Abstract
Patients with type 2 diabetes (T2DM) are usually obese and concurrent obesity results into activation of the renin–angiotensin-system (RAS) which is a risk factor for diabetic nephropathy (DN). Gene–gene interaction between acetyl-coenzymeA carboxylase beta (ACACβ) gene, which is involved in fatty acid metabolism and angiotensin II receptors (AGTR1) gene, which mediates RAS proteins actions on renal tissue, polymorphism with DN have not been studied earlier. The present study was designed with the aim to examine the association of an ACACβ (rs2268388) and AGTR1 (rs5186) gene polymorphism with the risk of DN in Asian Indians. 1,158 patients with T2DM belonging to two independently ascertained North Indian and one South Indian cohorts were genotyped for ACACβ (rs2268388) and AGTR1 (rs5186) polymorphism using real time PCR-based Taq-man assay and PCR–RFLP assays. In all the three cohorts, a significantly higher frequency of T allele and TT genotypes of ACACβ and C allele and CC genotypes of AGTR1 were found in patients with DN as compared to patients without nephropathy. Further, T allele of ACACβ and C allele of AGTR1 were found to be significantly associated with proteinuria, a hallmark of DN. We also found significant epistatic interactions between these two genes. TT genotypes of ACACβ gene and CC genotype of AGTR1 gene confers the risk of DN and both genes had significant epistatic interaction in Asian Indian patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a leading cause of end stage renal disease in developed as well as developing countries [1]. Diabetic nephropathy (DN) develops only in 35–40 % of diabetic patients and familial clustering of nephropathy in both type 1 and type 2 diabetes mellitus (T2DM) underlies the importance of genetic factors in the development and progression of DN [2].
The renin–angiotensin system (RAS) has been strongly implicated in the pathogenesis of progressive renal diseases [3]. RAS plays a central role in the regulation of blood pressure, sodium homeostasis, and renal hemodynamics. Its action is mediated primarily by angiotensin II which acts on type 1 angiotensin II receptors (AGTR1) in the vasculature, leading to vasoconstriction, and on the zona glomerulosa, where it stimulates the secretion of aldosterone and leading to mesengial fibrosis [4]. Adipocytes do have capacity to synthesize and secrete RAS proteins and hence, obesity is associated with increased aldosterone levels and it correlates with high blood pressure, high waist circumference, and low HDL cholesterol levels [5].
There are epidemiological evidences which indicate that obesity and increase in free fatty acid (FFA) levels are independent risk factors for development of DN [6]. The enzyme responsible for the metabolism of fatty acid is acetyl-coenzymeA carboxylase beta (ACACβ) which converts acetyl-CoA to malonyl-CoA and this product is known to inhibit carnitine palmitoyl-CoA transferase resulting in inhibition of mitochondrial beta oxidation of fatty acids [7]. Thus, activity of ACACβ gene results into accumulation of FFA in the cell resulting into lipotoxicity.
There is dearth of literature on association of AGTR1 gene polymorphism (rs5186) and DN in patients with T2DM, and furthermore, published literature shows inconsistent results. Eight studies have examined the role of AGTR1 (A1166C) gene polymorphism and risk of DN till date, of which two were from India and results were inconsistent across different populations [8–15]. In a study by Ahluwalia et al. [14], showed that the frequency of C allele of AGTR1 A1166C was higher in North Indian subjects with T2DM and CC genotype was associated with six-fold higher risk of DN. However, another study from India by Prasad et al. [15] did not find risk with A1166C and chronic renal insufficiency in Asian patients with T2DM.
Recently, a single nucleotide polymorphism (SNP) in intron 18 of the ACACβ gene was found to be associated with proteinuria in Japanese patients [16]. The frequency of the T allele for SNP rs2268388 was consistently higher in Japanese patients with T2DM and overt proteinuria. This finding was replicated in European Americans with T2DM associated ESRD [16]. Similar findings were also noted in Chinese patients with T2DM and DN [17].
Aforesaid, obesity is a state of RAS over activity. Therefore, we hypothesize that combinational effect of ACACβ gene and AGTR1 gene polymorphism may be more detrimental for renal tissue in patients with T2DM. The gene interaction between these two genes have not been studied till date and hence, the present study was designed with the aim to examine the association of an ACACβ (rs2268388) and AGTR1 (rs5186) gene polymorphism with susceptibility to DN in Asian Indians.
Methods
Study population
Two independently ascertained T2DM cohorts of North Indian origin, visiting Endocrinology and Nephrology Clinics of Postgraduate Institute of Medical Education and Research, Chandigarh, India between June 2006 and September 2007 (cohort 1) and July 2009 and April 2011 (cohort 2) were studied in this study. Cohort 1 consisted of 240 patients with DN and 255 T2DM patients without nephropathy (DM); cohort 2 had 260 patients with DN and 215 patients had DM. Replication studies were performed in a population of 92 patients with DM and 96 DN patients recruited from Madras Diabetes Research foundation (MDRF), Chennai, South India that formed cohort 3. All the subjects in both groups were age, and ethnicity matched. The research carried out was in compliance with the Helsinki Declaration.
Patients with diagnosis of T2DM and duration of DM ≥5 years were screened for the presence of nephropathy. DN was defined as (a) 24 h protein excretion >500 mg and or, (b) an urine albumin:creatinine ratio >300 μg/mg without any clinical or laboratory evidence of other kidney disease. T2DM patients and the absence of proteinuria (24 h protein <150 mg) and or urine albumin:creatinine ratio <30 μg/mg (measured on two consecutive occasions) formed DM group. Patients with type 1 diabetes (T1DM) and any known non-diabetic renal disease were excluded from the study.
Ethics statement
The study was approved by the Ethics committee of Post Graduate Institute of Medical Education and Research, Chandigarh, India and also by the Ethics committee of Madras Diabetes Research Foundation, Chennai, India.
Genotyping
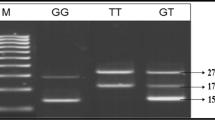
Genomic DNA was isolated from peripheral blood lymphocytes using proteinase K digestion and phenol chloroform method. The ACACβ gene polymorphism, (rs 2268388) was determined using real time PCR-based Taq-man assay (Applied Biosystems, Foster City, CA) following manufacturer’s instructions. The AGTR1 (rs5186) gene polymorphism was determined using the PCR–restriction fragment length polymorphism technique as described by Ahluwalia et al. [14]. Primers for the SNPs were designed using the Primer 3 software [18]. Positive and negative controls were used in each genotyping run, and 5 % of randomly selected samples were re-genotyped by other lab personnel with 100 % concordance. The genotypes were also confirmed by randomly sequencing some of the samples.
Statistical analysis
The statistical tests were performed, using the SPSS Inc., Chicago, IL version 11.0. We tested the genotype and allele frequencies for deviation from Hardy–Weinberg equilibrium (HWE) proportions using Hardy–Weinberg equilibrium calculator [19]. Discrete and continuous variables were compared between cases and controls using Pearson’s χ2 test and unpaired t test or Mann–Whitney U test as appropriate. Comparison of variables between different genotypes was performed using ANOVA for normally distributed data and Kruskal–Wallis test for skewed data. Power of the sample size (subjects) was calculated using the PAWE software (http://linkage.rockefeller.edu/pawe). Multivariate logistic regression was used to compute odds ratio for risk of nephropathy. p < 0.05 was considered as statistical significant. We also evaluated the gene–gene interactions among the large number of loci in our study using the multiple dimensionality reduction (MDR) method [20].
Results
Baseline characteristics of study participants
Baseline characteristics of the subjects with T2DM enrolled in the three cohorts are given in Table 1. In all the three cohorts, patients with DN had significantly higher systolic blood pressure, HbA1c, serum creatinine, and lower eGFR and duration of HT as compared to DM. The mean duration of T2DM was similar in DN and DM group.
Allele and genotype frequency of ACACβ (rs2268388) and AGTR1 (rs5186) gene polymorphism
Allele and genotype frequency of ACACβ and AGTR1gene polymorphism in all the three cohorts is shown in Table 2. The genotyping frequencies were in HWE for the three cohorts (p > 0.05) for AGTR1gene polymorphism, but were not in HWE for (p < 0.05) for ACACβ gene polymorphism.
The frequency of ‘T’ allele and TT genotype of ACACβ gene and ‘C’ allele and CC genotype of AGTR1 gene was significantly higher in DN patients as compared to DM. Patients with CT and TT genotypes of ACACβ gene were associated with nearly double and five times the risk for DN, respectively, compared to patients with CC genotype of ACACβ gene. Similarly, patients with AC and CC genotype of AGTR1gene were associated with nearly two-fold and six-fold increased risk for DN, respectively compared to patients with AA genotype.
Clinical characteristics of the study subjects according to AGTR1 A1166C (rs5186) and ACACβ (rs 2268388) genotypes
Patients with TT genotype of ACACβ and CC genotype of AGTR1 had significant overt proteinuria and lower eGFR in the all the three cohorts (Table 3). Further, in dominant model (CC vs CT + TT) genotypes, and (AA vs AC + CC) patients with CT + TT genotypes of ACACβ and AC + CC genotype of AGTR1 also showed higher proteinuria & lower eGFR suggesting an association of T allele of ACACβ and C allele of AGTR1 with DN (Table 3). We also observed similar association between genotype and proteinuria in the group of patients without DN.
Gene–gene interaction
We analyzed the gene–gene interactions among AGTR1 and ACACβ gene variants, evaluating the two-way SNP combinations using MDR approach. MDR approach is considered to be a better tool for estimating epistatic interactions, as compared conditional logistic regression [21]. (p < 0.012; based on 1000-fold permutation test). The two-locus model had a prediction error of <35 % and were significant (p < 0.05) in all the three cohorts, as determined empirically by permutation testing (Table 4). The two-locus model comprising AGTR1 and ACACβ SNPs was significant (p < 0.05) in all the three cohorts.
Discussion
We observed higher prevalence of T allele and TT genotype of ACACβ (rs2268388) and C allele and CC genotype of AGTR1 (rs5186) polymorphisms in three independently ascertained T2DM cohorts with DN, which were associated with proteinuria in patients with T2DM. Patients with TT genotypes of ACACβ gene and CC genotype of AGTR1 gene were associated with nearly five-fold and six-fold increase in risk for DN, respectively.
The activation of RAS has been strongly implicated in pathogenesis of DN [3]. Angiotensin II is a potent vasoconstrictor and it stimulates salt and water retention leading to systemic hypertension which is major risk factor for DN. Not only systemic RAS but local renal RAS is too important as studies have shown that mesangial cells and podocytes synthesize angiotensin II and express AGTR1 receptor. This results in renal hemodynamic alterations and mesangial cell proliferation thereby perpetuating renal tissue injury [22]. Pereira et al. [23] showed that hyperactivity of intracellular RAS induced overproduction of mesangial matrix components was reversed by AGTR1-receptor antagonist. Renal tissue RAS might be activated in the respect that ACE gene expression is upregulated in spite of a tendency to low renin expression in DN [24]. Strategies to control intracellular RAS activity may contribute to reduce the impact of diabetes on renal function and minimize the progression of chronic renal disease. Further investigations including assessment of disease stage and severity might provide further insight into the role of RAS in human DN. Mutant genotype of AGTR1 (rs5186) polymorphism probably increase the vascular responsiveness of angiotensin II or activation of AGTR1 receptors within the renal tissue leading to systemic as well as glomerular hypertension, proteinuria, and development of DN. Several studies have reported the association of AGTR1 gene polymorphism and development of hypertension [25] and this mutation predicts the response to angiotensin II blockers [26], thus strengthening the detrimental effect of A1166C AGTR1 mutation. AGTR1 A1166C polymorphism has been shown to be associated with an increased risk of microalbuminuria [8] and a faster progression of DN in patients with T2DM [10]; however, in contrast, data from several studies have observed no association between the A1166C polymorphism and diabetic renal disease [27]. The inconsistent findings suggest that a more complex model consisting of still poorly understood combinations of several RAS gene variants may be affecting disease susceptibility [25].
Our literature search revealed eight studies published across the world highlighting the role of AGTR1 (A1166C) for DN in T2DM [8–15]. Four studies implicated C allele or CC genotypes of AGTR1 as positive predictors of DN, while rest failed to show any association between the two. The conflicting results in above said studies were ascribed to different ethnic population, varying definition of DN, difference in sample size and a more complex model consisting of still poorly understood combinations of several AGTR1 gene variants may be affecting disease susceptibility [28]. The recent meta-analysis of 34 published studies on association of RAS genes and DN in both T1DM as well as in T2DM clearly demonstrated three-fold higher risk with CC genotype of AGTR1 (A1166C) for DN in patients with T2DM [29]. Our study also confirms the same; CC genotype of AGTR1 gene polymorphism was associated with nearly six times higher risk of DN.
Obesity has been shown as an independent risk factor for Chronic Kidney disease [6]. This has been linked to increased FFA turnover and defective mitochondrial beta oxidation in various tissues thereby resulting into accumulation of FFA in renal tissue exerting lipotoxicity [30]. Proposed mechanisms for renal lipotoxicity mediated injury with TT genotype of ACACβ include; (a) higher adipose tissue mass due to increased ACACβ activity is associated with increase in leptin, interleukin-6 levels and decrease in adiponectin levels resulting into worsening of hyperglycemia which leads to glucose mediated renal tissue injury [30]. (b) increased mRNA expression for renin–angiotensin–aldosterone system proteins by visceral adipose tissue, further perpetuates renal injury [31]. (c) Observational data also supports that hyper triglyceridemia, which is a component of obesity and metabolic syndrome is also associated with progression of nephropathy [32], and (d) mice model showed that ACACβ is expressed in glomerular tissues and expression of it is associated with increase in proinflammatory cytokines [33, 34]. Therefore, ACACβ-gene mutation confers the risk probably via lipotoxicity and hence, the risk is more in T2DM who are usually obese.
There were only two studies published so far on ACACβ gene polymorphism and development of DN [16, 17]. First published study by Maeda S and coworkers [16] showed higher frequency of the T allele for SNP rs2268388 in Japanese patients of T2DM with overt proteinuria, an observation that was replicated in European Americans patients of T2DM with ESRD and in a meta-analysis for Asians with T2DM and proteinuria. However, they failed to show the association of T allele with DN in patients with T1DM. This reflects different genetic susceptibility among T1DM and T2DM furthermore, T2DM subjects are obese and hence ACACβ polymorphism with enhanced activity may be more detrimental in patient with T2DM than T1DM. Another study by Chinese group also replicated the association between DN and rs2268388 in patient with T2DM, as seen in Japanese and European Americans [17]. We noted higher frequency of T alleles of ACACβ gene and TT genotype was associated with nearly five times higher risk of DN. In vitro functional analysis revealed that a 29-bp DNA fragment, including rs2268388, had significant enhancer activity in cultured human renal proximal tubular epithelial cells. Fragments corresponding to the disease susceptibility allele (T) had higher enhancer activity than those of the major allele. These results suggest that ACACβ gene is a strong candidate for conferring susceptibility for proteinuria in patients with T2DM [16]. Common polymorphisms of ACACβ gene rs2268388 has been shown to be associated with obesity and, independently, with T2DM in postmenopausal women, suggesting that the activity of ACACβ gene plays an important role in these disorders related to energy metabolism [35].
Till date, none of the studies have examined the additive effects of both genes on risk for DN. Therefore, we evaluated the gene–gene interaction of ACACβ (rs2268388) and AGTR1 (rs5186) genes to determine the significance of interactive and/or additional effects of variant combinations using MDR. We found significant epistatic interactions between ACACβ (rs2268388) and AGTR1 (rs5186) genes. However, we could not measure ACACβ and AGTR1 expression in these subjects as we did not have access to renal tissue from these subjects as renal biopsy from these subjects. However, we observed that T allele carriers of ACACβ and C allele carriers of AGTR1 also showed overt proteinuria, estimated decreased eGFR which are both markers of renal dysfunction, further suggesting an association of T allele with DN.
Significant deviation from HWE of genotype distribution in the present population in ACACβ (rs2268388) gene polymorphism is may be due to moderate population size or the allele is rare which can cause a random change in allele frequencies. High frequency of mutation occurring at the specific loci can also cause deviation from equilibrium of genotype distribution in the present population.
There are several strengths and limitations in our study. Recruitment of ethnically homogenous diabetic subjects, thus avoiding phenotyping errors and bias, large predetermined sample size for these variants to have a minimum power of 75 % which has been shown to be adequate for association studies [36], confirmation of the absence of microalbuminuria on two or more occasions for the control group to insure a good selection of control diabetic group are the major strength of the study. However, our study may be still underpowered for low-frequency SNP. Moreover, positive associations observed between ACACβ (rs2268388) and AGTR1 (rs5186) polymorphism and DN do not seem to be due to chance, as this association was replicated in three independent cohorts in our study. One of the limitations is cross-sectional design of the study. The present study fulfills most of the prerequisites for a good genetic association study as suggested by Bird et al. [37]. The positive association of ACACβ (rs2268388) and AGTR1 (rs5186) polymorphism with increased risk of DN in our study suggests that ACACβ and AGTR1 gene may be a critical contributor to the DN. Even though it may be a long way before the clinically relevant therapies targeting these genes or its promoters to prevent progression of DN come into play, but it certainly shows some hope in making the outcome better for patients who progress to ESRD secondary to diabetes related complications.
Conclusion
In conclusion, the TT genotype of ACACβ (rs2268388) and CC genotype of AGTR1 (rs5186) gene polymorphisms are independent risk factors for DN in Asian Indian patients with T2DM.
References
Ritz E, Rychlik I, Locatelli F, Halimi S (1999) End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 34:795–808
Vijay V, Snehalatha C, Shina K, Lalitha S, Ramachandran A (1999) Familial aggregation of diabetic kidney disease in type 2 diabetes in south India. Diabetes Res Clin Pract 43:167–171
Schmidt S, Ritz E (1997) Genetics of the renin–angiotensin system and renal disease: a progress report. Curr Opin Nephrol Hypertens 6:146–151
Remuzzi A, Puntorieri S, Alfano M, Macconi D, Abbate M, Bertani T et al (1992) Pathophysiologic implications of proteinuria in a rat model of progressive glomerular injury. Lab Invest 67:572–579
Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F et al (2006) Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension 48(2):239–245
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS (2006) Body mass index and risk for end-stage renal disease. Ann Intern Med 144(1):21–28
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ (2001) Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291:2613–2616
Fradin S, Goulet-Salmon B, Chantepie M, Grandhomme F, Morello R, Jauzac P et al (2002) Relationship between polymorphisms in the renin–angiotensin system and nephropathy in type 2 diabetic patients. Diabetes Metab 28(1):27–32
Osawa N, Koya D, Araki S, Uzu T, Tsunoda T, Kashiwagi A et al (2007) Combinational effect of genes for the renin–angiotensin system in conferring susceptibility to diabetic nephropathy. J Hum Genet 52:143–151
Tomino Y, Makita Y, Shike T, Gohda T, Haneda M, Kikkawa R (1999) Relationship between polymorphism in the angiotensinogen, angiotensin-converting enzyme or angiotensin II receptor and renal progression in Japanese NIDDM patients. Nephron 82:139–144
Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A (2006) Genetic polymorphisms of the renin–angiotensin system in end-stage renal disease. Nephrol Dial Transplant 21:979–983
Chang HR, Cheng CH, Shu KH, Chen CH, Lian JD, Wu MY (2003) Study of the polymorphism of angiotensinogen, anigiotensin-converting enzyme and angiotensin receptor in type II diabetes with end-stage renal disease in Taiwan. J Chin Med Assoc 66(1):51–56
Lin J, Hu FB, Qi L, Curhan GC (2009) Genetic polymorphisms of angiotensin-2 type 1 receptor and angiotensinogen and risk of renal dysfunction and coronary heart disease in type 2 diabetes mellitus. BMC Nephrol 10:9
Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, Sud K, Khullar M (2009) ACE variants interact with the RAS pathway to confer risk and protection against type 2 diabetic nephropathy. DNA Cell Biol 28:141–150
Prasad P, Tiwari AK, Prasanna KM, Ammini AC, Gupta A (2006) Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Med Genet 7:1–9
Maeda S, Kobayashi MA, Araki S, Babazono T, Freedman BI et al (2010) A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet 6:e1000842
Tang SC, Leung VT, Chan LY, Wong SS, Chu DW, Leung JC et al (2010) The acetyl-coenzyme A carboxylase beta (ACACB) gene is associated with nephropathy in Chinese patients with type 2 diabetes. Nephrol Dial Transplant 25:3931–3934
Rozen S, Helen J, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
http://www.genes.org.uk/software/hardy-weinberg.shtml. Accessed 17 Feb 2012
http://www.multifactordimensionalityreduction.org. Accessed 20 Feb 2012
Ritchie M, Hahn L, Roodi N, Bailey R, Dupont W, Parl F et al (2001) Multifactor-domensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet 69:138–147
Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P et al (2004) Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65:30–39
Pereira LG, Arnoni CP, Maquigussa E, Cristovam PC, Dreyfuss J, Boim MA (2012) (Pro)renin receptor: another member of the system controlled by angiotensin II? J Renin Angiotensin Aldosterone Syst 13(1):1–10
Tadashi K, Shigeyuki W, Shinichi M, Makoto M, Chikako A, Yasukazu M et al (2006) Tissue gene expression of renin–angiotensin system in human type 2 diabetic nephropathy. Diabetes Care 29:848–852
Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E et al (1994) Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension 24(1):63–69
Miller JA, Thai K, Scholey JW (1999) Angiotensin II type 1 receptor gene polymorphism predicts response to losartan and angiotensin II. Kidney Int 56:173–180
Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A et al (1996) Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int 50:657–664
Ruggenenti P, Bettinaglio P, Pinares F, Remuzzi G (2008) Angiotensin converting enzyme insertion = deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol 3(5):511–525
Ding W, Wang F, Fang Q, Zhang M, Chen J, Gu Y (2012) Association between two genetic polymorphisms of the renin–angiotensin-aldosterone system and diabetic nephropathy: a meta-analysis. Mol Biol Rep 39:1293–1303
Wahba IM, Mak RH (2007) Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2:550–562
Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA et al (2002) Overexpression of the renin–angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens 15:381–388
Bagby SP (2004) Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol 15:2775–2791
Nishida Y, Yorioka N, Oda H, Yamakido M (1997) Effect of lipoproteins on cultured human mesangial cells. Am J Kidney Dis 29:919–930
Kobayashi MA, Watada H, Kawamori R, Maeda S (2010) Overexpression of acetyl-coenzyme A carboxylase beta increase proinflammatory cytokines in cultured human renal proximal tubular cells. Clin Exp Nephrol 14:315–324
Riancho JA, Vázquez L, García-Pérez MA, Sainz J, Olmos JM, Hernández JL et al (2011) Association of ACACB polymorphisms with obesity and diabetes. Mol Genet Metab 104(4):670–676
Wacholder S, Chanock M, Garcia-Closas L, El Ghormli N (2004) Rothman assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 96:434–442
Bird TD, Jarvik GP, Wood NW (2001) Genetic association studies. Neurology 57(7):1153–1154
Acknowledgments
The part of this study was funded by Research Society for the Study of Diabetes in India (RSSDI, Grant No. 1228 to VNS). Authors are thankful to study participants and acknowledge the help rendered by Dr. Sanjay Bhadada, Dr. Pinaki Dutta, Dr. Rama Walia, Dr. Sonika, Dr. Anupam, Mr. Ravinder, and Mr. Sunil.
Conflict of interest
No competing interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, V.N., Cheema, B.S., Sharma, R. et al. ACACβ gene (rs2268388) and AGTR1 gene (rs5186) polymorphism and the risk of nephropathy in Asian Indian patients with type 2 diabetes. Mol Cell Biochem 372, 191–198 (2013). https://doi.org/10.1007/s11010-012-1460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1460-2