Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor that functions as a transcription factor to regulate adipogenesis and metabolism by binding to PPAR response elements (PPAREs) in the promoter region of various target genes. Activation of PPARγ suppresses smooth muscle cell proliferation and migration. This chapter discusses the potential protective role of PPARγ and its downstream signaling cascades in the development of pulmonary arterial hypertension. Furthermore, the chapter also provides an overview on the cellular and molecular mechanisms involved in PPARγ-mediated inhibitory effect on pulmonary vascular remodeling, a major contributor to the elevated pulmonary vascular resistance in patients with pulmonary arterial hypertension.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Peroxisome proliferator-activated receptor γ (PPARγ) •
- nuclear receptor •
- pulmonary vascular remodeling •
- cell proliferation and migration
1 Introduction
Peroxisome proliferator-activated receptor γ (PPARγ) is one of a family of three nuclear receptors (PPARγ, -α, and -δ) that can function as transcription factors to regulate adipogenesis and glucose metabolism.1-3 On activation by mechanisms not well understood, PPARs heterodimerize with the retinoid X receptor (RXR) and bind to PPAR response elements (PPREs) in regulatory promoter regions of their target genes.4 Many of these are implicated in suppressing smooth muscle cell (SMC) proliferation and migration.4 For example, PPARγ activation blocks platelet-derived growth factor (PDGF) gene expression5 and induces the expression of lipoprotein-like receptor protein 1 (LRP1),6 the receptor necessary for apolipoprotein (Apo) E-mediated suppression of PDGF-BB signaling7,8 as discussed in this chapter. Moreover, PPARγ activation can induce apoptosis of SMCs by phosphorylation of the retinoblastoma (RB) gene9 and by increasing the proapoptotic protein Gadd 45.10 PPARs can also interact with signaling molecules to regulate gene expression independent of DNA binding. For example, PPARγ impairs phosphorylation (i.e., activation) of extracellular-regulated kinase (ERK),11,12 a mitogen-activated protein kinase (MAPK), downstream of PDGF-BB/PDGFR-β signaling. Moreover, activated PPARγ stabilizes the cyclin-dependent kinase inhibitor p27KIP19 and inhibits telomerase activity,13 retinoblastoma protein phosphorylation,9 and cell cycle progression associated with vascular SMC proliferation.9 By blocking important pathways downstream of activated PDGFR-β (i.e., phosphoinositol-3-kinase (PI3K))14 PPARγ agonists can also cause apoptosis of proliferating vascular cells.4,15 In addition, it is known that PPARγ ligands impair production of matrix metalloproteinases16 that can be activated by elastase.17 Our group has shown that inhibition of this proteolytic cascade not only prevents but also reverses advanced fatal pulmonary artery hypertension (PAH) in rats.18
In endothelial cells (ECs), PPARγ activation reduces levels of endothelin 1 (ET-1)19 and the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA),20,21 factors that are implicated in both insulin resistance and in the pathobiology of PAH.21 PPARγ has anti-inflammatory properties that include suppression of factors implicated in PAH, such as vascular cell adhesion molecule (VCAM), interleukin 6,22,23 fractalkine,24,25 and monocyte chemoattractant protein 1.26 PPARγ also protects ECs against apoptosis27,28 and may also promote EC proliferation and migration through production of endothelial nitric oxide synthase (eNOS)29 as well as hemoxygenase (HO) 1.30 In contrast, HO-1 represses SMC proliferation,30 consistent with PPARγ-mediated repression of proliferation and migration of arterial SMCs in culture31,32 and in animal models.33
Thus, extrapolating from data in systemic vascular disease2 suggests that impaired activation of PPARγ transcriptional targets could lead to the pathology of PAH. This is reinforced by studies showing that the levels of PPARγ and its putative transcriptional target ApoE are reduced on complementary DNA (cDNA) microarrays from lung tissues and vessels in patients with PAH.34,35 There is supporting evidence that links PPARγ with transcription of ApoE. A functional PPRE is present in the ApoE promoter,36 conditional disruption of the PPARγ gene in mice results in decreased ApoE expression in macrophages,37 and PPARγ activation leads to ApoE messenger RNA (mRNA) expression and protein secretion in an adipocyte cell line.38
2 Insulin Resistance, Pulmonary Hypertension, and PPARγ Agonist Treatment
Mice that are null for ApoE are made insulin resistant by being fed a high-fat diet. We showed that these mice have PAH in association with abnormal muscularization of distal arteries, and that the muscularization could be reversed following treatment with rosiglitazone, a PPARγ agonist39 (Fig. 29.1). It was interesting that the female cohort of ApoE-/- mice had less-severe PAH as judged by lower levels of right ventricular systolic pressure, right ventricular hypertrophy, and muscularized distal arteries. We attributed this phenotype to the fact that the females had higher levels of adiponectin. The mechanism appears to be related to the fact that testosterone inhibits secretion of adiponectin,40 and adiponectin can sequester PDGF.41 We subsequently showed that adiponectin, a target of PPARγ-mediated transcriptional activity in adipocytes can repress pulmonary artery SMC (PASMC) proliferation in response to growth factors as does ApoE (Fig. 29.2).
Four-week treatment with the PPARγ agonist rosiglitazone reverses PAH, increases plasma adiponectin, and induces insulin sensitivity. Measurements of plasma adiponectin (a), right ventricular systolic pressure (RVSP) (b), blood glucose (c), right ventricular hypertrophy (RVH) (d), plasma insulin (e), and muscularization of alveolar wall arteries (f). Nineteen-week-old male C57Bl/6 and ApoE-/- mice, all on high fat (HF) diet for 15 weeks, were used. Bars represent mean ± standard error (SEM) (n = 4-16 as indicated in column graphs). *P < 0.05; **P < 0.01; and ***P < 0.001. Reproduced with permission39
Recombinant ApoE (a) and adiponectin (b) inhibit PDGF-BB-induced (20 ng/ml) proliferation of murine PASMCs harvested from both C57Bl/6 and ApoE-/- mice. Bars represent mean ± SEM (n = 3). *P < 0.05; **P < 0.01; and ***P < 0.001. Reproduced with permission39
In unpublished data from our group, we have found that older mice that are null for ApoE also develop PAH in the absence of a high-fat diet or insulin resistance. We speculate that this is related to the loss of the protective effect of ApoE in suppressing episodic PDGF-BB-mediated SMC proliferation over time. It has been shown that ApoE can bind to low-density LRP1, also a target of PPARγ-mediated gene transcription.6 When this occurs, LRP1 targets the PDGFRβ for endocytosis,42-44 repressing its function as an SMC mitogen. However, in contrast to its adverse effects as a smooth muscle mitogen, PDGF is also important in normal cell viability, in maintaining pericytes, and in the prevention of vascular endothelial growth factor (VEGF) overexpression and aberrant angiogenesis.45 So, it could be proposed that PPARγ agonists may “fine-tune,” allowing PDGF-mediated EC survival and pericyte recruitment while repressing aberrant angiogenesis and abnormal muscularization of distal vessels as well as proliferation of SMCs.
In light of these experimental studies and in keeping with clinical observations related to obesity in the population of patients with PAH, we investigated whether insulin resistance was prevalent in patients with PAH. Studies reported by Zamanian et al.46 indicated a significantly higher proportion of female patients with PAH and insulin resistance when compared with the general population (45.7 vs. 21.5%), as judged by an abnormal elevation in the ratio of triglycerides to high-density lipoproteins.46 It is of further interest that this high evidence of insulin resistance did not correlate with obesity as judged by the body mass index (BMI) or relate to the hemodynamic severity of the disease but was associated with a poorer 6-month event-free survival (58 vs. 79%) (Fig. 29.3).
Kaplan-Meier 6-month event-free survival curve in pulmonary arterial hypertension (PAH) females. Insulin-sensitive (solid line) PAH females had significantly better outcome compared with their insulin-resistant (dashed line) counterparts (79% vs. 58%; P < 0.05). Events were defined as death, transplantation, or acute hospitalization due to PAH exacerbation or right heart failure. Reproduced with permission46
3 PPARγ and the Bone Morphogenetic Protein Pathway
Mutations in bone morphogenetic protein receptor (BMPR) II that cause loss of function of the receptor are associated with familial and sporadic idiopathic PAH47-49 and reduced expression of BMPR-II has been related to PAH regardless of etiology.50 It was therefore of interest that in studies investigating transcription factors that are regulated by signaling via BMPR-II, we identified PPARγ as a target.51 We showed enhanced DNA binding of PPARγ following stimulation of PASMCs with bone morphogenetic protein (BMP) 2. We then showed that the ability of BMP2 to repress PDGF-BB-mediated PASMC proliferation depended on PPARγ (Fig. 29.4). Most interesting was the observation that loss of the function of BMPR-II to repress PDGF-BB-mediated proliferation could be rescued by a PPARγ agonist (Fig. 29.4). We then showed that BMP2-mediated inhibition of PDGF-BB-induced SMC proliferation requires not only activation of PPARγ but also that of its target of transcription, ApoE. We showed that both PPARγ and ApoE act downstream of BMP2/BMPR-II in human and murine PASMCs and prevent their proliferation in response to PDGF-BB. Bone morphogenetic protein-2 (BMP2)-mediated PPARγ activation occurs earlier than Smad1/5/8 phosphorylation and therefore appears to be independent of this established signaling axis downstream of BMPR-II. The BMPR-II ligand BMP2 induces a decrease in nuclear phospho-ERK, and rapid nuclear shuttling and DNA binding of PPARγ, whereas PDGF-BB has the opposite effects. Both BMP2 and the PPARγ agonist rosiglitazone stimulate production and secretion of ApoE in PASMCs (Fig. 29.5). Moreover, BMP2-mediated suppression of PDGF-BB-induced proliferation was absent in SMCs from a patient with a BMPR-II mutation and PAH, but this inhibition could be restored with a rosiglitazone (Fig. 29.6).
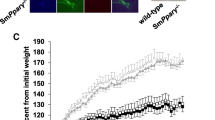
(a) PASMCs were seeded at 2.5 × 104 cells per well of a 24-well plate in 500 μl of growth medium and allowed to adhere overnight. The cells were washed with phosphate-buffered saline (PBS) prior to the addition of starvation media (0.1% fetal bovine serum [FBS]) and incubated for 24 h (murine pulmonary artery smooth muscle cells (PASMCs)) or 48 h human PASMC (HPASMCs) and then stimulated with PDGF-BB (20 ng/ml) for 72 h. BMP-2 (10 ng/ml) was added to quiescent cells 30 min prior to PDGF-BB stimulation. The PPARγ antagonist GW9662 (GW; 1 μM) was added 24 h prior to the addition of BMP2. Cells were finally washed twice with PBS, trypsinized, and counted in a hemocytometer (4 counts per well). Cell numbers in controls at time points 0 (CON) and 72 h were not significantly different. (b) Littermates, littermate control PASMCs; SMC PPARγ-/-, PASMCs isolated from SM22α Cre PPARγflox/flox mice. Bars represent mean ± SEM, n = 4 in (a) and 3 in (b). *P < 0.05; **P < 0.01; ***P < 0.001 as indicated; analysis of variance (ANOVA) with Bonferroni’s multiple-comparison test. Reproduced with permission51
Model: A novel antiproliferative BMP2/PPARγ/ApoE axis protects against PAH. This schema incorporates the findings described in our chapter and the literature to date as discussed. Fig. 29.5 (continued) (a) BMP2 inhibits SMC proliferation via PPARγ and ApoE. ApoE impairs PDGF-BB/MAPK signaling by binding to low-density lipoprotein (LDL) receptor-related protein (LRP), thereby initiating endocytosis and degradation of the LRP/PDGFR-β/PDGF-B complex. PPARγ induces LRP and other growth-inhibitory/proapoptotic genes in SMCs and inhibits cell cycle and other growth-promoting genes, such as telomerase, cyclin D1, and retinoblastoma protein. Moreover, PPARγ induces phosphatases that can directly inactivate phospho-ERK. (b) BMPR-II dysfunction promotes SMC proliferation and survival in PAH. Heightened PDGF-BB signaling leading to SMC proliferation is a key clinical feature of PAH. Deficiency of both ApoE and LRP enhances mitogenic PDGF-BB/MAPK signaling. Loss-of-function mutations in the BMPR-II gene will decrease endogenous PPARγ activity, leading to unopposed MAPK signaling, SMC proliferation and survival, and ultimately development of PAH. TF transcription factor. (c) PPARγ agonists can rescue BMPR-II dysfunction and reverse PAH. PPARγ agonists such as rosiglitazone or pioglitazone might reverse SMC proliferation and vascular remodeling in PAH patients with or without BMPR-II dysfunction via induction of ApoE and other growth-inhibitory/proapoptotic genes (as indicated) and through repression of growth-promoting genes (not shown). Reproduced with permission51
Antiproliferative effects of BMP2 and the PPARγ agonist rosiglitazone on PDGF-BB-induced proliferation of human wild-type and BMPR-II mutant PASMCs. Control PASMCs were isolated from surgical resection specimens derived from patients undergoing lobectomy or pneumonectomy for suspected lung tumor. Additional peripheral pulmonary arteries (<1- to 2-mm external diameter) were obtained from a patient undergoing heart-lung transplantation for familial PAH (FPAH) and known to harbor a mutation (W9X) in BMPR-II. The nature of the BMPR-II mutation, cell isolation, culture techniques, and cell counts is the same as shown in Fig. 29.1. HPASMCs were incubated for 48 h in starvation media (0.1% FBS) and then stimulated with PDGF-BB (20 ng/ml) for 72 h. BMP2 (10 ng/ml) or rosiglitazone (1 μM) were added to quiescent cells 30 min prior to PDGF-BB stimulation. Bars represent mean ± SEM (n = 3). **P < 0.01; ***P < 0.001 as indicated; ANOVA with Bonferroni’s multiple-comparison test. The number of PDGF-BB-stimulated cells was significantly higher than that of untreated control cells (P < 0.001). Reproduced with permission51
To determine whether, in addition to ApoE, other targets of PPARγ-mediated transcription in PASMCs were essential to inhibit the development of PAH, we made a mouse in which SM22-driven Cre was used to delete critical exons of a floxed PPARγ. This mouse had spontaneous PAH in the absence of a high-fat diet in association with muscularized distal arteries and right ventricular hypertrophy. Moreover, the pulmonary hypertensive response to chronic hypoxia is exaggerated in this mouse (Fig. 29.7).
PAH in mice with targeted deletion of PPARγ in SMCs. Thirteen- to 15-week-old mice underwent right ventricular (RV) catheterization, followed by organ harvest. (a) RVSP measurements. (b) Right ventricular hypertrophy (RVH), measured as ratio of the weight of the RV to that of the left ventricle (LV) plus septum (RV/LV + S). (c) Muscularization of alveolar wall arteries (Musc. Arteries Alv. Wall). (d) Representative photomicrographs of lung tissue (stained by Movat pentachrome) of 15-week-old mice showing a typical nonmuscular peripheral alveolar artery in a littermate control mouse. (e) A similar section in the SM22α Cre PPARγflox/flox (SMC PPARγ-/-) mouse shows an alveolar wall artery surrounded by a rim of muscle. (f-i) Immunohistochemistry in serial lung tissue sections from littermate control (CON) and SMC PPARγ-/- mice stained for smooth muscle α-actin (a-SMA) (f, g) and proliferating cell nuclear antigen (PCNA; h and i). Arrows in (i) indicate enhanced PCNA staining in PASMCs. Bars represent mean ± SEM (n = 5). ***P < 0.001 vs. control; unpaired two-tailed t test. Reproduced with permission51
We have bred the Tie2-expressing Cre mouse with the mouse in which PPAR is floxed. Our unpublished studies revealed that this mouse has a mild form of pulmonary hypertension under room air conditions but fails to show a heightened response to hypoxia. The mechanism does not appear to be related to ApoE but is associated with heightened signaling through PDGFRβ. While chronic hypoxia does not result in an exaggeration of PAH in these mice with EC deletion of PPARγ, reversal of PAH following exposure to chronic hypoxia is impaired.
Studies have been carried out in which PPARγ agonists have been used to inhibit and reverse hypoxia-induced PAH. If is of interest that PPARγ agonists can completely reverse structural changes in response to chronic hypoxia but not the elevation in pulmonary arterial pressure.52
PPARγ agonists appear to act favorably to facilitate suppression of proliferation in PASMCs, but it will be important to determine whether they also support endothelial growth and stability because our studies in cultured cells suggest that certain agents could impede PPARγ-mediated endothelial gene regulation.
References
He W, Barak Y, Hevener A et al (2003) Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A 100:15712-15717
Hevener AL, He W, Barak Y et al (2003) Muscle-specific PPARγ deletion causes insulin resistance. Nat Med 9:1491-1497
Lehrke M, Lazar MA (2005) The many faces of PPARγ. Cell 123:993-999
Marx N, Duez H, Fruchart JC, Staels B (2004) Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res 94:1168-1178
Zhang J, Fu M, Zhao L, Chen YE 2002) 15-Deoxy-prostaglandin J2 inhibits PDGF-A and -B chain expression in human vascular endothelial cells independent of PPARγ. Biochem Biophys Res Commun 298:128-132
Gauthier A, Vassiliou G, Benoist F, McPherson R (2003) Adipocyte low density lipoprotein receptor-related protein gene expression and function is regulated by peroxisome proliferator-activated receptor γ. J Biol Chem 278:11945-11953
Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J (2003) LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300:329-332
Newton CS, Loukinova E, Mikhailenko I et al (2005) Platelet-derived growth factor receptor-β (PDGFR-β) activation promotes its association with the low density lipoprotein receptor-related protein (LRP). Evidence for co-receptor function. J Biol Chem 280:27872-27878
Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE (2000) Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1 → S transition in vascular smooth muscle cells. J Biol Chem 275:22435-22441
Bruemmer D, Blaschke F, Law RE (2005) New targets for PPARγ in the vessel wall: implications for restenosis. Int J Obes Relat Metab Disord 29:S26-S30
Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL (2006) PPAR-γ inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol 290:H390-H397
Wakino S, Kintscher U, Liu Z et al (2001) Peroxisome proliferator-activated receptor γ ligands inhibit mitogenic induction of p21Cip1 by modulating the protein kinase Cδ pathway in vascular smooth muscle cells. J Biol Chem 276:47650-47657
Ogawa D, Nomiyama T, Nakamachi T et al (2006) Activation of peroxisome proliferator-activated receptor γ suppresses telomerase activity in vascular smooth muscle cells. Circ Res 98:e50-e9
Vantler M, Caglayan E, Zimmermann WH, Baumer AT, Rosenkranz S (2005) Systematic evaluation of anti-apoptotic growth factor signaling in vascular smooth muscle cells. Only phosphatidylinositol 3′-kinase is important. J Biol Chem 280:14168-14176
Bruemmer D, Yin F, Liu J et al (2003) Regulation of the growth arrest and DNA damage-inducible gene 45 (GADD45) by peroxisome proliferator-activated receptor γ in vascular smooth muscle cells. Circ Res 93:e38-e47
Worley JR, Baugh MD, Hughes DA et al (2003) Metalloproteinase expression in PMA-stimulated THP-1 cells. Effects of peroxisome proliferator-activated receptor-γ (PPARγ) agonists and 9-cis-retinoic acid. J Biol Chem 278:51340-51346
Nagase H, Enghild J, Suzuki K, Salvesen G (1990) Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl) mercuric acetate. Biochemistry 29:5783-5789
Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M (2000) Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6:698-702
Martin-Nizard F, Furman C, Delerive P et al (2002) Peroxisome proliferator-activated receptor activators inhibit oxidized low-density lipoprotein-induced endothelin-1 secretion in endothelial cells. J Cardiovasc Pharmacol 40:822-831
Wakino S, Hayashi K, Tatematsu S et al (2005) Pioglitazone lowers systemic asymmetric dimethylarginine by inducing dimethylarginine dimethylaminohydrolase in rats. Hypertens Res 28:255-262
Kielstein JT, Bode-Boger SM, Hesse G et al (2005) Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 25:1414-1418
Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE (2000) Inflammatory mechanisms in Alzheimer’s disease: inhibition of β-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. J Neurosci 20:558-567
Humbert M, Monti G, Brenot F et al (1995) Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151:1628-1631
Imaizumi T, Matsumiya T, Tamo W et al ( 2002) 15-Deoxy-D12,14-prostaglandin J2 inhibits CX3CL1/fractalkine expression in human endothelial cells. Immunol Cell Biol 80:531-536
Balabanian K, Foussat A, Dorfmüller P et al (2002) CX3C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 165:1419-1425
Ikeda Y, Yonemitsu Y, Kataoka C et al (2002) Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 283:H2021-H2028
Gensch C, Clever YP, Werner C, Hanhoun M, Böhm M, Laufs U (2007) The PPAR-γ agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis 192:67-74
Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM (2001) Biphasic effects of 15-deoxy-δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol 21:1846-1851
Cho DH, Choi YJ, Jo SA, Jo I (2004) Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) γ-dependent and PPAR γ-independent signaling pathways. J Biol Chem 279:2499-2506
Kronke G, Kadl A, Ikonomu E et al (2007) Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27:1276-1282
Goetze S, Xi XP, Kawano H et al (1999) PPARγ-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol 33:798-806
Benson S, Wu J, Padmanabhan S, Kurtz TW, Pershadsingh HA (2000) Peroxisome proliferator-activated receptor (PPAR)-γ expression in human vascular smooth muscle cells: inhibition of growth, migration, and c-fos expression by the peroxisome proliferator-activated receptor (PPAR)-γ activator troglitazone. Am J Hypertens 13:74-82
Law RE, Goetze S, Xi XP et al (2000) Expression and function of PPARγ in rat and human vascular smooth muscle cells. Circulation 101:1311-1318
Ameshima S, Golpon H, Cool CD et al (2003) Peroxisome proliferator-activated receptor γ (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92:1162-1169
Geraci MW, Moore M, Gesell T et al (2001) Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 88:555-562
Galetto R, Albajar M, Polanco JI, Zakin MM, Rodriguez-Rey JC (2001) Identification of a peroxisome-proliferator-activated-receptor response element in the apolipoprotein E gene control region. Biochem J 357:521-527
Akiyama TE, Sakai S, Lambert G et al (2002) Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and ApoE in macrophages and reduced cholesterol efflux. Mol Cell Biol 22:2607-2619
Yue L, Rasouli N, Ranganathan G, Kern PA, Mazzone T (2004) Divergent effects of peroxisome proliferator-activated receptor γ agonists and tumor necrosis factor α on adipocyte ApoE expression. J Biol Chem 279:47626-47632
Hansmann G, Wagner RA, Schellong S et al (2007) Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-γ activation. Circulation 115:1275-1284
Xu A, Chan KW, Hoo RL et al (2005) Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 280:18073-18080
Wang Y, Lam KS, Xu JY et al (2005) Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280:18341-18347
Swertfeger DK, Bu G, Hui DY (2002) Low density lipoprotein receptor-related protein mediates apolipoprotein E inhibition of smooth muscle cell migration. J Biol Chem 277:4141-4146
Boucher P, Gotthardt M (2004) LRP and PDGF signaling: a pathway to atherosclerosis. Trends Cardiovasc Med 14:55-60
Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J (2002) Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem 277:15507-15513
Wilkinson-Berka JL, Babic S, De Gooyer T et al (2004) Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol 164:1263-1273
Zamanian RT, Hansmann G, Snook S et al (2009) Insulin resistance in pulmonary arterial hypertension. Eur Respir J 33:318-324
Lane KB, Machado RD, Pauciulo MW et al (2000) Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 26:81-84
Deng Z, Morse JH, Slager SL et al (2000) Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67:737-744
Machado RD, Aldred MA, James V et al (2006) Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 27:121-132
Atkinson C, Stewart S, Upton PD et al (2002) Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105:1672-1678
Hansmann G, de Jesus Perez VA, Alastalo TP et al (2008) An antiproliferative BMP-2/PPARγ/ApoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118:1846-1857
Crossno JT Jr, Garat CV, Reusch JE et al (2007) Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292:L885-L897
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Humana Press, a part of Springer Science+Business Media, LLC
About this paper
Cite this paper
Rabinovitch, M. (2010). PPARγ and the Pathobiology of Pulmonary Arterial Hypertension. In: Yuan, JJ., Ward, J. (eds) Membrane Receptors, Channels and Transporters in Pulmonary Circulation. Advances in Experimental Medicine and Biology, vol 661. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-500-2_29
Download citation
DOI: https://doi.org/10.1007/978-1-60761-500-2_29
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-499-9
Online ISBN: 978-1-60761-500-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)