Abstract
Peroxisome proliferator activated receptor gamma coactivator 1α (PGC-1α) induced by hypoxia regulates mitochondrial biogenesis and oxidative stress. However, the potential role of PGC-1α in hypoxia-promoted proliferation of pulmonary arterial vascular smooth muscle cells (PASMCs) is completely unknown. In this study, we found that hypoxia significantly induced the expression of PGC-1α in cultured PASMCs and activated mitochondrial biogenesis through upregulation of nuclear respiratory factor-1 and mitochondria transcription factor A in a time-dependent manner. Knockdown of PGC-1α by siRNA abrogated hypoxia-induced PASMCs proliferation via the downregulation of PCNA, cyclinA, and cyclinE. Furthermore, we observed that PI3K/Akt signaling pathway was involved in hypoxia induced PGC-1α expression and PASMCs proliferation. Taken together, these datas reveal PGC-1α as the key regulator to mediate mitochondrial biogenesis and the proliferation of PASMCs at an early stage of hypoxic exposure. This process might bring to light a potential adaptive mechanism for PASMCs to minimize hypoxic damage and our novel findings provide new insight into the development of hypoxic pulmonary hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a severe disease of the pulmonary vasculature characterized by elevated pulmonary vascular resistance and progressive right ventricular hypertrophy causing right heart failure and eventually death [1, 2]. Persistent hypoxia is considered as one of the most predominant factors of PAH [3, 4]. The initial event of hypoxic pulmonary hypertension is acute pulmonary vasoconstriction and followed by pulmonary arteries vascular remodeling [5, 6]. In previous study, we have reported that hypoxia-stimulated excessive proliferation and inadequate apoptosis of pulmonary arterial vascular smooth muscle cell (PASMC) are important component of pulmonary vascular remodeling [7, 8]. However, the mechanisms of hypoxia-induced PASMCs proliferation remain largely unknown.
Mitochondria play a critical role in energy metabolism, cell proliferation, and apoptosis activity. An aberrant increase of mitochondrial biogenesis has been implicated in many diseases such as asthma and cancer [9, 10]. In addition, mitochondria biogenesis is stimulated by hypoxia as a response to the hypoxia damage [11]. Recently a variety of evidence obtained on human PAH and experimental PAH (including hypoxia models) clearly demonstrate that PASMCs and endothelial cells in pulmonary hypertensive conditions have impaired mitochondrial function, decreased mitochondrial oxidation, and metabolic shift to glycolysis, which is critical for increased proliferation, survival and pulmonary vascular remodeling [12–15]. However, a mitochondrial biogenic response is mounted. Especially at an early stage of hypoxic exposure the molecular mechanism by which hypoxia-induced mitochondrial biogenesis and dysfunction regulates the proliferation and function of PASMCs remains elusive.
Peroxisome proliferator activated receptor gamma coactivator 1α (PGC-1α) is a 795-amino acid protein, that mediates many metabolic functions through the interaction and activation of a large number of transcription factors including Peroxisome proliferator activated receptor gamma (PPARγ) [16], estrogen receptor-related α [17], myocyte enhancer factor-2 [18], and nuclear respiratory factor (NRFs) [19]. PGC-1α coordinates nuclear and mitochondrial gene expression into a program of mitochondrial biogenesis through regulating the NRF-1 and NRF-2, which transactivate genes for oxidative phosphorylation and mediate mtDNA transcription and replication via mitochondrial transcription factor A (TFAM) [20, 21]. Lack of oxygen can significantly induce expression of PGC-1α which then regulates mitochondrial biogenesis and oxidative stress [22–24]. Interestingly it was shown recently that PGC-1α dependent PPARγ signaling was down-regulated in human and experimental pulmonary hypertension. Furthermore, activation of PPARγ signaling suppressed smooth muscle cell excessive proliferation and migration, thus preventing pulmonary vascular remodeling [25].
Based on these previous studies, we hypothesized that hypoxia induces PGC-1α expression in PASMCs, which then regulates mitochondrial biogenesis and PASMCs proliferation. In this study, we examined the expression of PGC-1α and mitochondrial biogenesis in PASMCs subjected to hypoxia and observed the phenotypes of PASMCs. In addition, we explored the potential signaling pathways involved in the regulation of PGC-1α expression in hypoxic PASMCs. Our results showed for the first time that PGC-1α expression, mitochondrial biogenesis, and proliferation of PASMCs were significantly increased in PASMCs at an early stage of hypoxic exposure, which was markedly reversed by knockdown of PGC-1α. Furthermore, PI3K/Akt signaling pathway was identified to contribute to hypoxia induced PGC-1α expression and PASMCs proliferation.
Materials and methods
Materials
Antibodies against PGC-1α, TFAM, β-actin, PCNA, cyclinA, and cyclinE were purchased from Santa Cruz Biotechnology (CA, USA). Polyclonal antibody against NRF-1 was obtained from cayman (Ann Arbor, MI, USA). LY294002 were provided by Beyotime Institute of Biotechnology (Haimen, China). Enhanced chemiluminescence reagents were purchased from Amersham (Amersham, UK). Superscript First-Stand cDNA Synthesis Kit was obtained from Invitrogen (CA, USA). Bromodeoxyuridine (BrdU) Cell Proliferation Assay Test kit was purchased from Millipore (Billerica, MA, USA). Other regular reagents were from common commercial sources.
Animals
Adult male Wistar rats (180–250 g) obtained from the Experimental Animal Center of Harbin Medical University, which is fully accredited by the Institutional Animal Care and Use Committee (IACUC). The animals were maintained on a 12-h artificial light–dark cycle and housed in separate cages with standard rodent chow and water ad libitum. During our experiments, the rats were anesthetized with 4 % halothane, then chests were opened to take heart and lungs, pulmonary arteries were dissected under a stereo-microscope.
Cell culture and exposure hypoxia
PASMCs were separated according to our previously published protocol [8]. Cells were cultured using DMEM medium containing 20 % fetal bovine serum (FBS). Determined by Trypan Blue exclusion, cell viability was consistently >98 %. PASMCs purity of the primary cultures was confirmed by specific monoclonal antibodies raised against smooth muscle α-actin (Boehringer Mannbeim Germany). Cells were cultured in a humidified incubator with 5 % CO2 at 37 °C as normoxic control. After appropriate treatment, hypoxic group of cells were grown in a chambers equilibrated (3 % O2) for 24, 48, or 72 h, respectively. Passage 2–4 cells were used for all experiments.
RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted from cultured PASMCs using Trizol reagent according to the manufacturer’s instructions. The concentration of RNA was quantified from the optical density measured at 260 nm by ultraviolet spectrophotometry. Total RNA from each sample was reverse transcribed with the Superscript First-Stand cDNA Synthesis Kit (Invitrogen CA, USA). The Quantitative RT-PCR was performed with SYBR Green I on an ABI Prism 7300 sequence detection system (Applied Biosystem, Foster City, CA). β-Actin was used as a control. The specific primers sequences for Real-time Quantitative RT-PCR were designed and synthesized by Shinegene Co, Shanghai shown in Table 1.
Western blot analysis
After specific treatment, cells were washed twice with cold PBS and lysed on ice for 30 min in 0.3 ml lysis buffer (Tris 50 mM, pH7.4, NaCl 150 mM, TritonX-100 1 %, EDTA 1 mM and PMSF 2 mM), centrifugation at 13,500 rpm for 15 min at 4 °C, then the supernatants were collected as total protein, the protein concentrations were determined using the BCA method (Pierce, Rockford, IL, USA). The protocol for Western blot was used as previously described [7].
Electron microscopic study
At a confluence of 70–80 %, PASMCs were cultured in normoxic or hypoxic condition for 24 h after growth arrest. Then, the cells were detached with trypsin and centrifuged at 2,000 rpm 5 min. The cell pellets were fixed in 2 % glutaraldehyde/osmium tetroxide, after dehydration in ethanol and embedded with spurr resin. Thin sections were obtained from a microtome and viewed with a Philips transmission electron microscope. 20 randomly selected areas were photographed at 20,000× magnification.
siRNA transfection
To knockdown PGC-1α expression, PASMCs were transfected using small interfering RNA (accession numbers NM_031347.1), siPGC sequence: 5′-GCUCUUGAGAAUGGAUAUATT-3′ and non-targeted control siRNA (siNC) sequence: 5′-UUCUCCGAACGUGUCACGUTT-3′ which were designed and synthesized by Gene Pharma. Non-targeted control siRNA (siNC) was used to exam and optimize the efficiency of transfection and served as a negative control. siRNA was transfected into PASMCs using X-tremeGene siRNA transfection reagent (Roche, Mannheim, Germany) according to the manufacturer’s instructions. In brief, 2 μg siRNA and 10 μl X-tremeGene siRNA transfection reagent were incubated with serum-free medium for 5 min respectively, and mixed them together. After incubator for 20 min at room temperature, the mixture was added to cells. Following another culture for 6–8 h, cells were washed and subsequently cultured in DMEM containing 5 % FBS for 24 h. The efficiency of silencing was detected by western blot.
MTT assay
PASMCs were cultured in 96 well culture plates and starved for 24 h. Then, each group of cells received appropriate treatment. After 24 h, the cells were incubated with a medium containing the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), with a final concentration at 0.5 mg/ml. Finally, the MTT reaction was terminated via adding 150 μl DMSO into each well and incubation for 10 min at room temperature. The absorbance value was read at 570 nm using a spectrophotometer.
Cell proliferation assay
Cell proliferation was assayed with a commercially available 5-bromo-2′-deoxy-uridine (BrdU) labeling kit (Millipore Billerica, MA, USA) according to the manufacturer’s instruction. In brief, PASMCs were seeded in 96-well plates (about 1 × 105) 24 h, the cells were stopped growth for another 24 h. Then cells were transfected with non-targeted control siRNA (siNC) or targeted small interfering RNA (siPGC), respectively. After a 6-8 h culture, the medium was changed to DMEM containing 5 % FBS and 10 ng/ml BrdU. Then cells were exposed to normoxia or hypoxia for 24 h. After removal of the culture medium, the cells were fixed by the fixation reagent provided in the kit, then the cells were washed and incubated with mouse anti-BrdU monoclonal antibody, washed and added peroxidase goat anti-mouse IgG at room temperature, washed three times, and 100 μl substrates were added to each well and incubated in the dark. Finally, the reaction product was quantified by measurement of the absorbance at 450 nm using a spectrophotometer.
Statistics
The composite data were expressed as means ± SEM Statistical analysis was performed with Student’s t test or one-way ANOVA followed by Dunnett’s test where appropriate. Differences were considered to be significant at p ≤ 0.05.
Result
Hypoxia induced PGC-1α expression in cultured PASMCs
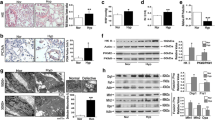
To identify whether hypoxia activates PGC-1α in cultured PASMCs, the mRNA and protein expression levels of PGC-1α were evaluated by real-time Quantitative RT-PCR and Western blot. The results showed that both PGC-1α mRNA and protein levels increased after exposed to hypoxia for 24 h, which continued to elevate until 48 h but then returned to control level at 72 h (Fig. 1). These results suggested that hypoxia induced expression of PGC-1α in cultured PASMCs in a time-dependent manner.
Hypoxia induced PGC-1α expression in cultured rat PASMCs. PGC-1α mRNA expression from PASMCs was measured by real-time Quantitative RT-PCR and at different time points under hypoxia, using β-actin as internal standard (a). Western blots of PGC-1α expression in PASMCs was analyzed after exposing to hypoxia for different time points (b). All of the values are denoted as means ± SEM (*P < 0.05 vs. hypoxia 0 h; # P < 0.05 vs. hypoxia 24 h n = 5). Data shown are representative of at least three independent experiments
Hypoxia activated mitochondrial biogenesis
To determine whether hypoxia promotes mitochondrial biogenesis in cultured PASMCs, the mitochondrial ultra-structure was examined by transmission electron microscope. The results indicated some damaged mitochondria surrounding the healthy ones, including medullary degeneration and vacuolar degeneration (Fig. 2a). By quantitative analysis, an apparently increased number of mitochondria were observed in PASMCs under hypoxia compared with normoxia (Fig. 2b).
Hypoxia activated mitochondrial biogenesis. The number of mitochondria and expressions of mitochondrial biogenesis-related genes were increased in cultured PASMCs exposed to hypoxia. Transmission electron microscope image of sections from cultured PASMCs after normoxia and hypoxia condition. Randomly selected areas were photographed at ×20,000 magnification (a). Quantitative analysis of the number of PASMCs mitochondria was tested in per photomicrograph (b). (*P < 0.05 vs. Normoxia) Nor Normoxia, Hyp hypoxia. Nu nucleus, arrows indicates mitochondria. NRF-1, TFAM mRNA were measured at the indicated times after hypoxia with real-time Quantitative RT-PCR (c and e). Protein expression of NRF1 and TFAM in PASMCs were measured using Western bolt at various time points after hypoxia (d and f). All of the values are denoted as means ± SEM from three or more separate experiments (*P < 0.05 vs. hypoxia 0 h; **P < 0.01 vs. hypoxia 0 h; # P < 0.05 and ## P < 0.01 vs. hypoxia 24 h n = 5)
To confirm the morphological changes we observed, next we detected the expression of nuclear respiratory factor-1 (NRF-1) and mitochondrial transcription factor A (TFAM), which are biomarkers of mitochondrial biogenesis. Real-time Quantitative RT-PCR and Western blot analysis showed that NRF-1 mRNA and protein expression levels increased in a time-dependent manner in PASMCs after exposed to hypoxia, beginning at 24 h and remaining significantly elevated at 48 h then returning to baseline after 72 h (Fig. 2c, d). Strikingly, hypoxia induced up-regulation of TFAM at both mRNA and protein levels after 24 h, and reached to maximum by 72 h (Fig. 2e, f). These data suggested that hypoxia activated mitochondrial biogenesis in PASMCs through upregulation of NRF-1 and TFAM in a time-dependent manner.
PGC-1α was required for hypoxia induced mitochondrial biogenesis in PASMCs
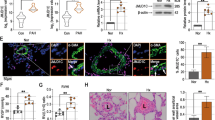
To further test whether PGC-1α is involved in hypoxia-mediated mitochondrial biogenesis and mitochondrial related gene expression in PASMCs, we performed RNA interference technology to knock down PGC-1α. The efficiency of the siRNA was measured by western blot analysis of PGC-1α protein. Our results showed that there were no differences between cells treated with siNC and untreated cells (Blank) or cells treated only with the transfection reagent (Mock). In contrast, PGC-1α small interfering RNA (siPGC) reduced PGC-1α protein expression remarkably compared with siNC (Fig. 3a). The mRNA and protein expression of NRF-1 and TFAM were detected in PASMCs with PGC-1α knocked down. The results showed that hypoxia-induced increase on mRNA and protein expression of NRF-1 and TFAM was significantly blocked by PGC-1α siRNA (Fig. 3b–e), suggesting that hypoxia-induced expression of NRF-1and TFAM was mediated by PGC-1α.
PGC-1α was required for hypoxia induced mitochondrial biogenesis in PASMCs. Western blots analyzed PGC-1α expression in untreated PASMCs (Blank), PASMCs treated with transfection vehicle alone (Mock), non-targeted small interfering RNA control (siNC), and siRNA targeted to PGC-1α (siPGC) (a). (*P < 0.05 vs. siNC n = 6). Knockdown of PGC-1α interfered with NRF1 and TFAM expression induced by hypoxia. NRF1 mRNA (b) and protein (c) expression were analyzed from normoxia with siNC, hypoxia with siNC, and hypoxia with siPGC. Nor Normoxia, Hyp hypoxia. In the same way, the mRNA and protein expressions of TFAM were shown (d, e). All values are denoted as mean ± SEM from three or more independent experiments (*P < 0.05 vs. normoxia with siNC, **P < 0.01 vs. normoxia with siNC, # P < 0.05 vs. hypoxia with siNC n = 6)
Knockdown of PGC-1α inhibited hypoxia induced proliferation of PASMCs
To investigate the role of PGC-1α in PASMCs proliferation at an early stage of hypoxia exposure, we employed loss of function approach to knock down PGC-1α in PASMCs. MTT assay demonstrated that cell viability was significantly increased by hypoxia compared with normoxic condition. However, knockdown of PGC-1α abrogated the cell viability induced by hypoxia (Fig. 4a). Similarly, to assess the population of PASMCs which are actively synthesizing DNA, a BrdU Cell proliferation assay was performed, which showed that hypoxia-induced DNA synthesis was also abolished by PGC-1α siRNA (Fig. 4b). Moreover, we analyzed the expression of proliferating cell nuclear antigen (PCNA) in PASMCs. Western blot analysis showed that hypoxia induced the up-regulation of PCNA, which was abolished by knockdown of PGC-1α (Fig. 4c). These results suggested that hypoxia induced cell proliferation of PASMCs was mediated partly by PGC-1α.
Effect of siPGC on PASMCs proliferation. After 24 h growth arrest, transfected with siRNA, PASMC cell viability was determined by MTT assay. Knockdown of PGC-1α inhibited the increase of cell viability by 24 h hypoxia exposure (a). 5-Bromodeoxyuridine (BrdU) incorporation was measured in a similar experimental protocol, which demonstrated that hypoxia-induced cell DNA synthesis was inhibited by transfecting with siPGC (b). The protein expression of PCNA (c) was analyzed by western blot using PASMCs under normoxia with siNC, hypoxia with siNC and hypoxia with siPGC. Nor normoxia, Hyp hypoxia. All of the values are denoted as mean as mean ± SEM from at least three separate experiments (*P < 0.05 vs. normoxia with siNC, # P < 0.05 vs. hypoxia with siNC n = 6)
Knockdown of PGC-1α inhibited hypoxia promoted cyclin expression
To further investigate the effect of PGC-1α in PASMCs proliferation at an early stage of hypoxia exposure, we sought to determine whether knockdown of PGC-1α in PASMCs affect the cell cycle progression. We checked cell cycle regulators cyclinA and cyclinE expression via western blot. The results showed that hypoxia induced the upregulation of cyclinA and cyclinE in control siRNA transfected PASMCs (siNC) but not in PGC-1α siRNA transfected PASMCs (siPGC) (Fig. 5). These results suggested that PGC-1α contributed to hypoxia-induced proliferation of PASMCs through promoting the cell cycle progression.
PGC-1α knockdown inhibited hypoxia promoted cyclin expression. The protein expression of cyclinA (a) and cyclinE (b) was analyzed by western blot using PASMCs under normoxia with siNC, hypoxia with siNC and hypoxia with siPGC. Nor normoxia, Hyp hypoxia. (*P < 0.05 vs. normoxia with siNC, # P < 0.05 vs. hypoxia with siNC; n = 6)
PI3K/Akt signaling pathway was involved in hypoxia-induced PGC-1α expression and PASMC proliferation
PI3K/Akt signaling play an important role in hypoxia-induce cell survival and proliferation [26]. To clarify whether PI3K/Akt signaling pathway is upstream of PGC-1α, we analyzed the effect of LY294002 (20 μM), an inhibitor of PI3K/Akt on hypoxia induced PGC-1α expression in PASMCs. The results showed that hypoxia-induced upregulation of PGC-1α expression at both mRNA and protein levels was abolished in the presence of LY294002 (Fig. 6a, b). Furthermore, hypoxia-induced upregulation of PCNA, cyclinA and cyclinE were all blocked by LY294002 (Fig. 6c, d).
PI3K/Akt signaling pathway was involved in hypoxia-induced PGC-1α expression and PASMC proliferation. The mRNA and protein expression of PGC-1α in cultured PASMCs from normoxia, hypoxia and hypoxia with LY294002 (20 μM) (a, b) was detected by real-time Quantitative RT-PCR and Western blots. The expressions of PCNA, cycinA and cyclinE in cultured PASMCs by western blot (c, d) were shown. Nor normoxia, Hyp hypoxia, LY LY294002, an inhibitor of PI3K/Akt. (*P < 0.05 vs. normoxia, # P < 0.05 vs. hypoxia; n = 6)
Discussion
PGC-1α as a key regulator of energy metabolism, mitochondrial biogenesis, and oxidative stress, is correlated with oxygen content. In this study we novel findings demonstrate that PGC-1α is significantly up-regulated in a time-dependent manner, which promotes mitochondrial biogenesis and cell proliferation during the early stage of hypoxia exposure. Furthermore, potential signaling pathway PI3K/Akt is found to be associated with the process.
Emerging reports show high expression of PGC-1α in cardiac myocytes and myocardium of TOF patients is hypoxia dependent [24]. Hypoxia stimulates PGC-1α expression, leading to angiogenesis in cultured skeletal muscle cells [23]. And PGC-1α expression is rapidly elevated in brain subcortex by transient hypoxia stimulates and then returned to baseline [27]. However, PGC-1α in hypoxic PASMCs is poorly known, although it was reported recently that PGC-1α dependent PPARγ signaling was implicated in pulmonary vascular remodeling [25]. In the current study, clearly proof is provided to demonstrate a time-dependent enhancement of PGC-1α expression in PASMCs by hypoxia exposure, which peaks after 24 h and decreases to control level at 72 h. However, the reason for prolong hypoxia results in loss of the adaptive increase of PGC-1α is unclear and may be related to oxidative stress or the development of a chronic inflammation.
It has been reported that transgenic over-expression of PGC-1α directly improves mitochondrial biogenesis mediating muscle fiber type determination [28]. In addition, PGC-1 expression is induced in the mouse heart resulting in increased cellular mitochondrial number and stimulated coupled respiration [20]. Given the fundamental role of PGC-1 in mitochondrial biogenesis, we wonder whether hypoxia-induced PGC-1α expression promotes mitochondrial biogenesis in cultured PASMCs. Transmission electron microscopy analysis showed that mitochondrial number was significantly higher in cultured PASMCs under hypoxia condition. As basilic mitochondria transcription factor, TFAM binds to the D loop of mitochondrial DNA and actives mitochondria DNA replication and transcription, which has been realized to be regulated by PGC-1α through coactivation with NRF-1 [21]. Our findings reveal TFAM and NRF-1 are rapidly increased in PASMCs after hypoxia stimulation, while PGC-1α knockdown abolishes hypoxia-induced NRF-1 and TFAM expression. Our results provide evidence that PGC-1α is essential for the induction of mitochondrial biogenesis at early stage of hypoxia in cultured PASMCs.
The acute and chronic responses to hypoxia include the angiogenesis and cell proliferation. These responses may sustain oxygen supply to tissues, whereas excessive proliferation of PASMCs contributes to the pathogenesis of pulmonary vascular remodeling [7]. In the present study, our result demonstrates that PGC-1α knockdown strikingly inhibited hypoxia-induced DNA synthesizing, cell viability, and PCNA expression of PASMCs. Moreover, similar result shows that blockage of PGC-1α inhibits both hypoxia-induced cyclinA and cyclinE expression. Based on these data, we speculate that the up-regulated PGC-1α expression evokes mitochondrial biogenesis and PASMCs proliferation during the early period of hypoxia exposure, which process could be an important adaptive mechanism in attempt to optimize oxygen utilization, meet the energy demand and minimize hypoxic damage. This is a very early event, whereas prolonged challenges to the PASMCs might cause different changes. Some data on human PAH samples and chronic hypoxia models show impairment of mitochondrial and PPARγ signaling. Therefore, it is worthy to investigate the role of PGC-1α in chronic hypoxic pulmonary artery remodeling in future studies.
PI3K/Akt signaling is crucial in many cellular adaptive processes. Hypoxia activated PI3K/Akt pathway is required for the proliferation of human pulmonary vascular smooth muscle cells [26]. Blockade of Akt activity is reported to inhibit PGC-1α activity but had no effect on expression levels of PGC-1α [29]. However, it is suggested that PI3K/Akt is a downstream effector of PGC-1α [30]. To clarify these conflicting results, we employ Akt inhibitor LY294002 to examine its effect on PGC-1α expression in PASMCs. The results show that it inhibits PGC-1α upregulation at both mRNA and protein levels. We also confirm that blockade of PI3K/Akt pathway inhibits the upregulation of cyclin and PCNA by hypoxia in cultured PASMCs. These results suggest that PI3K/Akt pathway positively regulates PGC-1α expression, which then mediates downstream effectors.
In conclusion, our results indicate for the first time that hypoxia induces early PGC-1α expression and mitochondrial biogenesis in cultured PASMCs in a time-dependent manner, and PGC-1α promotes proliferation of PASMCs during the early stage of hypoxia exposure. We also find that PI3 K/AKT pathway is involved in PGC-1α up-regulation and cell proliferation by hypoxia. These findings reveal PGC-1α as the key regulator for mitochondrial biogenesis and the proliferation of PASMCs during the early stage of hypoxia. It might bring to light a potential adaptive mechanism for PASMCs to minimize hypoxic damage and provide new insight into the development of hypoxic pulmonary hypertension.
References
Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX (2004) Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 68(2):75–103
Humbert M, Sitbon O, Simonneau G (2004) Treatment of pulmonary arterial hypertension. N Engl J Med 351(14):1425–1436
Meyrick B, Reid L (1978) The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest 38(2):188–200
Vender RL (1994) Chronic hypoxic pulmonary hypertension. Cell biology to pathophysiology. Chest 106(1):236–243
Stenmark KR, Fagan KA, Frid MG (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99(7):675–691
Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA (2008) Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation 117(16):2114–2122
Ma C, Li Y, Ma J, Liu Y, Li Q, Niu S, Shen Z, Zhang L, Pan Z, Zhu D (2011) Key role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in pulmonary vascular remodeling and vascular angiogenesis associated with hypoxic pulmonary hypertension. Hypertension 58(4):679–688
Wang Z, Tang X, Li Y, Leu C, Guo L, Zheng X, Zhu D (2008) 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmacol 588(1):9–17
Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, Berger P (2007) Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med 204(13):3173–3181
Isidoro A, Martínez M, Fernández PL, Ortega AD, Santamaría G, Chamorro M, Reed JC, Cuezva JM (2004) Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J 378:17–20
Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39(11):3057–3063
Rehman J, Archer SL (2010) A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol 661:171–185
Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman J (2010) Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121(24):2661–2671
Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK (2008) Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294(2):H570–H578
Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM (2010) Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176(3):1130–1138
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839
Huss JM, Kopp RP, Kelly DP (2002) Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem 277(43):40265–40274
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98(7):3820–3825
Jørgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H (2005) Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB 19(9):1146–1148
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98(1):115–124
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91(4):1309–1313
Shoag J, Arany Z (2010) Regulation of hypoxia-inducible genes by PGC-1 alpha. Arterioscler Thromb Vasc Biol 30(4):662–666
Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451(7181):1008–1012
Zhu L, Wang Q, Zhang L, Fang Z, Zhao F, Lv Z, Gu Z, Zhang J, Wang J, Zen K, Xiang Y, Wang D, Zhang CY (2010) Hypoxia induces PGC-1α expression and mitochondrial biogenesis in the myocardium of TOF patients. Cell Res 20(6):676–687
Rabinovitch M (2010) PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol 661:447–458
Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP (2002) PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283(2):L354–L363
Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA (2008) Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28(9):2015–2024
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418(6899):797–801
Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC (2008) VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J 22(9):3264–3275
Han S, Ritzenthaler JD, Sun X, Zheng Y, Roman J (2009) Activation of peroxisome proliferator-activated receptor beta/delta induces lung cancer growth via peroxisome proliferator-activated receptor coactivator gamma-1alpha. Am J Respir Cell Mol Biol 40(3):325–331
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 31071007, 30370578), Science and Technique Foundation of Harbin (Nos. 2008AA3AS097, 2006RFXXS029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jingjing Rao and Jing Li contributed equally to this study.
Rights and permissions
About this article
Cite this article
Rao, J., Li, J., Liu, Y. et al. The key role of PGC-1α in mitochondrial biogenesis and the proliferation of pulmonary artery vascular smooth muscle cells at an early stage of hypoxic exposure. Mol Cell Biochem 367, 9–18 (2012). https://doi.org/10.1007/s11010-012-1313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1313-z