Abstract
Congenital heart disease (CHD) is the most common birth defect in humans. Genetic causes for CHD remain largely unknown. T-box transcription factor 18 (TBX18) gene is expressed in the developing heart, including myocardium of the left ventricle and interventricular septum. Epicardial cells expressing TBX18 gene contribute to the cardiac fibroblast and smooth muscle cells. We speculated that the DNA sequence variants (DSVs) within TBX18 gene promoter may mediate CHD development by affecting TBX18 levels and the cardiac gene regulatory network. In this study, we genetically and functionally analyzed the TBX18 gene promoter in patients with ventricular septal defects (VSD) (n = 326) and ethnic-matched healthy controls (n = 327). Three novel heterozygous DSVs (g.85474435del, g.85474418C>T, and g.85473965C>G) and one single nucleotide polymorphism (g.85474871C>T, rs77693245) were identified in VSD patients, but none in the controls. Functional analysis revealed that the DSVs (g.85474871C>T, g.85474435del, and g.85473965C>G) significantly decreased the transcriptional activities of the TBX18 gene promoter. The effect of DSV (g.85474418C>T) on the TBX18 gene promoter was marginal, but not significant. Therefore, the DSVs within the TBX18 gene promoter identified in VSD patients may be involved in the VSD etiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart disease (CHD) is the most common human birth defect that affects about 1–2 % of live births, and the true prevalence may be much higher [1]. Even with successful correction surgeries, morbidity and mortality of CHD patients are still significantly higher than the general populations. The main causes of death are later cardiac complications, including arrhythmias, coronary heart disease, and heart failure, likely due to genetic defects [2, 3]. To date, mutations in cardiac transcription factor genes, such as GATA transcription factor 4 (GATA4), T-box transcription factor 5 (TBX5), and NK2 transcription factor related, locus 5 (NKX2-5), have been implicated in a small portion of familial and isolated CHD [4]. However, genetic causes for isolated CHD remain largely unknown.
TBX transcription factors, which share a highly conserved DNA-binding domain, play critical roles in several processes during the embryonic development [5]. In mammals, six members of TBX transcription factor family (TBX1, TBX18, and TBX20 of the TBX1 subfamily, and TBX2, TBX3, and TBX5 of the TBX2 subfamily) are essential in the developing heart. From the linear heart tube to the chambered heart, TBX1, TBX5, and TBX20 act as transcriptional activators of chamber myocardial genes, whereas TBX2, TBX3, and TBX18 function as repressors for a similar set of the target genes [6]. Genetic studies in animals demonstrate that TBX1, TBX5, and TBX20 act as dosage-sensitive regulators in the heart development [6]. We have hypothesized that the DNA sequence variants (DSVs) within TBX gene promoters may mediate CHD development by changing TBX factor levels. In previous studies, we had identified a number of DSVs within regulatory regions of the TBX1, TBX5, and TBX20 genes in CHD patients [7–9].
TBX18 factor is required for mammalian development [6]. In mice, TBX18 gene is widely expressed in various tissues, including the developing heart and limb buds [10]. In the developing heart, TBX18 gene is expressed in multiple sites, including the proepicardium, epicardium, mesenchymal progenitors, and the myocardium of the sinus venosus region, with the highest level in the epicardium [10]. Targeting deletion and genetic lineage studies in animal have revealed that TBX18 is essential for the formation of the sinus horn and the myocardial differentiation of the sinoatrial node [11–13]. TBX18 gene has been reported to be expressed in the myocardium of the left ventricle and the interventricular septum [14]. TBX18-expression progenitors also develop into the cardiac fibroblasts and coronary smooth muscle cells [15, 16]. A recent study showed that TBX18 directly converts cardiomyocytes into pacemaker cells [17].
Although TBX18 has been proposed as one of the candidate genes for split hand/foot malformation [18], mutations in TBX18 gene are not associated to human diseases. The heterozygous TBX18 null mutation mouse is born normal and fertile. The homozygous mutant TBX18 mouse dies shortly after birth with a shortened body and severe deformations [19]. In TBX18-deficient mouse embryos, development of the sinus horn in the heart is blocked and the myocardial differentiation of sinus venous region is delayed. A smaller head of sinoatrial node and less distinct boundary between sinoatrial node and atrial myocardium are observed [11, 13]. Therefore, we speculated that changed levels of the TBX18 gene, rather than mutations that change amino acids of TBX18 protein, may mediate the development of CHD, particularly ventricular septal defect (VSD). In the present study, we genetically analyzed the TBX18 gene promoter in large cohorts of VSD patients and ethnic-matched healthy controls.
Materials and methods
Patients and controls
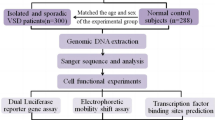
The VSD patients (n = 326, male 151, female 175, age range from 3 months to 50 years, median age 4.42 years) were recruited from the Division of Cardiac Surgery, Jining Medical University Affiliated Hospital, Jining Medical University, Jining, Shandong, China. VSD patients were diagnosed according to medical records, physical examination, electrocardiograms, and three-dimensional echocardiography. Healthy controls (n = 327, male 263, female 64, age range from 2 months to 41 years, median age 3.75 years) were recruited from the same hospital. VSD patients and controls with familial CHD history were excluded. This study was approved by the Human Ethic Committee of Jining Medical University Affiliated Hospital. Informed consent was obtained from the subjects and guardians.
Sequencing
Genomic DNA was extracted from peripheral leukocytes. Two overlapped DNA fragments, covering the TBX18 gene promoter (~1250 bp upstream to the transcription start site), were generated with PCR. The primers were designed based on genomic sequence of the human TBX18 gene (NCBI accession number: NC_000006.11) and are shown in Table 1. The DNA fragments were bi-directionally sequenced with BigDye® Terminator v3.0 reagents and a 3730 DNA Analyzer (Applied Biosystems, Foster city, CA, USA). The sequences were aligned and compared with the wild-type sequence of the TBX18 gene promoter. For heterozygous deletion DSV, the DNA fragment was subcloned into T vector and directly sequenced. Frequencies of the DSVs in VSD patients and controls were analyzed and compared with SPSS v13.0. P < 0.05 was considered statistically significant.
Functional analysis
Wild-type and variant TBX18 gene promoters (1222 bp, from 85475148 to 85473927 in NC_000006.11) were generated by PCR and subcloned into XhoI and HindIII sites of a luciferase reporter vector (pGL3-basic) to construct expression vectors. PCR primers are shown in Table 1. Expression vectors were transiently transfected into human embryonic kidney cells (HEK-293) and luciferase activities were measured using a dual-luciferase reporter assay system. According to the NCBI UniGene database, the TBX18 gene is expressed in the human kidney (NCBI UniGene, Hs.251830). In animals, the TBX18 gene is also expressed in the kidney cells [10]. Therefore, HEK-293 cells were used for functional analysis. All experiments were repeated at least three times.
Statistical analysis
Quantitative data are represented as mean ± SE and compared by Student’s t test. The distributions of DSVs were compared between VSD patients and controls by χ 2 test using SPSS v13.0. A P < 0.05 was considered statistically significant.
Results
The TBX18 gene promoters were bi-directionally sequenced in VSD patients (n = 326) and healthy controls (n = 327). Six novel DSVs and four single nucleotide polymorphisms (SNPs) were identified. Distribution of the DSVs and SNPs is summarized in Table 2. Locations are indicated in Fig. 1a. Three heterozygous DSVs, g.85474435del, g.85474418C>T, and g.85473965C>G, and one SNP, g.85474871C>T (rs77693245), were identified in four VSD patients, but none in the controls. Among these VSD patients, three patients were diagnosed with perimembranous VSD and one with muscular VSD. Three heterozygous DSVs, g.85474952A>T, g.85474648G>C, and g.85474119C>T, and one SNP, g.85474877C>G (rs77884863), were only found in the controls (Fig. 1b). The remaining two SNPs, g.85474974C>A (rs74555338) and g.85474947C>T (rs74934763), were found in VSD patients and controls with similar frequencies (P > 0.05).
The DSVs within the TBX18 gene promoter in VSD patients and controls. a Schematic representation of the DSVs. The numbers represent the sequence of TBX18 genomic sequences (NCBI accession number: NC_000006.11). The transcription starts (+1) at 85473899 in the first exon. B. Chromatograms of the DSVs. The DSVs only identified in VSD patients or controls are depicted. The orientations are indicated. For each DSV, the top panel shows wild type and bottom panel heterozygous. DSVs are marked with arrows. b Deletion DSV is underlined and marked
Sequence analysis of the TBX18 gene promoter with TESS (transcription element search software program, University of Pennsylvania, USA) suggested that putative binding sites for transcriptional factors were modified, interrupted, or created by the DSVs. For example, the SNP, g.85474871C>T (rs77693245), modified a binding site for zinc finger protein E4F1, abolished a binding site for activating transcription factor (ATF), and created a binding site for X-box-binding protein 1 (XBP-1). The deletion DSV (g.85474435del) abolished the binding sites for serum response factor (SRF), Ets-2 factor, and enhancer-binding factor to the E1A core motif (EF-1A). The DSV (g.85474418C>T) abolished a weak site for P300 protein and created a weak SP1 element. The DSV (g.85473965C>G) modified a binding site for non-histone nuclear protein 1 (NHP-1) and created a site for general transcription factor II-I (TFII-I).
To functionally analyze the variant TBX18 gene promoters, reporter gene expression constructs containing wild-type or variant TBX18 gene promoters (pGL3-WT, pGL3-85474947T, pGL3-85474648C, pGL3-85474871T, pGL3-85474435del, pGL3-85474418T, and pGL3-85473965G) were transfected into HEK-293 cells and dual-luciferase activities were determined. The results showed that DSVs g.85474871C>T (rs77693245), g.85474435del, and g.85473965C>G significantly decreased the transcriptional activities of the TBX18 gene promoter (P < 0.01). The effect of the DSV (g.85474418C>T) was marginal, but not significant. In contrast, the SNP (g.85474947C>T, rs74934763), which was found in both VSD patients and controls, and the DSV (g.85474648G>C), which was only identified in the controls, did not affect the transcriptional activities of the TBX18 gene promoter (P > 0.05) (Fig. 2). Therefore, these results suggested that the DSVs identified in VSD patients may alter the transcriptional activities of the TBX18 gene promoter.
Transcriptional activities of the wild type and DSVs within the TBX18 gene promoters. Wild-type and variant TBX18 gene promoters were inserted into reporter gene vector pGL3-basic to generate expression vectors. These vectors were then transfected into HEK-293 cells and dual-luciferase activities were measured. The transcriptional activity of wild-type TBX18 gene promoter was designated as 100 %. The data are represented as mean ± SE from three independent transfection experiments, each in triplicate. WT, wild type. Lanes 1, pGL3-85474947T, which was used as an internal negative control; 2, pGL3-85474648C; 3, pGL3-85474871T; 4, pGL3-85474435del; 5, pGL3-85474418T; and 6, pGL3-85473965G. P < 0.01, compared to pGL3-WT
Discussion
In this study, we genetically and functionally analyzed the TBX18 gene promoter in VSD patients and controls. We found three novel heterozygous DSVs and one SNP within the TBX18 gene promoter in VSD patients, but none in the controls. Out of the four DSVs, three DSVs significantly decreased and one DSV marginally decreased the transcriptional activities of the TBX18 gene promoter. Thus, these DSVs may be involved in the VSD etiology by altering the TBX18 gene transcription and changing TBX18 levels. Since mutations in TBX18 gene have not been reported, we for the first time linked the TBX18 gene DSVs to CHD patients.
The human TBX18 gene has been mapped to chromosome 6q14–q15 [20]. Little is known about the expression and regulation of the TBX18 gene. In a human embryonic stem cell line, TBX18 is directly regulated by OCT4 factor [21]. In human tumor cells, TBX18 is methylated by DNA methyltransferases 3B (DNMT3B) [22]. In this study, the DSVs within the TBX18 gene promoter identified in VSD patients may change TBX18 levels in the developing heart by interfering with binding sites for transcription factors.
To date, few downstream target genes of TBX18 have been identified. TBX18 functions as a transcriptional repressor by binding to T-box element and groucho protein, a transcriptional corepressor. TBX18 interacts with GATA4 and NKX2-5 and competes with TBX5 to directly regulate the cardiac natriuretic peptide precursor type a (NPPA) gene [23]. In postnatal cardiomyocytes, TBX18 represses CX43 gene expression [24]. TBX18 is also required for repressing transforming growth factor beta receptor (TGF-beta) and Notch signaling in the embryonic epicardium [25]. TBX18, interacting with SIX1, synergistically regulates development of the smooth muscle cells [26]. TBX18 cooperates with paired box transcription factor PAX3 in maintaining anterior–posterior somite polarity [27]. Therefore, changed TBX18 levels may interfere with its downstream target genes and its interaction with other factors and molecules in the heart development, contributing to VSD.
In conclusions, we identified novel and functional heterozygous DSVs within the TBX18 gene promoter in VSD patients. These DSVs may be involved in the VSD etiology by changing the levels of TBX18, one component of cardiac gene regulatory network. The findings would deepen our understanding of the genetic causes and molecular mechanisms for CHD and provide the basis for designing novel therapies for adult CHD patients.
References
Pierpont ME, Basson CT, Benson DW Jr et al (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115:3015–3038
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8:50–60
Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP, Vliegen HW, Grobbee DE, Mulder BJ (2010) Mortality in adult congenital heart disease. Eur Heart J 31:1220–1229
Kathiresan S, Srivastava D (2012) Genetics of human cardiovascular disease. Cell 148:1242–1257
Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE (2005) T-box genes in vertebrate development. Annu Rev Genet 39:219–239
Greulich F, Rudat C, Kispert A (2011) Mechanisms of T-box gene function in the developing heart. Cardiovasc Res 91:212–222
Qiao Y, Wanyan H, Xing Q, Xie W, Pang S, Shan J, Yan B (2012) Genetic analysis of the TBX20 gene promoter region in patients with ventricular septal defects. Gene 500:28–31
Shan J, Pang S, Qiao Y, Ma L, Wang H, Xing Q, Wanyan H, Wu G, Yan B (2012) Functional analysis of the novel sequence variants within TBX5 gene promoter in patients with ventricular septal defects. Transl Res 160:237–238
Wang H, Chen D, Ma L, Meng H, Liu Y, Xie W, Pang S, Yan B (2012) Genetic analysis of the TBX1 gene promoter in ventricular septal defects. Mol Cell Biochem 370:53–58
Kraus F, Haenig B, Kispert A (2001) Cloning and expression analysis of the mouse T-box gene TBX18. Mech Dev 100:83–86
Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AF, Kispert A (2006) Formation of the venous pole of the heart from an Nkx2–5-negative precursor population requires TBX18. Circ Res 98:1555–1563
Mommersteeg MT, Domínguez JN, Wiese C, Norden J, de Gier-de Vries C, Burch JB, Kispert A, Brown NA, Moorman AF, Christoffels VM (2010) The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc Res 87:92–101
Wiese C, Grieskamp T, Airik R, Mommersteeg MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF, Kispert A, Christoffels VM (2009) Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by TBX18 and TBX3. Circ Res 104:388–397
Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A (2009) TBX18 and the fate of epicardial progenitors. Nature 458:E8–E9
Cai CL, Martin JC, Sun Y et al (2008) A myocardial lineage derives from TBX18 epicardial cells. Nature 454:104–108
Grieskamp T, Rudat C, Lüdtke TH, Norden J, Kispert A (2011) Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ Res 108:813–823
Kapoor N, Liang W, Marbán E, Cho HC (2013) Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of TBX18. Nat Biotechnol 31:54–62
Niedrist D, Lurie IW, Schinzel A (2009) 4q32–q35 and 6q16–q22 are valuable candidate regions for split hand/foot malformation. Eur J Hum Genet 17:1086–1091
Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A (2004) The T-box transcription factor TBX18 maintains the separation of anterior and posterior somite compartments. Genes Dev 18:1209–1221
Yi CH, Terrett JA, Li QY, Ellington K, Packham EA, Armstrong-Buisseret L, McClure P, Slingsby T, Brook JD (1999) Identification, mapping, and phylogenomic analysis of four new human members of the T-box gene family: EOMES, TBX6, TBX18, and TBX19. Genomics 55:10–20
Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, Groth D, Lehrach H, Burdon T, Adjaye J (2007) Analysis of Oct 4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells 25:500–510
Ghoshal K, Motiwala T, Claus R, Yan P, Kutay H, Datta J, Majumder S, Bai S, Majumder A, Huang T, Plass C, Jacob ST (2010) HOXB13, a target of DNMT3B, is methylated at an upstream CpG island, and functions as a tumor suppressor in primary colorectal tumors. PLoS One 5:e10338
Farin HF, Bussen M, Schmidt MK, Singh MK, Schuster-Gossler K, Kispert A (2007) Transcriptional repression by the T-box proteins TBX18 and TBX15 depends on Groucho corepressors. J Biol Chem 282:25748–25759
Kapoor N, Galang G, Marbán E, Cho HC (2011) Transcriptional suppression of connexin43 by TBX18 undermines cell–cell electrical coupling in postnatal cardiomyocytes. J Biol Chem 286:14073–14079
Greulich F, Farin HF, Schuster-Gossler K, Kispert A (2012) TBX18 function in epicardial development. Cardiovasc Res 96:476–483
Nie X, Sun J, Gordon RE, Cai CL, Xu PX (2010) SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development 137:755–765
Farin HF, Mansouri A, Petry M, Kispert A (2008) T-box protein TBX18 interacts with the paired box protein PAX3 in the development of the paraxial mesoderm. J Biol Chem 283:25372–25380
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81070173) and the Shandong Provincial Natural Science Foundation (ZR2010HM111).
Author information
Authors and Affiliations
Corresponding author
Additional information
Liming Ma, Jianjun Li, and Yumei Liu contributed equally to the study.
Rights and permissions
About this article
Cite this article
Ma, L., Li, J., Liu, Y. et al. Novel and functional variants within the TBX18 gene promoter in ventricular septal defects. Mol Cell Biochem 382, 121–126 (2013). https://doi.org/10.1007/s11010-013-1725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1725-4