 6 h of CLP or sham operation, the infusion of rhEPO or vehicle was started. The rats were deeply anesthetized with sodium pentobarbital (120 mg/kg, i.p.) followed by cervical dislocation after rhEPO delivery, and hippocampal samples were collected for western blot. Other animals were recruited to the behavior study later. For the in vitro study, cells were incubated with LPS (1.0 μg/ml), 10 μM of LY294002 (Calbiochem, La Jolla, CA), 20 nM of Rapamycin (Tocris, Ellisville, MO), 20 μM of SH-5 (Calbiochem, Merck KGaA, Darmstadt, Germany), or vehicle (0.01 M of PBS) for 24 h.
Western blot
The hippocampal tissues and neuronal cultures were subjected to lysis buffer. The concentrations of sample were determined by Bradford Protein Assay (Bio-Rad, Hercules, CA). Samples were separated by 10–15 % SDS-PAGE and transferred to PVDF membranes by electroelution. The membranes were blocked by 2 % bovine serum albumin for 1 h, and then incubated overnight at 4 °C with the appropriate primary antibodies followed by washes and incubation with HRP-conjugated appropriate secondary antibodies. The membranes were washed and processed with an enhanced chemiluminescence kit, and then the results were developed using Kodak (Fuji, Tokyo, Japan) radiography film. Actin was probed and normalized as a loading control. Bands were digitally scanned and analyzed using ImageJ software (developed by the National Institutes of Health; available at: http://rsb.info.nih.gov/ij/).
TUNEL-staining
Genomic DNA fragmentation was determined by the TUNEL assay according to the protocol of manufacturer. In Brief, neuron-bearing coverslips were fixed, and permeabilized with 0.01 % Triton X-100 in PBS containing 1 % sodium citrate. The cells were then incubated (30 min, 37 °C) with the TUNEL reaction mixture. The coverslips were washed, and incubated with Rhodamine (TRITC)-Streptavidin (Jackson, West Grove, PA, USA) at 37 °C for 1 h. The nuclei were stained with DAPI. Twenty fields per culture and three separate cultures were conducted in each experimental group. Immunofluorescence was captured by Laser scanning confocal microscope (Zeiss, Germany).
Behavioral study
Open-field exploration
The rats were allowed to recover from CLP or sham surgery for 1 week before evaluation of the behavioral study. The open-field exploration test was performed to determine the rats’ emotional responses to a novel environment. The rats were placed in the center of a dimly lit chamber of the open-field apparatus (100 x 100 x 50 cm). Movements of the animals were tracked by an automatic monitoring system. Activity was measured as the total distance traveled (meters) in 10 min.
Inhibitory avoidance training and test
Before behavior training, all animals were habituated to the experimental arena for 20 min per day for consecutive 3 days in the absence of stimulus objects. The IA apparatus is a trough-shaped alley (15cm deep, 20cm wide at the top, and 6.5cm wide at the floor). When the animal turned to face the door, the door was lifted out of the way to reveal the dark shock compartment. After the rat crossed from the illuminated to the dark compartment of the alley, the door was closed, and a single foot shock (0.5 mA for 2 s) was delivered. All animals were trained once a day for 5 consecutive days. The IA test was performed on the sixth day. All animals were tested only once. Latency was defined as the time taken to enter the dark compartment, which reflects emotional memory retention.
Morris water maze
The spatial learning and memory functions were evaluated by Morris water maze (MWM) as previously described with some modifications [21]. The place trials were employed to assess the rats’ ability to learn the spatial relationship between distant cues and the escape platform (submerged 2.5 cm, not visible). All rats received 4 trials each day for 4 consecutive days. The rats were released randomly facing the pool wall, and were allowed 60 s to find the platform upon which they sat for 30 s. If a rat did not find the platform, then it was guided gently to the platform and allowed to stay there for 30 s. The traveled distance (swimming length), swimming speed, and time taken to reach the platform (latency) were recorded for later analysis. The probe test was conducted to determine the capability of spatial memory retention. One day after the last place trial, the platform was removed, and the rats were started to swim in a same quadrant facing the pool wall, and all rats were allowed to swim for 60 s. We recorded the swimming time spent in each quadrant, as well as the number of crossings over the place where the platform used to be. Movements of the rat in MWM were tracked by a video-monitoring system for further analysis.
Statistical analysis
All data are presented as mean ± SEM. Comparisons among multiple groups involved one-way analysis of variance followed by Tukey’s multiple comparison testing. Specially, we analyzed the place trials of the MWM using two-way analysis of variance followed by Bonferroni multiple comparison testing. Significant differences were considered to be P < 0.05 through all experiments.
Results
Administration of rhEPO prevents neuronal apoptosis through regulating Bcl-2 and Bad
To evaluate the possible neuroprotective role of rhEPO in sepsis, the rhEPO was delivered 5 units per day consecutively for 7 days after CLP surgery. We found that the hippocampal expression of Bcl-2 was inhibited, and the Bad was enhanced in CLP rats (P < 0.05, Fig. 1a, b, lane 2). Administration of rhEPO significantly improved the changes of Bcl-2 and Bad (P < 0.05, Fig. 1a, b, lane 3) in CLP rats, while rhEPO alone had no effects on the expressions of Bcl-2 and Bad in sham surgery group. To confirm this result, the different doses of rhEPO (25, 50, and 100 ng/mL) or vehicles were incubated with hippocampal neurons before LPS treatment (1.0 μg/mL for 24 h). We found that LPS treatment triggered significant neuronal apoptosis (about 32.3 %, P < 0.01, Fig. 1c). Addition of 50 or 100 ng/mL of rhEPO statistically reduced the neuronal apoptosis to 21.2 % (P < 0.05) and 14.2 % (P < 0.01) respectively (Fig. 1c). 100 ng/ml of rhEPO treatment was selected for the subsequent in vitro study.
Treatment with rhEPO ameliorates neuronal apoptosis both in vitro and in vivo (a) and (b). Five units of rhEPO or vehicle (saline) were applied consecutively for 7 days after CLP surgery. The hippocampal tissues were collected for detecting the protein levels of Bcl-2 and Bad by western blot. Actin expression was re-probed as a loading control. The histogram represents the relative expressions of Bcl-2 and Bad (n = 5). c The neuronal cultures were treated with different doses of rhEPO (0, 25, 50 or ,100 ng/ml), and then co-incubated with or without 1 μg/ml of LPS for 24 h. Genomic DNA fragmentation was examined by the TUNEL assay. The neuronal apoptosis was determined by counting the TUNEL-positive neurons in a blinded manner. We evaluated 20 microscopic fields per culture and three separate experiments. Error bars reflect SEM. We determined statistical significance as *P < 0.05 and # P < 0.01
The phosphorylated AKT is involved in the neuronal protective role of rhEPO against sepsis
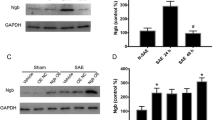
We next detected the total AKT (t-AKT) and phosphorylated AKT (p-AKT-Ser-473) expressions by western blot in the presence or absence of rhEPO of CLP rats. We found that p-AKT expression was suppressed (P < 0.05, Fig. 2a), and the t-AKT expression was unchanged in CLP rats. Delivery of rhEPO rescued the p-AKT level was as expected (P < 0.05, Fig. 2a, lane 3). To specify the role of p-AKT in sepsis, LY294002 (10 μM) or SH-5 (20 μM), inhibitors of PI3K/AKT signaling, was co-incubated with LPS (1.0 μg/ml)-treated neuronal cultures for 24 h. We found that both LY294002 and SH-5 reversed the neuronal protective effects of rhEPO assessed by determining the protein levels of Bcl-2 and Bad (P < 0.05, Fig 2b, c).
rhEPO prevents neuronal apoptosis by regulating the phosphorylated AKT level a After CLP surgery, 5 units of rhEPO were delivered each day consecutively for 7 days, and the hippocampal tissues were subjected for probing the protein expressions of total AKT and p-AKT-Ser-473. The histogram reflects the relative quantity of p-AKT-Ser-473 expression (n = 4–6). b, c The hippocampal neuronal cultures were treated with LY294002 (10 μM) or SH-5 (20 μM), and then further co-incubated with or without rhEPO (100 ng/ml) before LPS treatment (1.0 μg/ml for 24 h). The whole cell protein was extracted to detect the protein levels of Bcl-2 and Bad. The histogram represents the relative protein expressions (n = 5–6). Error bars reflect SEM. The statistical significance was determined as *P < 0.05
Treatment with rhEPO protects brain against sepsis by controlling phosphorylated mTOR
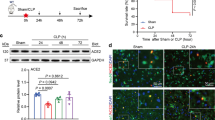
We found that hippocampal-phosphorylated mTOR (p-mTOR-Ser-2448) level was reduced in CLP rat (P < 0.05, Fig. 3a, lane 2). Treatment with rhEPO (5 U per day for 7 days) restored the p-mTOR expression (P < 0.05, Fig. 3a, lane 3). In addition, administration of rhEPO also rescued the expression of phosphorylated p70S6K (p-p70S6K-Thr-389), a downstream target of mTOR (P < 0.05, Fig. 3c, d). To further explore the involvement of mTOR signaling in sepsis, we transfected the mTOR siRNA in LPS-treated neuronal cultures. The transfection efficiency is shown in Fig. 3e, f. We found that the neuronal protective effects of rhEPO were abolished by the p-mTOR inhibitor, Rapamycin (20 nM), or mTOR siRNA transfection (P < 0.05, Fig. 3g).
rhEPO protects brain against sepsis through modulating phosphorylated mTOR a Administration of rhEPO (5 U/day consecutively for 7 days) was performed after the CLP or sham surgery. The hippocampal protein expressions of p-mTOR-Ser-2448, mTOR, and Actin were determined by western blot. b The histogram represents the relative protein expression of p-mTOR-Ser-2448 (n = 4). c The rhEPO (5 U) was infused into rat lateral ventricle consecutively for 7 days after surgery. The hippocampal tissues were collected for probing the expressions of p70S6K and p-p70S6K-Thr-389 by western blot. d Representative image reflects the quantitative analysis of p-p70S6K-Thr-389 expression (n = 4). e The mTOR siRNA transfection efficiency was shown by western blot. The nonspecific scrambled siRNA was transfected as a negative control. f Representative image reflects the quantitative analysis of mTOR expression (n = 4). g The hippocampal neuronal cultures were treated with Rapamycin (20 nM) or mTOR siRNA transfection, and combined with rhEPO (100 ng/ml) during LPS incubation (1.0 μg/ml for 24 h). The neuronal apoptosis was evaluated in a blinded manner 24 h later. Error bars indicate SEM. We determined statistical significance as *P < 0.05 and # P < 0.01
Application of rhEPO improves the emotional and spatial cognitive disabilities in CLP rats
The behavior study was conducted 1 week after the CLP surgery (n = 18, 22, and 20 in control, CLP, and CLP + EPO group, respectively). In open-field exploration, the total distance traveled (meters) in 10 min was similar among groups (Control group: 33.5 ± 3.2 m; CLP group: 30.9 ± 4.1 m and CLP + EPO group: 32.6 ± 3.5 m respectively, P > 0.05), suggesting that rhEPO delivery may not alter the locomotor activity. In IA test, the CLP rats exhibited a worse performance, and had a lower latency to the illuminated compartment (P < 0.05, Fig. 4a). However, CLP rats treated with rhEPO were comparable to those of the sham group (P > 0.05, Fig 4a). In the place trials of MWM, we detected no significant differences in terms of average swimming speed (P > 0.05, Fig. 4b), confirming that rhEPO delivery had no effects on locomotor activity. However, the CLP rats spent significantly longer time to find the submerged platform at trial days 3 and 4 than those in the control group (P < 0.05, Fig. 4c). Rats in the CLP + rhEPO group were comparable to those in the control group in terms of latency to find the submerged platform (P > 0.05, Fig. 4c). Analysis of swimming length in place trials coincided with latency (data not shown). In the probe test, CLP rats spent significantly less time in searching the target quadrant than those in the control group (P < 0.05, Fig. 4d). Animals applied with rhEPO performed similar to the control rats (P > 0.05, Fig. 4d). Similar results were obtained by analyzing the number of crossings (data not shown). These results indicate that sepsis induces emotional and cognitive deficits that can be attenuated by the administration of rhEPO.
Application of rhEPO improves emotional and spatial cognitive disorders in CLP rats The behavior studies were performed one week after surgery (n = 18, 22, and 20 in Control, CLP, and CLP + EPO group, respectively). a IA test was chosen to assess the function of emotional memory of experimental rats. Latency to enter the dark compartment was recorded as memory retention. b In place trials of MWM, the average swimming speeds were calculated. c In place trials of MWM, the latency was determined as the time to reach the submerged platform. d For the probe test, we removed the platform and rats started to swim (1 min) in the same quadrant facing the pool wall. The histogram represents the percentage of swimming time spent in the target quadrant. Statistical significance was determined as *P < 0.05. Data are expressed as mean ± standard error of the mean
Discussion
Septic-associated encephalopathy is clinically characterized by changes in mental status that range in severity from reversible, transient encephalopathy, to irreversible brain damage [22]. In CLP treated rat model, studied herein, we showed that septic rats exhibited emotional and spatial cognitive deficits paralleling with neuronal apoptosis. Moreover, application of rhEPO prevented neuronal apoptosis and dramatically improved the brain dysfunction without any influence on locomotor activity. In addition, we demonstrated that AKT/mTOR signaling is likely involved in the neuroprotective effects of rhEPO. These results extended the notion that rhEPO plays a critical role in regulating neuronal survival against sepsis.
Although endogenous EPO is produced mostly by adult kidney and fetal liver, its receptors are expressed extensively in the CNS, including hippocampus [23]. In addition to its role in erythropoiesis, several studies have investigated that the administration of exogenous EPO in animal models can protect brain against hypoxic-ischemic injury [20, 24]. Furthermore, exogenous EPO also possess the neuroprotective role in several neurodegenerative diseases, such as Parkinson and Alzheimer diseases [25, 26]. Our data support the hypothesis that rhEPO improved neuronal apoptosis and hippocampus-dependent cognitive impairments in septic model. Other beneficial effects of rhEPO administration on sepsis were reported to be relevant to inhibition of tissue hypoxia and nitric oxide production [27].
As regards to neuroprotection, high-dose of rhEPO (ranging from 1,000 to 30,000 U/kg) was used in the previous literature, which is well above the range used to treat anemia (about <500 U/kg). Only a small fraction (<2 %) of circulating rhEPO crossed the blood–brain barrier (BBB), peaking 3 h after its application [28]. To address this issue, we employed a continuous lateral ventricle microinjection for 7 days to ensure the stable concentration of rhEPO in the brain. High-dose of rhEPO was examined in adult stroke model, and has not been associated with complications [29]. In neonates, long-term rhEPO treatment of anemia has been studied extensively, and no adverse outcomes were found [30]. We had postulated that sepsis-induced cognitive disorders may also benefit from the high-dose of systemic rhEPO employment. In fact, the preliminary data from our lab illustrated that the cognitive disorders of septic rats had improved statistically after systemic administration of high-dose rhEPO (500–1,000 U/kg, i.p.). Just to focus on the role of neuroprotection of rhEPO, the minipump implantation and lateral ventricle rhEPO microinjection were chosen in the subsequent experiments.
The key mechanism for controlling cell survival, division, and metabolism is the phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) pathway [31]. In the present study, the neuronal protective role of rhEPO was abrogated by LY294002, SH-5, and rapamycin or mTOR siRNA transfection, strongly indicating that AKT/mTOR signaling was involved. Notably, AKT activation controls the cell survival also through phosphorylation of multiple downstream effector proteins, such as glycogen synthase kinase-3b, Bad, Bid, Bax, caspase-9, FOXO, and possibly apoptosis-inducing factor [32–34] which may also contribute to the improvement of neuronal apoptosis in sepsis. Further studies are needed to comprehensively examine the underlying mechanisms of neuronal protection of rhEPO in SAE.
The proteins of Bcl-2 family play a pivotal role in neuronal apoptosis. As a proapoptotic Bcl-2 family member, Bad can be phosphorylated through AKT, bind to the cytosolic protein 14-3-3 to release Bcl-xl, and allow Bcl-xl to bind to the pro-apoptotic protein Bax [35–37]. Hypophosphorylated Bad interacts with and neutralizes the prosurvival Bcl-2 family proteins, which frees Bax and Bak to induce apoptosis at the mitochondria [38]. As an antiapoptotic protein, Bcl-2 prevents cell apoptosis while the proapoptotic members such as Bad, can lead to impairment of mitochondrial membrane potential, cytochrome c release, and caspase-3 activation [39]. Our results illustrated that the enhanced PKB/AKT signaling exert its neuroprotective effects through triggering the cascade of intrinsic antiapoptotic pathway.
In conclusion, exogenous EPO manifests antiapoptotic role in CNS through mitochondria mechanism in SAE model. The AKT/mTOR pathway is likely involved in this process. The rhEPO treatment may serve as a potential strategy for SAE clinical therapy.
Abbreviations
- CLP:
-
Cecal ligation and peroration
- LPS:
-
Lipopolysaccharide
- mTOR:
-
Mammalian target of rapamycin
- MWM:
-
Morris water maze
- rhEPO:
-
Recombinant human erythropoietin
- SAE:
-
Sepsis-associated encephalopathy
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling
References
Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T (2009) Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med 37:S331–S336
Siami S, Annane D, Sharshar T (2008) The encephalopathy in sepsis. Crit Care Clin 24:67–82
Comim CM, Rezin GT, Scaini G, Di-Pietro PB, Cardoso MR, Petronilho FC, Ritter C, Streck EL, Quevedo J, Dal-Pizzol F (2008) Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion 8:313–318
Joana da Costa P, Santiago APS, Amâncio RT, Galina A, Oliveira MF, Bozza FA (2008) Sepsis induces brain mitochondrial dysfunction. Crit Care Med 36:1925–1932
Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schäfers M, Heneka MT (2009) NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci 29:14177–14184
Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R (1997) Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 76:105–116
Morishita E, Narita H, Nishida M, Kawashima N, Yamagishi K, Masuda S, Nagao M, Hatta H, Sasaki R (1996) Anti-erythropoietin receptor monoclonal antibody: epitope mapping, quantification of the soluble receptor, and detection of the solubilized transmembrane receptor and the receptor-expressing cells. Blood 88:465–471
Digicaylioglu M, Bichet S, Marti H, Wenger R, Rivas L, Bauer C, Gassmann M (1995) Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA 92:3717–3720
McPherson RJ, Juul SE (2008) Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci 26:103–111
Kao R, Xenocostas A, Rui T, Yu P, Huang W, Rose J, Martin CM (2007) Erythropoietin improves skeletal muscle microcirculation and tissue bioenergetics in a mouse sepsis model. Crit Care 11:R58
Koroglu T, Yilmaz O, Ozer E, Baskin H, Gokmen N, Kumral A, Duman M, Ozkan H (2006) Erythropoietin attenuates lipopolysaccharide-induced splenic and thymic apoptosis in rats. Physiol Res 55:309
Kumral A, Baskin H, Yesilirmak DC, Ergur BU, Aykan S, Genc S, Genc K, Yilmaz O, Tugyan K, Giray O (2007) Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neonatology 92:269–278
Mitra A, Bansal S, Wang W, Falk S, Zolty E, Schrier RW (2007) Erythropoietin ameliorates renal dysfunction during endotoxaemia. Nephrol Dial Transplant 22:2349–2353
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Bao H, Jacobs-Helber S, Lawson A, Penta K, Wickrema A, Sawyer S (1999) Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells). Blood 93:3757–3773
Tramontano A, Muniyappa R, Black A, Blendea M, Cohen I, Deng L, Sowers J, Cutaia M, El-Sherif N (2003) Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun 308:990–994
Kaech S, Banker G (2007) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JCF, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Sakanaka M, Wen T-C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R (1998) In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA 95:4635–4640
Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IAS, Antunes LM (2010) Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology 113:1099–1108
Ebersoldt M, Sharshar T, Annane D (2007) Sepsis-associated delirium. Intensive Care Med 33:941–950
Maiese K, Li F, Chong ZZ (2004) Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci 25:577
Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M (2000) Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol 401:349–356
Kanaan NM, Collier TJ, Marchionini DM, McGuire SO, Fleming MF, Sortwell CE (2006) Exogenous erythropoietin provides neuroprotection of grafted dopamine neurons in a rodent model of Parkinson’s disease. Brain Res 1068:221–229
Assaraf MI, Diaz Z, Liberman A, Miller WH Jr, Arvanitakis Z, Li Y, Bennett DA, Schipper HM (2007) Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol 66:389–398
Aoshiba K, Onizawa S, Tsuji T, Nagai A (2009) Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit Care Med 37:889–898
Haiden N, Klebermass K, Cardona F, Schwindt J, Berger A, Kohlhauser-Vollmuth C, Jilma B, Pollak A (2006) A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics 118:180–188
Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck H, Breiter N, Jacob S, Knerlich F (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8:495–505
Aher S, Ohlsson A (2006) Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 3:CD004868
Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P (2008) Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 8:393–412
Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA (2008) PKB and the mitochondria: AKTing on apoptosis. Cell Signal 20:21–30
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Rokutanda S, Fujita T, Kanatani N, Yoshida CA, Komori H, Liu W, Mizuno A, Komori T (2009) Akt regulates skeletal development through GSK3, mTOR, and FoxOs. Dev Biol 328:78–93
Cai W, Rudolph JL, Harrison SM, Jin L, Frantz AL, Harrison DA, Andres DA (2011) An evolutionarily conserved Rit GTPase–p38 MAPK signaling pathway mediates oxidative stress resistance. Mol Biol Cell 22:3231–3241
Koh P-O (2011) Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett 498:105–109
Maiese K, Chong ZZ, Li F, Shang YC (2008) Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol 85:194–213
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3 K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36:320–328
Lei G, Xia Y, Johnson KM (2007) The role of Akt-GSK-3β signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacol 33:1343–1353
Conflict of interest
The authors have no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, GB., Ni, YL., Zhou, XP. et al. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem 385, 125–132 (2014). https://doi.org/10.1007/s11010-013-1821-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1821-5