Abstract
Some of the effects of tumor necrosis factor alpha (TNF-α) are suggested to be mediated by oxidative stress. It has also been reported that dietary supplements of olive oil result in a reduction in LDL, oxidative stress, and blood pressure and these effects are attributed to oleic acid (OA)—a major component of olive oil. The objective of this study was to examine the beneficial effects of OA against TNF-α-induced oxidative stress and cardiomyocytes injury. Isolated cardiomyocytes from adult rat hearts were treated as follows: (A) control; (B) OA (50 μM); (C) TNF-α (10 ng/ml); and (D) TNF-α + OA. After 4 h of the treatment, cells were assessed for oxidative stress, cellular damage, viability, and apoptosis. Cardiomyocytes treated with TNF-α showed a significant increase (P < 0.05) in reactive oxygen species, decrease in the viability of cells, and increase in creatine kinase release. All these TNF-α-induced changes were prevented by OA. TNF-α also caused a significant increase in the expression of apoptotic proteins Bax, Caspase 3 and PARP cleavage, Bnip3, and TGF-β , whereas OA modulated these changes. It is suggested that TNF-α induced oxidative stress mediates cardiomyocyte cell damage which is prevented by OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since heart disease is the leading cause of death globally and costing billions of dollars [1], a better understanding of its basis as well as management is very important. In patients with myocardial infarction (MI), the heart undergoes remodeling characterized by hypertrophy which is a compensatory response to preserve cardiac function [2]. Despite this compensatory response, in some patients there is the occurrence of heart failure [2]. MI induces inflammatory cascade which is associated with the infiltration of mononuclear cells and neutrophils in the infarcted area as well as release of pro-inflammatory cytokines and chemokines [3]. One of the pro-inflammatory cytokines that is elaborated after MI, is tumor necrosis factor alpha (TNF-α), which may play a critical role in cardiac remodeling and heart failure [4, 5].
Protection against cardiovascular diseases in Mediterranean region is suggested to be due to the consumption of unsaturated fatty acid diet which contains high percentage of oleic acid (OA) [6]. OA, a monounsaturated fatty acid, is a potent antioxidant and a major component of olive oil, especially the extra virgin oil [7]. However, OA is also present in many other products including nuts, seeds, and olives [8–10]. Rationale for the beneficial effects of OA is multipronged but strictly anecdotal. A large clinical trial with more than 74,000 women, and a follow up of 20 years, revealed that consumption of Mediterranean food containing OA, significantly reduced the risk for developing heart diseases [11]. The prevention of coronary heart disease by OA is suggested to be due to the suppression of oxidative stress [7]. OA is also protective against heart lipotoxicity by boosting the accumulation of triglycerides [12]. Other benefits of OA include a reduction in blood pressure, lowering of LDL cholesterol, and antimicrobial activity [13]. It is also reported to stimulate peroxisome proliferator-activated receptor (PPARγ) [14] which may inhibit the expression of TNF-α [15].
In order to better characterize the beneficial effects of OA, we investigated in detail the protective role of OA against TNF-α induced oxidative stress, apoptosis, and cell damage using isolated rat cardiomyocytes.
Materials and methods
Isolation of adult rat cardiomyocytes and treatments
All protocols in this study were ethically approved by the University of Manitoba Animal Care Committee and are according to Canadian Council on Animal Care. Adult rat cardiomyocytes were isolated from male Sprague–Dawley rats (250–300 g) and cultured according to methods well established in our laboratory [16, 17].
The viable cardiomyocytes were incubated overnight in M199 medium supplemented with 10 μg/ml gentamicin and 0.25 μg/ml amphotericin B (Invitrogen Canada) and were divided into four groups: (A) control; (B) OA (50 μM) treated; (C) TNF-α (10 ng/ml) treated; and (D) TNF-α (10 ng/ml) + OA (50 μM) treated. In some experiments, 25 μM H2O2 (Sigma) was used as positive control for oxidative stress. OA used in this study was from Sigma and the purity was ≥99 % (GC). For groups A, B, and C, cardiomyocytes were incubated for 4 h and for group D there was a 30 min pretreatment with 50 μM OA followed by 4 h incubation with TNF-α added. These concentrations were based on our pilot studies using TNF-α (0.5–50 ng/ml), OA (50–200 μM), H2O2 (25–100 μM), and using different exposure times (1, 2, 4, 6, 8, 12, 18, and 24 h).
Measurement of ROS production
Assessment of reactive oxygen species (ROS) levels in cardiomyocytes as an indicator of oxidative stress was done using the method described previously [18]. Control and treated cardiomyocytes were washed with warm PBS (37 °C) and then incubated with 10 μM solution of the fluorescent dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Invitrogen) dissolved in warm PBS. Cardiomyocytes were incubated for 30 min at 37 °C and were protected from light. After capturing the fluorescent images of the cardiomyocytes with Olympus BX 51 fluorescent microscope, on average 10 fields per plate, the fluorescent intensity was analyzed using the software (Image Pro Plus).
Assessment of cardiomyocytes viability and damage
Trypan blue: M199 (1:1) were added to control and treated cells for 5 min, and the cells were observed for viability under Olympus microscope equipped with colored Infinity X camera (Lumenera Corporation) and were analyzed using Infinity software. Viable cells excluded the dye and remained colorless, whereas the dead cells retained the blue dye.
Cardiomyocytes damage was assessed indirectly by measuring the creatine kinase (CK) release in the medium after treatment. A commercially available kit for measuring the creatine kinase activity through spectrophotometric assay was used according to the manufacturer specifications (Stanbio Laboratory, Boerne, TX). In brief, after 4 h of treatment, 25 μl of culture medium from control and treated groups were added to 1 ml of prewarmed reconstituted CK reagent and the change in absorbance was recorded at 1 min intervals for 3 min at 340 nm.
Apoptosis and related proteins
For examining DNA fragmentation, one of the hallmarks of apoptosis, control and treated cardiomyocytes were washed with warm PBS and fixed with 4 % paraformaldehyde for 30 min. After fixation, paraformaldehyde was discarded and cardiomyocytes were washed with PBS and incubated with Hoechst 33258 (1 μg/ml) for 10 min protected from light exposure. After staining of cardiomyocytes nuclei, plates were examined using fluorescent microscope (Olympus, BX 51).
Pro-apoptotic proteins Bax, cleaved Caspase 3, cleaved PARP, Bnip3, and TGF-β and anti-apoptotic protein Bcl-xL expression levels were determined by western blotting using specific antibodies (Cell Signaling Technology). For protein analysis, control and treated cardiomyocytes were washed with warm PBS, then scrapped gently from the plates, and homogenized with radioimmunoprecipitation assay buffer (RIPA buffer) containing protease (Roche Diagnostics) and phosphatase inhibitor cocktail (Santa Cruz). Protein concentration in each sample was determined using albumin standards and dye from Bio-RAD Laboratories [19]. Protein samples (35 μg) were subjected to SDS-PAGE at 120 V for 90 min and were transferred on polyvinylidene fluoride (PVDF) (Roche Diagnostics) at 300 mV for 90 min or 30 mV overnight. The protein bound to PVDF membrane was detected using Pierce ECL western blotting substrate and bands were visualized using X-ray films (Thermo Scientific). Proteins bands were quantified using image analysis software (Quantity One, Bio-Rad Laboratories).
Statistical analysis
All experiments were done in duplicates for each treatment group and repeated five times (N = 5). Data are expressed as the mean ± SEM. Groups were compared by one-way analysis of variance (ANOVA), and Bonferroni’s test was performed to identify differences between groups. P value of ≤0.05 was considered significant.
Results
Oxidative stress assessment
Levels of oxidative stress in isolated cardiomyocytes were assessed by the measurement of ROS subsequent to the exposure of TNF-α and these data are shown in Fig. 1. The production of endogenous ROS in TNF-α treated cardiomyocytes was increased significantly to 163 % (P < 0.05). There was no significant difference in ROS production between control and OA-treated cardiomyocytes. However, treatment of cardiomyocytes with OA significantly prevented the TNF-α-induced increase in ROS production. H2O2 (25 μM) was used as a positive control for inducing oxidative stress, and was able to increase ROS significantly to 166 % (P < 0.05), which was also blunted by OA (data not shown).
Effects of the exposure to TNF-α (10 ng/ml), OA (50 μM), and TNF-α + OA for 4 h on ROS generation in adult rat cardiac myocytes using DCFDA dye: Upper panel fluorescence microscope images; lower panel is a fluorescent intensity analysis. H2O2 (25 μM) was used as a positive control. N = 5, *P < 0.05, significantly different from control
Assessment of cell viability and damage
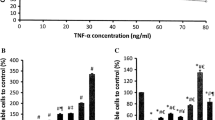
Exposure of cardiomyocytes to TNF-α decreased cell viability significantly (P < 0.05) to 43 % as compared to control (Fig. 2a1, a2). Treatment with OA alone had no effect on cell viability compared to control. However, treatment with OA prevented TNF-α-induced cell death significantly (P < 0.05) (Fig. 2a1, a2). TNF-α increased creatine kinase (CK) to 148 % in the medium from damaged cardiomyocytes compared to control group (Fig. 2b). There was no significant difference between cell leakage from cardiomyocytes treated with OA and the control group. Whereas, OA treatment ameliorated the effect of TNF-α-induced cardiomyocytes damage significantly (P < 0.05) (Fig. 2b).
Apoptosis
Nuclear fragmentation
TNF-α caused a significant increase (P < 0.05) in the number of apoptotic cardiomyocytes to 22.4 % as compared to control value of 7.5 %. There was no significant difference between control and OA-treated group in the number of apoptotic cells. However, treatment with OA prevented the TNF-α induced apoptosis (Fig. 3a, b). H2O2 (25 μM) was used as a positive control and it caused 25.6 % increase in apoptosis.
Apoptosis in isolated cardiac myocytes by Hoechst 33258 staining after cells had been treated with TNF-α (10 ng/ml), OA (50 μM), and TNF-α + OA for 4 h. H2O2 (25 μM) was used as a positive control. Nuclear fragmentation pointed in a. b represents quantitative analysis of apoptotic cells. N = 5, *P < 0.05, significantly different from control
Expression of pro-apoptotic proteins
TNF-α significantly increased (P < 0.05) the expression of pro-apoptotic proteins and these data are shown in (Fig. 4). As compared to control, TNF-α significantly increased the Bax/Bcl-xL ratio (1.53); cleaved Caspase 3 (140 %); cleaved PARP (148 %); Bnip3 (152 %); and TGF-β (150 %). Expression of these pro-apoptotic proteins in control and OA-treated group was comparable without any significant change. However, treatment with OA ameliorated these TNF-α-induced pro-apoptotic proteins expression significantly (P < 0.05).
Western blot analysis of the effects of treatment with TNF-α (10 ng/ml), OA (50 μM), and TNF-α + OA for 4 h in adult rat cardiac myocytes on: a pro-apoptotic Bax and anti-apoptotic Bcl-xL protein expression, b cleaved Caspase 3, c cleaved PARP, d TGF-β expression, and e Bnip3 expression. All expressions of proteins were assessed using arbitrary units. N = 5, *P < 0.05, significantly different from control
Discussion
Mediterranean diet rich in olive oil and thus OA, is known to result in a better cardiovascular health [7]. For example, it has been shown to reduce oxidative stress, enhance the antioxidant capacities in the body [20], reduce damage and dysfunction of endothelium, and enhance its regenerative capacity [21–23]. The diet has also been reported to preserve the left ventricular systolic function [24], reduce carotid artery stiffness [25], and reduce inflammation and risk of cardiovascular diseases [26]. Data in the present study provides evidence that OA-reduced oxidative stress, caused by TNF-α, may provide the cellular basis of some of the beneficial effects of OA.
It has been shown that plasma levels of TNF-α are high in patients with large MI [27]. Ischemia induces production of TNF-α which is associated with myocardial dysfunction and necrosis [28]. It is also reported that TNF-α and oxidative stress increased in patients with heart failure [29, 30]. Although, treatment of patients with TNF-α blockers like infliximab, etanercept, and adalimumab failed to offer protection [31], left ventricular assist devices (LVADs) and cardiac resynchronization therapy (CRT) resulted in a decrease in TNF-α levels in patients with CHF [32].
In heart failure in rats, subsequent to MI, we have reported a significant increase in TNF-α in the early stages of heart failure [5]. Moreover, transgenic mice, overexpressing TNF-α, developed congestive heart failure [33]. We also showed that TNF-α-induced increase in oxidative stress is due to the suppressed expression of antioxidant enzymes in isolated cardiomyocytes [34, 35]. In addition, an increase in ROS through the activation of a cytosolic pathway [36] as well as through a mitochondrial pathway [37] has also been reported. All these studies suggest a causative role of TNF-α in the production of oxidative stress. An increase in cardiomyocyte oxidative stress due to TNF-α exposure was also detected in the present study.
TNF-α caused an upregulation of NFκB and p38 MAPK phosphorylation, and downregulation of ERK ½ phosphorylation which led to activation of apoptotic pathway [17, 35]. In the present study, TNF-α not only increased oxidative stress, but also increased apoptosis as shown by increased expression of pro-apoptotic proteins: Bax/Bcl-xL ratio, cleaved Caspase 3, cleaved PARP, Bnip3, and TGF-β. TNF-α-induced upregulation of Bax has also been shown in previous studies [37, 38]. A balance between pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-xL appears to be critical to determine the cell fate either undergoing apoptosis or survival [39, 40]. In this study, similar cellular changes was seen, when we used H2O2 as a major source of oxidative stress.
In this study, OA was able to mitigate the TNF-α-induced increase in the expression of pro-apoptotic proteins; Bax/Bcl-xL ratio, cleaved Caspase 3, cleaved PARP, Bnip3, and TGF-β. This effect is most likely due to the quenching ability of OA and a reduction in TNF-α-induced oxidative stress. It is known that addition of oleate suppresses palmitate-induced JNK phosphorylation (associated with increased mitochondrial ROS production and apoptosis), mitochondrial DNA damage, decreased cellular ATP, and Caspase 3 cleavage in skeletal muscle cells [41]. OA has also been shown to increase the gene expression of adiponectin in cardiomyocytes which had a protective role against cardiac hypertrophy by a PPARγ-dependent autocrine mechanism [42]. Treatment of human umbilical vein endothelial cells with OA reduced TNF-α-induced ROS significantly, and this effect was not due to increased activity of enzymes governing ROS, but it was suggested to be due to the scavenging of free radicals through its double bond [43]. It is pointed out that at very high concentration of 200 μM, OA also had detrimental effects [44]. However, at lower concentration of 50 μM, OA modulated mitochondria oxidative stress in human umbilical vein endothelial cells (Human ECV-304 cells) via epidermal growth factor receptor-dependent activation of glutathione peroxidase and enhanced ROS degradation [45]. At this low concentration of 50 μM, we also noted a reduction in oxidative stress. That this may be the basis of its beneficial effects, is also supported by the fact that H2O2-induced cardiomyocyte injury was reduced by OA. Among different possibilities discussed here with respect to the potential mechanism of OA protection, its quenching ability and scavenging of the free radicals appear to be supported by our data.
OA prevented the cleavage of both Caspase 3 and PARP, induced by stearic acid on human aortic endothelial cells [46] as well as palmitic acid-induced apoptosis in rat cardiomyocytes [47, 48]. Yamasaki et al. [49] reported that OA prevented apoptosis induced by trans10, cis12 isomer of conjugated linoleic acid through p38 MAP kinase-dependent pathway. ERK inhibition prevented the anti-apoptotic properties of free fatty acids in murine enteroendocrine cell line STC-1, suggesting the importance of ERK activation in the protection from apoptotic cell death [50]. Hoechst test was used in our study to confirm apoptosis induced by TNF-α treatment which was prevented significantly by OA. In this study, cardiomyocytes treated with TNF-α showed leakage of CK, which was prevented significantly by OA. Earlier, we have reported that oxidative stress due to H2O2 increased CK activity [34]. Use of H2O2 as a positive control in this study provided further evidence for the role of oxidative stress in cardiac myocytes injury. Use of Trolox—a water-soluble antioxidant, has also been reported to decrease TNF-α-induced oxidative stress as well as cardiomyocyte injury [17, 35, 51]. Thus, TNF-α-induced oxidative stress appears to be the cause of apoptosis as well as cardiomyocytes membrane injury, which was prevented by OA. Furthermore, such a protection by OA is due to a reduction in oxidative stress.
References
WHO (2011) The top 10 causes of death. In: WHO. http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Accessed 05 Mar 2012
Pfeffer MA, Pfeffer JM, Lamas GA (1993) Development and prevention of congestive heart failure following myocardial infarction. Circulation 87(IV):120–125
Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG (2004) Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164:665–677
Nian M, Lee P, Khaper N, Liu P (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94:1543–1553
Kaur K, Sharma AK, Singal PK (2006) Significance of changes in TNF-alpha and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. Am J Physiol Heart Circ Physiol 291:H106–H113
de Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N (1998) Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med 158:1181–1187
Owen RW, Giacosa A, Hull WE, Haubner R, Wurtele G, Spiegelhalder B, Bartsch H (2000) Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol 1:107–112
Lowry RR, Tinsley IJ (1966) Oleic and linoleic acid interaction in polyunsaturated fatty acid metabolism in the rat. J Nutr 88:26–32
Herrera MD, Perez-Guerrero C, Marhuenda E, Ruiz-Gutierrez V (2001) Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar–Kyoto and spontaneously hypertensive rats. Br J Nutr 86:349–357
Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr 55:171–178
Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB (2009) Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 119:1093–1100
Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100:3077–3082
Waterman E, Lockwood B (2007) Active components and clinical applications of olive oil. Altern Med Rev 12:331–342
Khodadadi I, Griffin B, Thumser A (2008) Differential effects of long-chain fatty acids and clofibrate on gene expression profiles in cardiomyocytes. Arch Iran Med 11:42–49
Jiang C, Ting AT, Seed B (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–86
Kirshenbaum LA, Thomas TP, Randhawa AK, Singal PK (1992) Time-course of cardiac myocyte injury due to oxidative stress. Mol Cell Biochem 111:25–31
Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK (2009) IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res 82:59–66
Swift LM, Sarvazyan N (2000) Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. Am J Physiol Heart Circ Physiol 278:H982–H990
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Yubero-Serrano EM, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Tasset-Cuevas I, Santos-Gonzalez M, Caballero J, Garcia-Rios A, Marin C, Gutierrez-Mariscal FM, Fuentes F, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J (2011) Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age (Dordr) 33:579–590
Fuentes F, Lopez-Miranda J, Perez-Martinez P, Jimenez Y, Marin C, Gomez P, Fernandez JM, Caballero J, Delgado-Lista J, Perez-Jimenez F (2008) Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr 100:159–165
Rallidis LS, Lekakis J, Kolomvotsou A, Zampelas A, Vamvakou G, Efstathiou S, Dimitriadis G, Raptis SA, Kremastinos DT (2009) Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am J Clin Nutr 90:263–268
Marin C, Ramirez R, Delgado-Lista J, Yubero-Serrano EM, Perez-Martinez P, Carracedo J, Garcia-Rios A, Rodriguez F, Gutierrez-Mariscal FM, Gomez P, Perez-Jimenez F, Lopez-Miranda J (2011) Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr 93:267–274
Chrysohoou C, Panagiotakos DB, Aggelopoulos P, Kastorini CM, Kehagia I, Pitsavos C, Stefanadis C (2010) The Mediterranean diet contributes to the preservation of left ventricular systolic function and to the long-term favorable prognosis of patients who have had an acute coronary event. Am J Clin Nutr 92:47–54
Aizawa K, Shoemaker JK, Overend TJ, Petrella RJ (2009) Effects of lifestyle modification on central artery stiffness in metabolic syndrome subjects with pre-hypertension and/or pre-diabetes. Diabetes Res Clin Pract 83:249–256
Esposito K, Ciotola M, Giugliano D (2006) Mediterranean diet, endothelial function and vascular inflammatory markers. Public Health Nutr 9:1073–1076
Maury CP, Teppo AM (1989) Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J Intern Med 225:333–336
Squadrito F, Altavilla D, Zingarelli B, Ioculano M, Calapai G, Campo GM, Miceli A, Caputi AP (1993) Tumor necrosis factor involvement in myocardial ischaemia-reperfusion injury. Eur J Pharmacol 237:223–230
De Biase L, Pignatelli P, Lenti L, Tocci G, Piccioni F, Riondino S, Pulcinelli FM, Rubattu S, Volpe M, Violi F (2003) Enhanced TNF alpha and oxidative stress in patients with heart failure: effect of TNF alpha on platelet O2-production. Thromb Haemost 90:317–325
Tsutamoto T, Wada A, Matsumoto T, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Yamamoto T, Horie H, Sugimoto Y, Kinoshita M (2001) Relationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol 37:2086–2092
Scheinfeld N (2004) A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatol Treat 15:280–294
Orrego CM, Nasir N, Oliveira GH, Flores-Arredondo JH, Cordero-Reyes AM, Loebe M, Youker KA, Torre-Amione G (2011) Cellular evidence of reverse cardiac remodeling induced by cardiac resynchronization therapy. Congest Heart Fail 17:140–146
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM (1997) Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81:627–635
Kaur K, Sharma AK, Dhingra S, Singal PK (2006) Interplay of TNF-alpha and IL-10 in regulating oxidative stress in isolated adult cardiac myocytes. J Mol Cell Cardiol 41:1023–1030
Dhingra S, Sharma AK, Singla DK, Singal PK (2007) p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 293:H3524–H3531
Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, Kim JH (2000) Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem 275:32357–32362
Ghavami S, Eshraghi M, Kadkhoda K, Mutawe MM, Maddika S, Bay GH, Wesselborg S, Halayko AJ, Klonisch T, Los M (2009) Role of BNIP3 in TNF-induced cell death—TNF upregulates BNIP3 expression. Biochim Biophys Acta 1793:546–560
Kim JY, Kim YJ, Lee S, Park JH (2011) BNip3 is a mediator of TNF-induced necrotic cell death. Apoptosis 16:114–126
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Shinoura N, Yoshida Y, Asai A, Kirino T, Hamada H (1999) Relative level of expression of Bax and Bcl-XL determines the cellular fate of apoptosis/necrosis induced by the overexpression of Bax. Oncogene 18:5703–5713
Yuzefovych L, Wilson G, Rachek L (2010) Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 299:E1096–E1105
Amin RH, Mathews ST, Alli A, Leff T (2010) Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARgamma-dependent autocrine mechanism. Am J Physiol Heart Circ Physiol 299:H690–H698
Massaro M, De Caterina R (2002) Vasculoprotective effects of oleic acid: epidemiological background and direct vascular antiatherogenic properties. Nutr Metab Cardiovasc Dis 12:42–51
Krieglstein J, Kewitz T, Kirchhefer U, Hofnagel O, Weissen-Plenz G, Reinbold M, Klumpp S (2010) Damage of guinea pig heart and arteries by a trioleate-enriched diet and of cultured cardiomyocytes by oleic acid. PLoS ONE 5:e9561
Duval C, Auge N, Frisach MF, Casteilla L, Salvayre R, Negre-Salvayre A (2002) Mitochondrial oxidative stress is modulated by oleic acid via an epidermal growth factor receptor-dependent activation of glutathione peroxidase. Biochem J 367:889–894
Harvey KA, Walker CL, Xu Z, Whitley P, Pavlina TM, Hise M, Zaloga GP, Siddiqui RA (2010) Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J Lipid Res 51:3470–3480
de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M (1997) Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38:1384–1394
Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Eppenberger HM, Spinas GA, Donath MY (2001) Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes 50:2105–2113
Yamasaki M, Tachibana H, Yamada A, Ochi Y, Madhyastha H, Nishiyama K, Yamada K (2008) Oleic acid prevents apoptotic cell death induced by trans10, cis12 isomer of conjugated linoleic acid via p38 MAP kinase dependent pathway. In Vitro Cell Dev Biol Animal 44:290–294
Katsuma S, Hatae N, Yano T, Ruike Y, Kimura M, Hirasawa A, Tsujimoto G (2005) Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem 280:19507–19515
Dhingra S, Bagchi AK, Ludke AL, Sharma AK, Singal PK (2011) Akt regulates IL-10 mediated suppression of TNFalpha-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS ONE 6:e25009
Acknowledgments
The study was supported by an operating Grant from the Canadian Institutes of Health Research. P. K. Singal is the holder of the Naranjan S. Dhalla Chair in Cardiovascular Research supported by the St. Boniface Hospital and Research Foundation. Abd Al-Rahman Al-Shudiefat is supported by University of Manitoba Graduate Fellowship/Manitoba Health Research Council Studentship Award.
Conflict of interest
A. Al-Shudiefat, A. Sharma, A. Bagchi, S. Dhingra, and P. Singal have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Shudiefat, A.A., Sharma, A.K., Bagchi, A.K. et al. Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Mol Cell Biochem 372, 75–82 (2013). https://doi.org/10.1007/s11010-012-1447-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1447-z