Abstract
Melatonin is an indoleamine secreted by the pineal gland that shows multiple tasks. This ubiquitously acting free radical scavenger has recently been shown to stimulate the production of reactive oxygen species (ROS) in tumour cells, making them undergo apoptosis, whilst it prevents apoptosis in healthy cells. The mechanisms by which melatonin exerts these dual actions are, however, not yet clearly understood. Thus, the aim of this study was to further investigate how melatonin can enhance oxidative stress-induced apoptosis in a leukaemia cell line. The results show that melatonin increased the apoptotic effects of H2O2 in human myeloid HL-60 cells as assessed by cellular viability, mitochondrial permeability transition induction, mitochondrial membrane depolarization, ROS generation, caspases 3, 8 and 9 activity, phosphatidylserine externalization, and DNA fragmentation techniques. When healthy leucocytes were exposed to H2O2, melatonin increased the viability of the cells. Taken together, the findings indicate that melatonin is a potential physiological tool capable of protecting healthy cells from chemotherapy-induced ROS production as well as inducing tumour cell death. Because cancer cells manifest increased oxidative stress as a result of their elevated metabolism, the use of melatonin may be useful in impairing their ROS buffering capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin is an indolamine mainly synthesized by the pineal gland. It possesses a wide spectrum of biological effects and is ubiquitously distributed from unicells to human [1, 2]. An increasing number of studies have reported the important role of melatonin in preventing apoptosis in healthy cells [3]. Evidence shows that melatonin decreases apoptosis in healthy cells because of its antioxidant and free radical scavenging abilities [4, 5]. However, the mechanisms by which melatonin exerts its oncostatic actions are not fully understood yet. Surprisingly, melatonin is able to stimulate reactive oxygen species (ROS) production in tumour cells [6, 7], making them undergo apoptosis [8]. However, this remains controversial since some reports claim that melatonin is able to prevent apoptosis of tumour cells both in vitro [9, 10] and in vivo [11, 12]. Melatonin has been reportedly shown to promote apoptotic cell death through the generation of ROS in several cancer cells including human myeloid HL-60, CMK, Jurkat, Molt-4 tumour cells [7, 13] lymphoma cells [14] and HT-29 human colorectal cancer cells [15]. Moreover, the treatment with melatonin reduced the antioxidant defenses in tumour cells depleting glutathione and increasing ROS production [16].
It is well known that some of the most common chemotherapeutic agents induce ROS generation, increasing their toxicity to the cells. This is the case of hydrogen peroxide (H2O2) [17]. Since some tumours develop resistance to ROS induced by chemotherapeutics [18] and taking into account the dual antioxidant and pro-oxidant actions of melatonin in normal and tumour cells, respectively, this molecule could be used in chemotherapy treatments to induce ROS and increase their effects within the tumour cell, protecting normal cells from ROS-induced cell death. The rationale behind is that anticancer agents simultaneously applied with melatonin seem to cause less damage in normal cells [19] and to enhance the efficacy of the treatment [20], although little is known about this issue [21].
Thus, the aim of this study was to further investigate how melatonin can enhance oxidative stress-induced apoptosis in a leukaemia cell line. For that purpose, we have investigated the effects of melatonin on H2O2-induced apoptosis in human myeloid HL-60 cells in culture, analysing cellular viability, mitochondrial permeability transition (MPT) induction, mitochondrial membrane depolarization, ROS generation, caspases 3, 8 and 9 activity, phosphatidylserine (PS) externalization and DNA fragmentation.
Materials and methods
Materials
HL-60 15-12 cell lines (ECACC No 88120805) were purchased from The European Collection of Cell Cultures (ECACC) (Dorset, U.K.). Foetal bovine serum (FBS) and penicillin/streptomycin were obtained from HyClone (Aalst, Belgium). l-Glutamine and RPMI 1640 medium were obtained from Cambrex (Verviers, Belgium). Melatonin, N-acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (AC-DEVD-AMC) and Ficoll-Histopaque separating medium were obtained from Sigma (Madrid, Spain). N-acetyl-Leu-Glu-His-Asp-7-amido-4-trifluoromethylcoumarin (AC-LEHD-AMC), Z-Asp-Glu-Val-Asp-chloromethylketone (Z-DEVD-CMK), N-acetyl-Leu-Glu-His-Asp-chloromethylketone (Ac-LEHD-CMK), N-acetyl-Leu-Glu-Thr-Asp-AFC (Ac-LETD-AFC) and Z-Ile-Glu-Thr-DL-Asp-fluoromethylketone (Z-IETD-FMK) were obtained from Bachem (Bubendorf, Switzerland). Dihydrorhodamine-123 (DHR-123) and tetramethylrhodamine methyl ester (TMRM) were obtained from Molecular Probes (Oregon, USA). In situ cell death detection kit was obtained from Roche Diagnostics GmbH (Mannheim, Germany). Annexin binding buffer, propidium iodide (PI) and annexin V-FITC were obtained from Immunostep (Salamanca, Spain). Hoechst 33342 was purchased from Calbiochem (San Diego, CA, USA). All other reagents were of analytical grade.
Cellular culture
HL-60 cells are a human promyelocytic leukaemia cell line. Cells were grown in RPMI 1640 medium supplemented with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 1.25% DMSO at 37°C under a humidified condition of 95% air and 5% CO2. Cells were routinely plated at a density of 3 × 105 cells/ml into fresh flasks, and the viability was <95% in all the experiments as assayed by the trypan-blue exclusion method. Cells were pretreated with 1 mM melatonin for 16 h and then treated with 500 μM H2O2 for 2 h at 37°C, under 5% CO2. Melatonin was dissolved in absolute ethanol to give a 200 mM stock solution which was further diluted in RPMI 1640 medium to yield a 1-mM melatonin concentration resulting in a 0.5% ethanol concentration after being added to the cells. This concentration of ethanol did not modify the cell viability, and it was used as control value.
Human leucocytes isolation
Venous blood was drawn from healthy volunteers (20–55 year-old) of both genders under informed consent according to a procedure approved by Local Ethical Committees and in accordance with the Declaration of Helsinki. Human leucocytes were separated from whole blood using Ficoll-Histopaque density centrifugation. After centrifugation at 600×g for 30 min, peripheral blood mononuclear cells were isolated from the Histopaque-1077/1119 upper interphase and maintained in RPMI-1640 medium for 1 h to allow the adherence of monocytes so as to obtain a pure lymphocyte preparation, as previously described [22]. Similarly, peripheral blood polymorphonuclear cells were harvested from the lower interphase, and residual erythrocytes were then lysed by short treatment of neutrophil pellet with an ice-cold isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4) so as to get a neutrophil-enriched preparation, as previously described [22]. Cell purity was routinely above 98 and 97% in lymphocytes and neutrophils, respectively, after Diff-Quick staining. Once purified, both neutrophils and lymphocytes were collected and washed in phosphate-buffered saline (PBS) and centrifuged at 480×g for 15 min. The supernatant was then discarded, and the cell pellet was gently resuspended in RPMI-1640 medium.
Cell viability assay
The metabolic activity of the cells was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described [7]. In brief, cells were seeded in a 96-well plate at a density of 5 × 105 cells per well. Then, cells were treated with melatonin at the indicated doses and times. After the treatments, the medium was removed and MTT was added into each well, and then incubated for 60 min at 37°C. Treatments were carried out in triplicate. Optical density was measured in an automatic microplate reader (Tecan Infinite M200) at a test wavelength of 490 nm and reference wavelength of 650 nm to negate the effect of cell debris. The data are presented as percentage above control (untreated samples).
MPT pore opening assay
To test the effects of melatonin on H2O2-induced MPT pore, cells were loaded with calcein-AM in the presence of cobalt chloride to quench fluorescence from all cellular domains except from within mitochondria. Although this protocol does not distinguish between calcein efflux and cobalt influx, it is consistent with induction of the permeability transition pore [23]. Opening was induced by 500 μM H2O2 in cells pretreated with 1 mM melatonin for 16 h or vector. MPT pore opening was indicated by a reduction in mitochondrial calcein signal and was measured with an automatic microplate reader (Tecan Infinite M200) with excitation wavelength of 488 nm and emission at 515 nm. Treatments were carried out in triplicate. Data are expressed as fractional changes of emitted fluorescence above the baseline values.
Determination of mitochondrial membrane potential
The quantification of mitochondrial membrane depolarization was carried out by measuring the fluorescence intensity of the membrane-permeant cationic probe TMRM. The resulting signal of TMRM-stained cells thus represents only the probe accumulated in intact mitochondria. A collapse on mitochondrial membrane is indicated by a decrease in output fluorescence. Briefly, cells (1 × 106 cells/ml) were washed once with PBS and loaded with 1 μM TMRM by incubation at 37°C for 30 min. The fluorescence intensity of TMRM was measured in an automatic microplate reader (Tecan Infinite M200). Excitation was set at 443 nm and emission at 575 nm. Treatments were carried out in triplicate. The data are presented as fold increase over the pretreatment level (experimental/control).
Measurement of intracellular ROS levels
Intracellular ROS production was quantified by measuring the fluorescence intensity of rhodamine-123 (Rh-123). Briefly, cells (1 × 106 cells/ml) were washed once with PBS and loaded with 2 μM DHR-123 by incubation at 37°C for 30 min. This probe is a non-fluorescent, cell-permeable compound; once inside the cell, it turns fluorescent upon oxidation to yield Rh-123, fluorescence being proportional to ROS generation. The fluorescence intensity of Rh-123 was measured in an automatic microplate reader (Tecan Infinite M200). Excitation was set at 488 nm and emission at 543 nm. Treatments were carried out in triplicate. The data are presented as fold increase over the pre-treatment level (experimental/control).
Assay for caspase activity
To determine caspases 3, 8 and 9 activities, stimulated or resting cells were pelleted and washed once with PBS. After centrifugation, cells were resuspended in PBS at a concentration of 1 × 107 cells/ml; 15 μl of the suspension were added to a microplate, and mixed with the appropriate peptide substrate dissolved in a standard reaction buffer which was composed of 100 mM HEPES, pH 7.25, 10% sucrose, 0.1% CHAPS, 5 mM DTT, 0.001% NP40 and 40 μM caspase-3 substrate AC-DEVD-AMC or 100 μM caspase-8 substrate AC-LETD-AFC. For caspase-9, the reaction buffer was composed of 0.1 M mes hydrate, pH 6.5, 10% PEG, 0.1% CHAPS, 5 mM DTT, 0.001% NP40 and 100 μM of caspase-9 substrate AC-LEHD-AMC. Substrate cleavage was measured with a microplate reader (Tecan Infinite M200) with excitation wavelength of 360 nm and emission at 460 nm. Preliminary experiments reported that caspases 3, 8 or 9 substrate cleaving was not detected in the presence of the inhibitors of caspases 3, 8 or 9, Z-DEVD-CMK, Z-IETD-FMK or AC-LEHD-CMK, respectively. Treatments were carried out in triplicate. The data were calculated as fluorescence units/mg protein and presented as fold increase over the pre-treatment level (experimental/control).
In situ detection of DNA fragmentation by TUNEL and Hoechst staining
At the end of the treatments, HL-60 cells were harvested and washed once with PBS. The cells were then fixed with 4% paraformaldehyde (in PBS, pH 7.4, at least 6 h, RT) and air-dried on slides for 24 h. Afterwards, the air-dried cells were washed twice with PBS, and incubated in permeabilization solution (0.1% Triton-X-100 in 0.1% sodium citrate, 15 min, RT). The permeabilization solution was then removed, and TUNEL reaction mixture (50 μl) was added and the cells were incubated (1 h, 37°C) in a humidified chamber. The cells were washed again with PBS and counterstained with Hoechst 33342 (1 μg/ml) in PBS for 5 min to identify cellular nuclei. The incidence of apoptosis was assessed under an epifluorescence microscope (BX51, Olympus Spain S.A.U., Barcelona, Spain) using a FITC filter. Cells with TUNEL-positive nuclei were considered apoptotic. Hoechst staining was used to determine the total number of cells in a field. A minimum of five fields per slide were used to calculate the percentage of apoptotic cells, which was expressed as fold-increase over the pre-treatment level (experimental/control).
Annexin V staining
Cells were harvested and washed twice with PBS and centrifuged at 500×g for 5 min, then the supernatant was discarded, and the pellet was resuspended in 95 μl annexin V-binding buffer at a density of 105–106 cells/ml containing annexin V-FITC. Cells were analysed by cytometry (Cytomycs FC-500 Beckman-coulter) after addition of PI. Each sample was tested 3–5 times in independent experiments. Annexin V binds to those cells that express PS on the outer layer of the cell membrane, and PI stains the cellular DNA of those cells with a compromised cell membrane. This allows for live cells (unstained with either fluorochrome) to be discriminated from early apoptotic cells (stained only with annexin V) late apoptotic or necrotic cells (stained with both annexin and PI) [23].
Statistical analysis
Data are presented as mean ± standard error of mean (SEM) and analysis of statistical significance was performed using Student’s t-test. 2α < 0.05 was considered to indicate a statistically significant difference (P < 0.05).
Results
Melatonin improves the viability of healthy cells and augments cell death in tumour cells
In order to compare the effects of melatonin on the mitochondrial activity (i.e., metabolic activity) on H2O2-exposed cells, both tumour cells (HL-60 cells) and healthy cells (human neutrophils and lymphocytes) were used to perform the MTT reduction assay. As shown in Fig. 1a, a decrease in the MTT reduction was observed when the cultured cells were incubated with 1 mM melatonin for 16 h and/or 500 μM H2O2 for 2 h with respect to untreated cells. In addition, melatonin decreased the HL-60 cells viability induced by H2O2 (Fig. 1a; P < 0.05). On the other hand, the effects of H2O2 on healthy cells after the pre-treatment with melatonin were opposite. As shown in Fig. 1b 1 mM melatonin for 16 h increased the viability of leucocytes with respect to the untreated cells (P < 0.05). Although the metabolic activity dropped because of the treatment with H2O2 (Fig. 1b; P < 0.05), melatonin was able to prevent this drop and restored the metabolic activity of neutrophils and lymphocytes to values similar to those obtained at the controls (Fig. 1b; P < 0.05). These results emphasize the dual behaviour of the indolamine when in the presence of healthy leucocytes or leukaemia cells, supporting the idea that melatonin may reduce the side effects of drugs in healthy cells in terms of production of oxidative stressors and act at the same time as a cytotoxic agent in tumour cells.
Melatonin improves the viability of healthy cells and augments cell death in tumour cells. HL-60 cells (a) or neutrophils and lymphocytes (b) were treated with 1 mM melatonin (Mel) or the vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was estimated as described under Materials and Methods section. Values are presented as means ± SEM of 5–8 separate experiments, which were carried out in triplicate, and expressed as fold-increase over the pre-treatment level (experimental/control). *P < 0.05 compared to control values. ♦ P < 0.05 compared to melatonin or H2O2 alone values
Melatonin enhances the H2O2-induced mitochondrial disruption in tumour cells
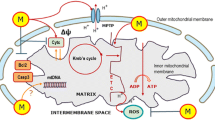
Opening of MPT pore is considered a key event in cell death. We previously reported that both melatonin and H2O2 compromise mitochondrial stability in leukaemic cells [24, 25]. To check the effects of melatonin pre-treatment on mitochondria integrity compromised by H2O2, we performed MPT pore opening assay and TMRM loading. MPT causes mitochondria to release apoptogenic molecules from intermembrane space into cytoplasm [26]. Figure 2a displays a higher slope in calcein fluorescence decrease induced by 500 μM H2O2 when cells were previously treated with 1 mM melatonin for 16 h. Therefore, MPT pore in tumour cells becomes more sensitive to H2O2 induction when cells are previously treated with melatonin. It is also well known that MPT leads to the loss of mitochondrial membrane potential (Δψ) [26]. Figure 2b shows a significant loss of Δψ, indicated by a decrease in TMRM fluorescence when cells were exposed to 1 mM melatonin for 16 h, and then 500 μM H2O2 was added for 2 h, with respect to the values obtained when cell were incubated with either melatonin or H2O2 values (P < 0.05). These findings support the hypothesis that melatonin enhances the H2O2-evoked mitochondrial disruption in tumour cells.
Melatonin enhances the H2O2-induced mitochondrial disruption in HL-60 cells. a Cells were pre-incubated with 1 mM melatonin (Mel) or the vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 (as the arrow indicated). Mitochondrial permeability transition (MPT) induction assay was performed as described under “Materials and methods” section. Changes in fluorescence were expressed as means ± SEM of 4-6 separate experiments of fractional changes of emitted fluorescence (F/F0). *P < 0.05 compared to H2O2 alone. b Cells were pre-incubated with 1 mM melatonin (Mel) or vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h. Mitochondrial membrane potential and cell treatment were carried out as described under “Materials and methods” section. Values are presented as means ± SEM of 5–8 separate experiments, which were carried out in triplicate, and expressed as fold-change over the pre-treatment level (experimental/control). *P < 0.05 compared to control values. ♦ P < 0.05 compared to melatonin or H2O2 alone
Melatonin increases H2O2-induced ROS generation in tumour cells
Since ROS are generated in mitochondria because of the uncoupling of electron transport chain as a step of apoptosis intrinsic pathway, and consequently ROS can induce oxidative damage of cell macromolecules which leads to apoptosis, we also evaluated the effect of melatonin on the production of intracellular ROS induced by H2O2. As shown in Fig. 3, treatment of cells for 16 h with 1 mM melatonin or for 2 h with 500 μM H2O2 raised the production of intracellular ROS, as revealed by the increase in Rh-123 fluorescence. Cell pre-treatment with 1 mM melatonin caused a significant elevation of H2O2-induced ROS generation with respect to the control, and to when cells were treated with melatonin or H2O2 alone (P < 0.05).
Melatonin increases H2O2-induced ROS generation in HL-60 cells. Dihydrorhodamine-123-loaded HL-60 cells were pre-incubated with 1 mM melatonin (Mel) or vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h. Intracellular ROS production was determined by changes of rhodamine-123 (Rh-123) fluorescence as described in “Materials and methods” section. Values are presented as means ± SEM of 5–8 separate experiments, which were carried out in triplicate, and expressed as fold-increase over the pretreatment level (experimental/control). *P < 0.05 compared to control values. ♦ P < 0.05 compared to melatonin or H2O2 alone
Caspase activation by H2O2 is enhanced by melatonin in tumour cells
Caspase-3 plays an essential executioner role in apoptosis in which caspases 8 and 9 are involved as critical upstream activators. To study the effects of cell pre-treatment with melatonin on H2O2-induced caspase activation, we treated HL-60 cells with 1 mM melatonin for 16 h and then 500 μM H2O2 were added for 2 h. Our results showed that melatonin augmented substantially the H2O2-induced caspase-3 activity (P < 0.05; Fig. 4). We observed similar results when the activities of activators caspases 8 and 9 were evaluated (P < 0.05; Fig. 4). In addition, when leukaemia cells were treated with melatonin or H2O2 separately, caspases 3, 8 and 9 activities increased significantly compared to the controls (P < 0.05; Fig. 4).
Caspase activation by H2O2 is enhanced by melatonin. HL-60 cells were treated with 1 mM melatonin (Mel) or vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h, caspases 3, 8 and 9 activities were estimated as described under “Materials and methods” section. Values are presented as means ± SEM of 5–8 separate experiments and expressed as fold-increase over pretreatment levels (experimental/control). *P < 0.05 compared to control values. ♦ P < 0.05 compared to melatonin or H2O2 alone
Melatonin augments the H2O2-induced DNA fragmentation in tumour cells
Chromatin cleavage is an idiosyncrasy of apoptosis, given that, during apoptosis, some endogenous nucleases are activated cleaving DNA into internucleosomal fragments of ~200 pb. TUNEL assay is a well-established method for detection of DNA cleavage, a relatively late apoptotic marker [25]. We assessed the amount of DNA fragmentation in the presence of H2O2 to verify whether melatonin enhanced the programmed cell death. Treatment of HL-60 cells with both 1 mM melatonin for 16 h and 500 μM H2O2 for 2 h produced a substantial increase in the proportion of cells depicting DNA fragmentation (P < 0.05; Fig. 5a). Importantly, a higher proportion of cells depicting DNA fragmentation was found when cells were pre-incubated with 1 mM melatonin before H2O2 treatment as compared to H2O2 treatment alone (P < 0.05; Fig. 5a), thereby reflecting that melatonin pre-treated leukaemia cells are much more vulnerable to apoptosis induced by H2O2.
Melatonin augments the H2O2-induced DNA fragmentation in HL-60 cells. Cells were pre-treated with 1 mM melatonin (Mel) or vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h. To evaluate the proportion of cells depicting DNA fragmentation, TUNEL assay was performed and DNA fragmentation was estimated as described under “Materials and methods” section. a Right and central panels: pictures of Hoechst 33342 nuclear stain (blue) and TUNEL-positive cells (green), respectively. Left panel: differential interference contrast (DIC) images of cultured cells using a 40× objective. Scale bars: 15 μm. b Values inside the central panel are presented as means ± SEM of three separate experiments and expressed as fold-increase over the pre-treatment level (experimental/control). *P < 0.05 compared to control values. ♦ P < 0.05 compared to melatonin or H2O2 alone
Melatonin moves HL-60 cells apoptosis forward later apoptotic stages caused by H2O2
Lastly, to check the different cell apoptotic stages under melatonin and H2O2 treatments, HL-60 cells annexin V-FITC/PI staining was performed. Figure 6 show different regions where cells are located depending on PS externalization (annexin V positivity) and porosity of membrane (PI positivity). Despite apoptotic and necrotic cells show annexin V/PI positivity, a higher permeability for PI is easily differentiated in necrosis cases, showing a population of cells with upper location than late apoptosis (Fig. 6). Control samples (Fig. 6; upper-left section) showed most cells in the living cell region. The low percentage of cells showing positivity was due to spontaneous cell death. Cells treated with 1 mM melatonin displayed an increase in annexin V/PI positivity in both the late apoptosis and necrosis regions (Fig. 6; upper-right section). Although an increase in the percentage of cells at the necrosis region was observed, similar results were obtained when cells where treated with 500 μM H2O2 for 2 h (Fig. 6; lower-left section). When cells were treated with 1 mM melatonin for 16 h and 500 μM H2O2, double-stained cells displayed a location mainly at the necrosis area (Fig. 6; lower-right section), being a low percentage of cells located at the living cell or early apoptosis regions. These results strongly suggest that the toxicity of H2O2 may be raised by melatonin.
Melatonin moves cell leukaemia apoptosis forward the later apoptotic stages caused by H2O2. Cells were pre-treated with 1 mM melatonin (Mel) or vehicle (0.5% ethanol) for 16 h and then were stimulated with 500 μM H2O2 for 2 h. To evaluate the stages of apoptosis, cells were stained with annexin V-FITC and propidium iodide (PI) and analysed by flow cytometry as described under “Materials and methods” section. Data are representative of three independent experiments
Discussion
The presence of melatonin regulates the expression of clock genes which are responsible of haemorhythmicity. Lack of regulation of internal clock gene could make cancer appear [27]. In addition, an increasing number of studies report that melatonin induces a significant, strong inhibition in tumour cells. This may be also the case of its precursor [28]. As previously described, melatonin disrupts mitochondrial function and generates intracellular ROS [7, 24]. A recent report supports the fact that melatonin-induced ROS was one of the main effects to evoke apoptosis in HL-60 cells, given that other antioxidant agents were able to reduce apoptotic events [11]. Experimental evidence shows that tumour cells are under an elevated oxidative stress, compared to healthy cells. Such a huge stress is due to metabolic alterations and subsequent increased ROS production [29–31]. A moderate increase in ROS promotes cellular proliferation and mutations, and moreover contributes to develop anticancer drug resistance. The activity of antioxidant intracellular enzymatic defenses, including superoxide dismutase (SOD), glutathione-S-transferase (GST) or glutathione peroxidase is elevated in cancer tissues [32]. In fact, the expression of antioxidant enzymes, like SOD, GST or catalase, shows an adaptational increase under the elevated oxidative stress in cancer cells [33]. It is thought that the increase in the amount of antioxidant agents may contribute to anticancer drug resistance during the disease progression. Since ROS are able to produce strong cellular injures, a further ROS insult could provide a chance to evoke cell death much more efficiently, when the antioxidant intracellular machinery has reached its maximum, and even counteract drug resistance, which has been reported for some cancers [17]. Melatonin has been shown not only to generate intracellular ROS in tumour cells [7, 16], but also to increase the apoptotic effects of anticancer agents that generate intracellular ROS. In order to discard actions of anticancer drugs different from those mediated by ROS generation, 500 μM H2O2 was used in this study to emulate chemotherapeutically caused oxidative stress in HL-60 cells pre-treated with 1 mM melatonin. Our findings revealed a reinforcing apoptotic action of the indoleamine, when leukaemia HL-60 cells were treated with the oxidative agent. Such intensification is due, at least in part, to the supplementary effects of melatonin on oxidative stress. It is well known that mitochondria produce ROS when Δψ decreases by MPT induction. Other processes, including deficiency of mitochondrial antioxidant occur simultaneously with MPT. Such damage in mitochondria causes the release of apoptotic factors that trigger caspase-9 activation. In this regard, treated HL-60 cells with melatonin may represent a further oxidative insult, which is increased when an oxidative agent, like H2O2, is added with the indoleamine. Melatonin and H2O2 have been previously shown to induce mitochondrial damage [25, 34], but interestingly, in our study, when cells were treated simultaneously with both substances, a significant elevation in mitochondrial malfunction parameters (MPT induction, loss of Δψ, intracellular ROS production, diminished metabolic capacity and higher activation of caspase-9) could be detected in comparison to the mitochondrial injury provoked by these substances alone. Taken together, these results strongly indicate that mitochondria were seriously affected when combining both treatments, which activated the intrinsic apoptotic pathway. In addition, it was also observed that the extrinsic pathway was also activated through caspase-8 activity when leukaemia cells were treated. These results are in agreement with those of Martín-Renedo and colleagues [35] who reported a melatonin-induced increase in caspase-8 activity in hepatocarcinoma cells. Also, a recent study has shown that increases in intracellular ROS regulate the activation of caspase-8 during apoptosis [36]. In our study, increases in caspase-8 activity were obtained in cells treated with either H2O2 or melatonin or in combination of both agents. Melatonin likely increases the H2O2-induced oxidative stress causing an increased activation of caspase-8. This caspase-8 activation might be due to the strong pro-oxidant activity in tumour cells, as shown by Wolfler et al. [11] in Jurkat cells. In addition, melatonin has been shown to lead to the promotion of Fas-induced cell death. Pre-incubation with the indolamine may augment the susceptibility of cells to additional oxidant agents, including H2O2. In fact, melatonin has been reported to increase Fas-receptor expression in cultured cancer endothelial cells [37].
It is known that the initiator caspases 8 and 9 are able to activate the executioner caspase-3, evoking an irreversible apoptotic stage that leads to cell death. Our results showed the activation of initiator caspases and the resulting increase in caspase-3 activity in the presence of H2O2 in melatonin-pretreated cells. The toxic effect of melatonin in tumour cell lines has been widely reported [15, 34, 35]. In this study, a higher number of cells with fragmented DNA, i.e., the number of apoptotic cells, were observed when cells were treated with both H2O2 and melatonin, as shown by TUNEL assay techniques. As expected, most HL-60 cells were found at the final apoptotic stages (late apoptosis or necrosis). This indicates that the treatment had a relevant toxicity in leukaemia cells. The findings of this study in leukaemia cells are contrary to those described by Chetsawang et al. [38] in neuroblastoma, where it was suggested that melatonin displays a protector role in tumour cells similar to that exerted in normal cells. An MTT assay was carried out to evaluate the metabolic capacity of healthy leucocytes, and therefore their viability, with melatonin and H2O2 treatments. In agreement with Juknat et al. [39], we observed that the pre-treatment with melatonin was able to reverse H2O2 toxic effects both in lymphocytes and neutrophils. However, when HL-60 cells were pre-treated with melatonin before H2O2, the viability diminished considerably.
In summary, melatonin augmented oxidative stress in tumour cells and reduced oxidative stress in healthy cells. Many chemotherapeutic agents generate ROS as one of their mechanisms to kill cancer cells. Cancer cells possess increased oxidative stress levels as a result of their elevated metabolism. Consequently, the expression of antioxidant enzymes and their activities are severely increased. Our findings indicate that melatonin may be a potential tool able to protect healthy cells from chemotherapy-induced ROS and saturate, at the same time, the antioxidant buffering capacity of cancers cells due to the generation of ROS induced by the indoleamine in this type of cells.
References
Reiter RJ, Tan DX, Fuentes-Broto L (2010) Melatonin: a multitasking molecule. Prog Brain Res 181:127–151
Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009) Phytomelatonin: a review. J Exp Bot 60:57–69
Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF (2007) Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res 43:389–403
Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ (2003) Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci 60:1407–1426
Cos S, Mediavilla MD, Fernández R, González-Lamuño D, Sánchez-Barceló EJ (2002) Does melatonin induce apoptosis in MCF-7 human breast cancer cells in vitro? J Pineal Res 32:90–96
Pirozhok I, Meye A, Hakenberg OW, Fuessel S, Wirth MP (2010) Serotonin and melatonin do not play a prominent role in the growth of prostate cancer cell lines. Urol Int 84:452–460
Dziegiel P, Podhorska-Okolow M, Surowiak P, Ciesielska U, Rabczynski J, Zabel M (2003) Influence of exogenous melatonin on doxorubicin-evoked effects in myocardium and in transplantable Morris hepatoma in rats. In Vivo 17:325–328
Pawlikowski M, Winczyk K, Karasek M (2002) Oncostatic action of melatonin: facts and question marks. Neuro Endocrinol Lett 23:24–29
Sainz RM, Mayo JC, Reiter RJ, Antolín I, Esteban MM, Rodríguez C (2000) Melatonin regulates glucocorticoid receptor: an answer to its antiapoptotic action in thymus. FASEB J 13:1547–1556
Espino J, Bejarano I, Ortiz A, Lozano GM, García JF, Pariente JA, Rodríguez AB (2010) Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil Steril 94:1915–1917
Wolfler A, Caluba HC, Abuja PM, Dohr G, Schauenstein K, Liebmann PM (2001) Prooxidant activity of melatonina promotes Fas-induced cell death in human leukemic Jurkat cells. FEBS Lett 502:127–131
Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2011) Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol 108:14–20
Büyükavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, Savaşan S (2006) Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol 20:73–79
Paternoster L, Radogna F, Accorsi A, Cristina Albertini M, Gualandi G, Ghibelli L (2009) Melatonin as a modulator of apoptosis in B-lymphoma cells. Ann NY Acad Sci 1171:345–349
Wenzel U, Nickel A, Daniel H (2005) Melatonin potentiates flavone-induced apoptosis in human colon cancer cells by increasing the level of glycolytic end products. Int J Cancer 116:236–242
Albertini MC, Radogna F, Accorsi A, Uguccioni F, Paternoster L, Cerella C, De Nicola M, D’Alessio M, Bergamaschi A, Magrini A, Ghibelli L (2006) Intracellular pro-oxidant activity of melatonin deprives U937 cells of reduced glutathione without affecting glutathione peroxidase activity. Ann NY Acad Sci 1091:10–16
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Tien Kuo M, Savaraj N (2006) Roles of reactive oxygen species in hepatocarcinogenesis and drug resistance gene expression in liver cancers. Mol Carcinog 45:701–709
Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH (2002) Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol 283:H254–H263
Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, Wang H (2010) Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol 16:1473–1481
Casado-Zapico S, Rodriguez-Blanco J, García-Santos G, Martín V, Sánchez-Sánchez AM, Antolín I, Rodriguez C (2010) Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res 48:72–80
Espino J, Bejarano I, Redondo PC, Rosado JA, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2010) Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: evidence for the involvement of mitochondria and Bax activation. J Membr Biol 233:105–118
Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Benardi P, di Lisa F (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734
Bejarano I, Redondo PC, Espino J, Rosado JA, Paredes SD, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB (2009) Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J Pineal Res 46:392–400
Bejarano I, Espino J, González-Flores D, Casado JG, Redondo PC, Rosado JA, Barriga C, Rodríguez AB, Pariente JA (2009) Role of calcium signal on hydrogen peroxide-induced apoptosis in human myeloid HL-60 cells. Int J Biomed Sci 5:246–256
Tsujimoto Y, Nakagawa T, Shimizu S (2006) Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 1757:1297–1300
Berger J (2008) A two-clock model of circadian timing in the immune system of mammals. Pathol Biol (Paris) 56:286–291
Sánchez S, Sánchez CL, Paredes SD, Rodríguez AB, Barriga AB (2008) The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland in rats. J Appl Biomed 6:177–186
Toyokuni S, Okamoto K, Yodoi J, Hiai H (1995) Persistent oxidative stress in cancer. FEBS Lett 358:1–3
Hileman EO, Achanta G, Huang P (2001) Superoxide dismutase: anemerging target for cancer therapeutics. Expert Opin. Ther. Targets 5:679–710
Hileman EO, Liu J, Albitar M, Keating MJ, Huang P (2004) Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 53:209–219
Kanbagli O, Ozdemirler G, Bulut T, Yamaner S, Aykac-Toker G, Uysal M (2000) Mitochondrial lipid peroxides and antioxidant enzymes in colorectal adenocarcinoma tissues. Jpn J Cancer Res 91:1258–1263
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R (2005) Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathways activation. J Pineal Res 39:425–431
Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J (2008) Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res 45:532–540
Perez-Cruz I, Cárcamo JM, Golde DW (2007) Caspase-8 dependent TRAIL-induced apoptosis in cancer cell lines is inhibited by vitamin C and catalase. Apoptosis 12:225–234
Suhara T, Fukuo K, Sugimoto T, Morimoto S, Nakahashi T, Hata S, Shimizu M, Ogihara T (1998) Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. J Immunol 160:4042–4047
Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P (2006) Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res 41:116–123
Juknat AA, Mendez M del V, Quaglino A, Fameli CI, Mena M, Kotler ML (2005) Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res 38:84–92
Acknowledgments
This study was supported by the Junta de Extremadura (PRI07-A024) and the Ministerio de Ciencia e Innovación-Fondo Europeo de Desarrollo Regional grants (BFU2007-60091 and BFU2010-15049). I. Bejarano and S.D. Paredes are the beneficiaries of grants from the Consejería de Economía, Comercio e Innovación-Fondo Social Europeo (Junta de Extremadura, PRE06070 and REI09009, respectively). J. Espino is a beneficiary of a grant from the Ministerio de Ciencia e Innovación (AP2009-0753).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bejarano, I., Espino, J., Marchena, A.M. et al. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukaemia HL-60 cells. Mol Cell Biochem 353, 167–176 (2011). https://doi.org/10.1007/s11010-011-0783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0783-8