Abstract
Vascular calcification due to elevated phosphate levels is the major contributor of cardiovascular dysfunction. The oxidative stress and gene expression events modulate the transdifferentiation of vascular smooth muscle cells into osteogenic phenotype. This present study intends to evaluate the dose-dependent effect of diosgenin, an antioxidant on high phosphate induced vascular calcification in adenine-induced chronic renal failure rats. High phosphate environment causes elevated calcium accumulation with related histological changes and alkaline phosphatase activity in aorta. Further it downregulates the activity of enzymatic antioxidants and elevates the level of lipid peroxidative markers. Moreover, the renal failure leads to reduced nitric oxide production. But, treatment with diosgenin at a dose of 10, 20, and 40 mg/kg given via oral gavages causes reversion of all the above events in a dose-dependent manner. The highest dose has shown more potential activity than other two doses, which has the ability to protect the alteration of liver markers and red blood cell antioxidant system without any adverse effects and it does not alter the kidney associated changes too. Finally, the Fourier transform infrared spectroscopy study strongly supports its ability to protect the macromolecules from oxidative stress. All the above evidences show that diosgenin has overall benefits against renal failure-induced vascular calcification-associated oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification is a prominent pathogenic event in aging, diabetes, chronic kidney disease (CKD), and inflammatory diseases. Vascular calcification is now considered to be an organized, regulatory process comparable to bone mineralization. The presence of various components associated with bone mineralization, such as bone morphogenetic protein (BMP), osteocalcin, osteopontin, osteoblast-like cells, and matrix vesicles in atherosclerotic lesions, supports this concept [1–3]. Vascular smooth muscle cell (VSMC) populations within the normal vascular media are responsible for maintaining proper vascular tone. Consequently, they exhibit a contractile phenotype which is characterized by the high expression of genes which encode proteins which are involved in maintaining myofilament structure and function. Studies on human VSMCs in vitro and in vivo have demonstrated that phenotypic change is often associated with the ability of VSMCs to acquire characteristics of a diverse range of mesenchymal lineages, including osteoblastic, chondrocytic, and adipocytic [4–7].
A previous study indicates that chronic inflammation and oxidative stress have a critical role in vascular calcification [8]. Oxidative stress occurs when oxidant production exceeds local antioxidant capacity, resulting in increased oxidation of important macromolecules, including proteins, lipids, carbohydrates, and nucleic acids and may sometimes drastically lead to tissue damage or changes via several different cellular molecular pathways [9]. In addition, reactive oxygen species (ROS) mediate increase in BMP2 expression and signaling, favouring osteogenesis [10]. Previous study reports that, Tempol, an antioxidant, ameliorated osteogenic transdifferentiation of VSMCs and arterial medial calcification in uremic rats, together with reduction in aortic and systemic oxidative stress levels [11].
Diosgenin, a steroidal saponin present in fenugreek (Trigonella foenum graecum) and other plants suppress inflammation, inhibit proliferation and induce apoptosis in a variety of tumor cells [12]. Figure 1 illustrates the structure of diosgenin. Studies have also suggested a lower incidence for coronary artery diseases and disorders related to estrogen deficiencies in humans who have a high consumption of diet rich in phytoestrogens (e.g., genistein, daidzein, and diosgenin) [13–16]. Recent study on the effect of diosgenin on VSMC under TNF-α-induced condition reports that diosgenin abrogated tumor necrosis factor-α (TNF-α) induced production of intracellular ROS and phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), extracellular signal-regulated kinase (ERK), jun N-terminal kinases (JNK), and protein kinase B (PKB/Akt). Diosgenin was also shown to inhibit nuclear factor-kappa B (NK-κB) activation induced by TNF-α [17]. Another finding suggested that diosgenin has beneficial role against aortic remodeling induced by oxidative stress in diabetic state, which was evident from the propensity of diosgenin to modulate the antioxidant defence and to decrease the lipid peroxidation in aorta [18]. Understanding the mechanisms of novel natural molecules that control SMC transdifferentiation to osteochondro progenitors and subsequent vascular calcification may help developing novel strategies that prevent or reverse vascular calcification. No sufficient work has been done until now to study the calcification prevention activity of diosgenin. Therefore, the present study was designed to determine the dose-dependent effect of diosgenin in adenine-induced chronic renal failure albino wistar rats.
Materials and methods
Animals and chemicals
Male albino Wistar rats, 7–10-weeks-old (weighing 180–220 g) were procured from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University. The experimental study was approved by the Ethical Committee of Rajah Muthiah Medical College and Hospital, Annamalai nagar, Tamil Nadu, India. Diosgenin was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). All other chemicals used in this study were of analytical grade obtained from Merck and HIMEDIA, India.
Vascular calcification and drug administration
Vascular calcification in wistar rats was induced by feeding the animals with a diet containing 0.75 % adenine for 5 weeks. Each of the following groups consisted of 6 animals. Diosgenin was administered everyday orally by dissolving it in corn oil (vehicle) from the beginning till end of the fifth week in parallel with adenine treatment.
-
Group I—Control (animals fed with rat chow)
-
Group II—Control + diosgenin 40 mg/kg body weight (b.w) of animals
-
Group III—Control animals fed with 0.75 % adenine—Chronic renal failure animals (CRF)
-
Group IV—CRF + diosgenin 10 mg/kg b.w of animals
-
Group V—CRF + diosgenin 20 mg/kg b.w of animals
-
Group VI—CRF + diosgenin 40 mg/kg b.w of animals
Blood collection and tissue homogenization
Blood was collected from orbital sinus with great care using a dry test tube and allowed to coagulate at ambient temperature for 40 min. Serum was separated by centrifugation at 224×g for 10 min. The blood, collected in a heparinized centrifuge tube, was centrifuged at 224×g for 10 min and the plasma was separated by aspiration. After the separation of plasma, the buffy coat, enriched in white cells, was removed and the remaining erythrocytes were washed three times with physiological saline. Erythrocytes were lysed with hypotonic phosphate buffer at pH 7.4. The hemolysate was separated by centrifugation at 350×g for 10 min and 0.5 mL of supernatant was used for the estimation of enzymatic antioxidants. Aorta tissues were sliced into pieces and homogenized in appropriate buffer in cold condition (pH 7.0) to give 20 % homogenate (w/v). The homogenate was centrifuged at 560×g for 10 min at 4 °C in a refrigerated centrifuge. The supernatant was separated and used for various biochemical estimations.
Aortic calcium accumulation
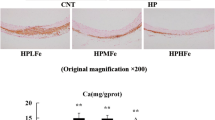
Aorta was perfused with saline, harvested, and cleaned. After drying and weighing, a small piece of aorta, extracted overnight in 1 M HCl and calcium content in the extract, was measured colorimetrically by o-cresolphthalein complexone method as described [19]
ALP activity in aorta
Aortic alkaline phosphatase was measured colorimetrically as the hydrolysis of p-nitrophenyl phosphate according to instructions given from the supplier (Fisher scientific, kerala). Aorta was homogenized in HEPES buffer on ice and centrifuged in a microfuge at 8,000×g for 5 min. Supernatant was removed for assay as described previously [20].
Plasma nitric oxide metabolites level
Nitrite and nitrate [stable nitric oxide metabolites] in the plasma samples were measured based on the Griess reaction [21].
Antioxidant enzyme activity and lipid peroxidation products in aorta and RBC
Aortic antioxidant enzyme assay and lipid peroxidation assays had been done for all the groups. But, the maximum dose of 40 mg/kg was found to have more pronounced effect than other two doses, so the high dose group only was included for RBC, plasma analysis, and further analysis. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were assayed by the methods of Kakkar et al. [22], Sinha [23], Rotruck et al. [24], respectively. Total protein was assayed by the method of Lowry et al. [25]. The level of thiobarbituric acid-reactive substances (TBARSs) and lipid hydroperoxides was estimated by the methods of Niehaus and Samuelson [26], Jiang et al. [27].
Serum biochemistry and kidney markers
Calcium was measured in serum by o-cresolphthalein complexone method and phosphorous content was measured by the ammonium molybdate method as previously described [19]. The serum urea, uric acid, and creatinine were estimated using the diagnostic kit based on the method of Fawcett and Scott [28], Caraway [29] and Jaffe [30], respectively.
Level of liver markers
The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were assayed using commercially available kit (Fisher scientific, Kerala). The activity of gamma glutamyl transferase (GGT) was measured by the method of Rosalki and Rau [31].
FTIR analysis
FTIR analysis was carried out as described previously [32]. A small and equal amount of aorta samples of 4 groups (Group 1, 2, 3, and 6) were homogenized and freeze dried. Samples were stored under −80 °C until further use. For FTIR analysis, the samples were mixed with KBr at ratio of 1:100. The mixture was then subjected to a pressure of 1,100 kg/cm2 to produce KBr pellets for use in FTIR spectrometer. Pellets of the same thickness were prepared by taking the same amount of sample and applying the same pressure. FTIR spectra of the region 4,000–400 cm−1 were recorded at the temperature of 25 ± 1 °C on a Nicolet-Avatar-360 FTIR spectrometer.
Histopathology analysis of aorta
Excised aorta samples were cleared of blood and immediately fixed in neutral buffered solution of 10 % formalin for 24 h. 5-μm thick tissue sections from aorta of each animal were prepared from processed paraffin-embedded samples. Sections were stained with Hematoxylin and Eosin for light microscopic examination. The cross-sectional area of aorta was evaluated from photographs of tissue sections taken at 40× magnification.
Statistical analysis
Values are given as mean ± SD for six rats in each group. Data were analyzed by one-way analysis of variance followed by Duncan’s multiple range test using SPSS version 11.5 (SPSS, Chicago, IL). The limit of statistical significance was set at P < 0.05.
Results
Aortic calcium accumulation
The results demonstrated that renal failure-induced high phosphate condition significantly increased calcification in rat aorta, whereas 40 mg/kg diosgenin significantly decreased aorta calcification than 10 and 20 mg/kg in a dose-dependent manner as illustrated in Fig. 2.
ALP activity in aorta
The results demonstrated that renal failure rats have shown significantly elevated ALP activity compared with control as shown in Fig. 3. However, the treatment of 40 mg/kg diosgenin more significantly decreased ALP activity in aorta than 10 and 20 mg/kg diosgenin groups.
Effects of diosgenin on nitric oxide metabolites
Figure 4 depicts the levels of nitric oxide metabolites (nitrite and nitrate) in plasma. Adenine-fed renal failure rats had significantly (P < 0. 05) decreased levels of total nitrite and nitrate in plasma and treatment with diosgenin significantly (P < 0.05) elevated the levels of nitric oxide metabolites in a dose-dependent manner with the maximum effect at 40 mg/kg.
Enzymatic antioxidants
The activities of SOD, CAT, and GPx in aorta and red blood cells (RBC) are presented in Tables 1 and 2, respectively. The activities of these enzymatic antioxidants significantly decreased in renal failure rats. Treatment with diosgenin significantly (P < 0.05) restored the activity of these enzymatic antioxidants in aorta in a dose-dependent manner. 40 mg/kg diosgenin treatment group has shown more effect on the above assays than the other two doses. So, further studies used the above dose only. Enzymatic antioxidant activity in RBC has shown that 40 mg/kg diosgenin exhibited significant protective effect.
Tables 1 and 2 portrays the levels of TBARS, lipid hydro peroxides in aorta and RBC, respectively. Levels of lipid peroxidation products in aorta and plasma were significantly elevated in renal failure rats, whereas treatment of diosgenin significantly reduced the lipid per oxidation products in a dose-dependent manner in aorta and the 40 mg/kg dose shown more effective. 40 mg/kg diosgenin treatment shows significant suppression of lipid peroxidation level in plasma.
Effect of diosgenin on serum calcium, phosphorous, and kidney function markers
Table 3 illustrates the effect of diosgenin on serum calcium and phosphorous level. Results have shown that phosphorous concentration was elevated significantly in the renal failure rats, but the calcium level was not altered. Treatment with diosgenin did not significantly alter the phosphorous and calcium level. Levels of renal function marker such as urea, uric acid, and creatinine were significantly elevated in serum. But, diosgenin does not have any significant impact on renal function markers
Effect of diosgenin on liver markers
Table 4 summarizes the effects of diosgenin on the activities of hepatic function marker such as aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and gamma glutamyl transferase in serum of rats. The activities of these pathophysiological marker enzymes were significantly elevated in renal failure rats. Treatment with diosgenin 40 mg/kg significantly declined these levels of hepatic markers toward normal. Moreover, the diosgenin-alone-treated group (group II) does not show any significant alteration.
Molecular alterations
The representative FTIR spectra of a control aorta with wave number of all the macromolecular bonding regions are shown in Fig. 5. The wave numbers were assigned from previous studies [33, 34]. Frequency shifts of macromolecules are illustrated in Table 5. The bands in 3,405 cm−1 region arose from N–H and O–H stretching modes of proteins and intermolecular hydrogen bonding. In the experiment, Amide A appeared at 3,405 cm−1 in control and the band frequency was significantly shift in renal failure rats, whereas the diosgenin treated protects the changes significantly and there was no significant change between control and diosgenin alone treated group. CH2 symmetric stretching of lipids/fatty acids appears at 2,868 cm−1 in control and in renal failure it was shifted increase in wave number significantly, whereas the diosgenin treated group significantly brought back the shift to control level. The most important molecular bonds of proteins, amide linkages were significantly altered in renal failure group, in which the amide I band was appeared in 1,655 cm−1. In the wave number of amide II linkage, the control group appeared at 1,544 cm−1 and the significant change in frequency shift was observed. But, this change was significantly protected by diosgenin in amide I, but not in amide II region.
Histopathology of aorta
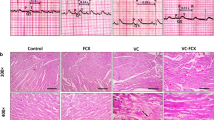
The histopathology study of aorta is shown in Fig. 6. Pictures of aorta exhibited that control and diosgenin-alone-treated groups have shown no pathogenic signature in the aortic medial layer and the normal arrangement of vascular cells were found, whereas aorta from CRF rat has shown patched calcification and calcium accumulation in the medial layer of vasculature. Diosgenin-treated CRF rats have shown no calcified region in the aorta. This indicates that diosgenin treatment in CRF rats has prevented the vascular calcification in aorta.
Histopathology of aorta. Control rat aorta shows normal structural arrangement (A), aorta from control + diosgenin 40 mg/kg shows no pathogenic signatures (B), CRF aorta shows patchy calcification occurred in medial layer vascular smooth muscle cell (C), CRF + diosgenin 40 mg/kg shows normal arrangement of cells in vasculature without any signature of vascular calcification (D)
Discussion
Previous reports have shown that high phosphate environment causes elevated calcium accumulation and ALP activity and VSMC transdifferentiation [35]. In this animal model, the results showing elevated serum phosphorus levels increased aortic calcium accumulation and showing marked elevation in ALP activity indicate the osteogenic conversion of aortic VSMCs. The treatment with diosgenin reduced this kind of VSMC transdifferentiation in a dose-dependent manner. The histopathology of aorta was consistent with the calcium quantification analysis. The histology shows that there was an intense accumulation of calcium in the medial layer of vasculature in CRF rat, whereas treatment with diosgenin in CRF rat has shown to prevent vascular calcification. Previous reports have shown that diosgenin abrogated TNF-α-induced production of intracellular ROS and phosphorylation of p38, ERK, JNK, and Akt. Diosgenin was also shown to inhibit NF-κB activation induced by TNF-α [17]. Moreover, the involvement of oxidative stress, p38 MAPK, ERK, and Akt in vascular calcification was already explored [36]. From this point of view, the protective action of diosgenin against VSMC transdifferentiation may be in partly due to its effects on the above pathways including oxidative stress. So, this study focused on the antioxidant potential of diosgenin.
Kidney function-related physiological parameters have shown that the phosphorus, urea, uric acid, and creatinine levels were elevated, but the treatment of diosgenin has no impact on these parameters indicating that it has no action or less renal protection potential against adenine-induced renal failure. Moreover, this indicates that the action of diosgenin against vascular calcification is independent of blood chemistry and most exactly depends on the direct action on VSMCs.
In the oxidative stress mechanistic point of view, the high phosphate environment causes mitochondrial dysfunction and generation of excess free radicals and oxidative stress [37]. Previous study have shown that calcifying VSMCs treated with inorganic phosphate (Pi) exhibited mitochondrial dysfunction, as demonstrated by the decreased mitochondrial membrane potential and ATP production; the disruption of mitochondrial structural integrity and concurrently increased the production of ROS [38]. Previous study reports that excess free radical generation can cause dysfunction of the enzymatic antioxidant system which is comprised of SOD, CAT, and GPx. Free radical-scavenging enzymes such as SOD, CAT, and GPx are the first line of cellular defense against oxidative injury. SOD reduces superoxide anion to form H2O2 and oxygen. CAT removes H2O2 by breaking it down directly to oxygen [39]. In this study, SOD, CAT, and GPx activity decreased significantly in the renal failure aortic calcification group, which might be due to an excessive formation of superoxide anions. Further, the decrease in enzyme activity can result in the decreased removal of free radicals and may favor oxidative stress driven calcification [40]. Treatment with diosgenin dose dependently increases the antioxidant enzymes activity in both aorta and in RBC; this indicates the antioxidant potential of diosgenin. Moreover, excess production of free radicals causes membrane lipid peroxidation and damage, which is already reported in a previous study on heart [41]. In this study, also, the lipid peroxidation products such as TBARS and LOOH were elevated significantly in both aorta and plasma, whereas the treatment of diosgenin dose dependently reduces the level of lipid peroxide markers. It was already shown that elevated oxidative stress in aorta causes increase in lipid peroxidation and treatment with diosgenin significantly restores the changes [18]. Further, a study on membrane-stabilizing effect of diosgenin, in experimentally induced myocardial infarction, shows that antilipid peroxidative potential of diosgenin may be due to its antioxidative activity [41].
Endothelium is also a considerable target in the pathogenesis of increased cardiovascular disease in CKD patients with hyperphosphatemia. Moreover, hyperphosphatemia impaired endothelial function by increasing ROS, inhibiting endothelial nitric oxide synthase (eNOS), increased oxidative stress, and apoptosis in endothelial cells [42]. In this study, the serum nitrite/nitrate level which is the indicator of nitric oxide production was reduced, but the antioxidant potential of diosgenin reversed the above changes in a dose-dependent manner.
The FTIR spectroscopy monitors the vibration modes of functional groups present in proteins, lipids, polysaccharides, and nucleic acids. Shifts in peak positions indicate the molecular changes associated with macromolecules in a physiological condition. In this analysis, the N–H stretching of proteins were changed significantly in calcification, this indicates that the intramolecular hydrogen bonding in proteins may be disturbed [33]. This effect might be due to the oxidative stress generated during calcification. This effect protected by diosgenin-treated group indicated its antioxidant potential against oxidative stress generated during calcification.
The wave number of fatty acid species changed significantly in renal failure aorta; this indicated the acyl chain modification of membrane lipids. The frequency of the CH2 bands of acyl chains depends on the degree of conformational disorder and level of flexibility. The position of these bands provides information about the lipid acyl chain flexibility (order/disorder state of lipids) [43]. The shift of the peak position of these bands to higher values indicated that increase lipid order and acyl chain flexibility. A previous study indicates that this kind of changes may be associated with oxidative stress [33]. The above shift significantly protected by diosgenin indicated its protective effect on lipid species through its antioxidant and anti lipid peroxidative potential in aorta [18]. This effect further supports antilipid peroxidation potential of diosgenin on vascular calcification related oxidative stress and lipid peroxidation in an indirect way.
Amide bands are the most important indicators of oxidative stress. The shift in amide I and amide II in renal failure calcification group compared with control indicates the excess production of free radicals and free radical-mediated protein damage. However, diosgenin treatment significantly protected the Amide I-associated changes, while this effect was not extended in amide II. According to the findings of a previous study, all the constituent amino acid side-chains in proteins are susceptible to free radicals, but some are more vulnerable than others. Thus, the exposure of proteins to free radical-generating systems may induce secondary structural changes. Secondary structure is stabilized by hydrogen bonding of the peptide backbone and interference with the functional groups of the peptide bonds may cause structural modifications. Further, the shift in amide I and II regions corresponds to the alpha-helix protein conformational change [44]. Thus, the above effect may directly indicate the antioxidant and preventive potential of diosgenin on protein modification.
In the present study, we found that diosgenin-inhibited chronic renal failure induced calcification by preventing oxidative stress independent of kidney protection and it extends its beneficial effects to RBC. The effect of diosgenin was dependent on dose and restoration of the antioxidant pathway through which it prevent the VSMC phenotype changes. Furthermore, this beneficial effect was supported by its preventive ability on nitric oxide production and macromolecular protection potential and its effects on liver makers showing its non-toxicity. Our results reveal novel pathways by which diosgenin deregulate renal failure-induced calcification. Thus, diosgenin will be used as a good therapeutic molecule for vascular calcification.
References
Anderson HC (1983) Calcific diseases. A concept. Arch Pathol Lab Med 107:341–348
Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Investig 91:1800–1809
Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS (2001) Bone formation and inflammation in cardiac valves. Circulation 103:1522–1528
Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weiss-berg PL, Edmonds ME (1999) Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100:2168–2176
Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM (2003) Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol 23:489–494
Davies JD, Carpenter KL, Challis IR, Figg NL, McNair R, Proudfoot D, Weissberg PL, Shanahan CM (2005) Adipocytic differentiation and liver X receptor pathways regulate the accumulation of triacyl-glycerols in human vascular smooth muscle cells. J Biol Chem 280:3911–3919
Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL (2003) Multilineage potential of cells from the artery wall. Circulation 108:2505–2510
Massy ZA, Mazière C, Kamel S, Brazier M, Choukroun G, Tribouilloy C, Slama M, Andrejak M, Mazière JC (2005) Impact of inflammation and oxidative stress on vascular calcifications in chronic kidney disease. Pediatr Nephrol 20:380–382
Sherki YG, Rosenbaum Z, Melamed E, Offen D (2006) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54:271–284
Johnson RC, Leopold JA, Loscalzo J (2006) Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99:1044–1059
Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, Noguchi H, Iida M, Tsuruya K, Kitazono T (2012) The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res 27:474–485
Shishodia S, Aggarwal BB (2005) Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene 25:1463–1473
Adlercreutz H, Markkanen H, Watanabe S (1993) Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342:1209–1210
Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155:381–386
Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P (2000) Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J Am Coll Cardiol 35:1977–1985
Au AL, Kwok CC, Lee AT, Kwan YW, Lee MM, Zhang RZ, Ngai SM, Lee SM, He GW, Fung KP (2004) Activation of iberiotoxin-sensitive, Ca2-activated Kchan-nels of porcine isolated left anterior descending coronary artery by diosgenin. Eur J Pharmacol 502:123–133
Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, Pyo S (2010) Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vasc Pharmacol 53:273–280
Pari L, Monisha P, Jalaludeen AM (2012) Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur J Pharmacol 691:143–150
Sutliff RL, Walp ER, El-Ali AM, Elkhatib S, Lomashvili KA, O’Neill WC (2011) Effect of medial calcification on vascular function in uremia. Am J Physiol Renal Physiol 301:F78–F83
Zhou Y-B, Jin S-J, Cai Y, Teng X, Chen L, Tang C-S, Qi Y-F (2009) Lanthanum acetate inhibits vascular calcification induced by vitamin D3 plus nicotine in rats. Exp Biol Med 234:908–917
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21:130–132
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidise. Science 179:588–590
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Niehaus WG, Samuelson B (1968) Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Caraway WT (1955) Determination of uric acid in serum by carbonate method. Am J Clin Pathol 25:840–845
Jaffe M (1886) Concerning the precipitate produced in normal urine by picric acid and a new reaction of creatinine. Z Physiol Chem 10:391–400
Rosalki SB, Rau D (1972) Serum-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta 39:41–47
Sivakumar S, Sivasubramanian J, Raja B (2012) Aluminium induced structural, metabolic alterations and protective effects of desferrioxamine in the brain tissue of mice: An FTIR study. Spectrochimica Acta Part A 99:252–258. doi:10.1016/j.saa.2012.09.036
Saravanakumar M, Manivannan J, Sivasubramanian J, Silambarasan T, Balamurugan E, Raja B (2012) Molecular metabolic fingerprinting approach to investigate the effects of borneol on metabolic alterations in the liver of nitric oxide deficient hypertensive rats. Mol Cell Biochem 362:203–209
Cakmak G, Togan I, Severcan F (2006) 17-Estradiol induced com-positional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: a comparative study with nonyl-phenol. Aquat Toxicol 77:53–63
Kircelli F, Peter ME, Sevinc Ok E, Celenk FG, Yilmaz M, Steppan S, Asci G, Ok E, Passlick-Deetjen J (2012) Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant 27:514–521
Shan PF, Lu Y, Cui RR, Jiang Y, Yuan LQ, Liao EY (2011) Apelin attenuates the osteoblastic differentiation of vascular smooth muscle cells. PLoS ONE 6(3):e17938. doi:10.1371/journal.pone.0017938
Kapustin A, Galkin A, Furmanik M, Hernandez AD, Shanahan C (2011) Elevated calcium and phosphate impair mitochondrial function in calcifying human vascular smooth muscle cell. Heart 97(24) doi:10.1136/heartjnl-2011-301156.19
Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, Ha CM, Choi YK, Lee SJ, Kim JY, Harris RA, Jeong D, Lee IK (2012) α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 16:273–286
Frank L, Massaro D (1980) Oxygen toxicity. Am J Med 69:117–126
Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y (2008) Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 283:15319–15327
Jayachandran KS, Vasanthi HR, Rajamanickam GV (2009) Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol Cell Biochem 327:203–210
Van TV, Watari E, Taketani Y, Kitamura T, Shiota A, Tanaka T, Tanimura A, Harada N, Nakaya Y, Yamamoto H, Miyamoto K, Takeda E (2012) Dietary phosphate restriction ameliorates endothelial dysfunction in adenine-induced kidney disease rats. J Clin Biochem Nutr 51:27–32
Severcan F (1997) Vitamin E decreases the order of the phospholipids model membranes in the gel phase: an FT-IR study. Biosci Rep 17:231–235
Rice-Evans CA, Diplock AT, Symos MCR (1991) Technique in free radical research. Elsevier, New York, pp 207–218
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manivannan, J., Barathkumar, T.R., Sivasubramanian, J. et al. Diosgenin attenuates vascular calcification in chronic renal failure rats. Mol Cell Biochem 378, 9–18 (2013). https://doi.org/10.1007/s11010-013-1588-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1588-8