Abstract
Regucalcin was discovered in 1978 as a calcium-binding protein that does not contain EF-hand motif of calcium-binding domain (Yamaguchi and Yamamoto Chem Pharm Bull 26:1915–1918, 1978). The name regucalcin was proposed for this calcium-binding protein, which can regulate various Ca2+-dependent enzyme activations in liver cells. The regucalcin gene is localized on the chromosome X, and the organization of the regucalcin gene consists of seven exons and six introns. AP-1, NF1-A1, and RGPR-p117 bind to the promoter region of the rat regucalcin gene and enhance transcription activity of regucalcin gene expression that is mediated through calcium signaling. Regucalcin plays a pivotal role in the keep of intracellular calcium ion (Ca2+) homeostasis due to activating Ca2+ pump enzymes in the plasma membrane (basolateral membrane), microsomes (endoplasmic reticulum), mitochondria, and nuclei of many cell types. Regucalcin has a suppressive effect on calcium signaling from the cytoplasm to the nucleus in the proliferative cells. Regucalcin has also been demonstrated to transport to the nucleus, and it can inhibit Ca2+-dependent protein kinase and protein phosphatase activities, Ca2+-activated deoxyribonucleic acid (DNA) fragmentation, and DNA and ribonucleic acid (RNA) synthesis in the nucleus. Overexpression of regucalcin suppresses cell death and apoptosis in the cloned rat hepatoma cells induced by various signaling factors. Regucalcin can inhibit the enhancement of cell proliferation due to hormonal stimulation. Regucalcin plays an important role as a regulatory protein in cell signaling system, and it is proposed to play a pivotal role in keep of cell homeostasis and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium ion (Ca2+) plays an important role in the regulation of many cell functions. Ca2+ can regulate muscle contraction, neurotransmission, hormone secretion, cell mitosis, and gene expression. A role as second messengers of Ca2+ in cells for hormonal stimulation comes into notice. Calcium signal is transmitted to intracellular responses, which are mediated through a family of calcium-binding protein and protein kinase C [1, 2]. Liver metabolism is regulated by an increase in Ca2+ in the cytoplasm of liver cells due to hormonal stimulation [3–5]. The effect of Ca2+ is amplified through calmodulin and protein kinase C [1–5]. Calcium signaling is important in the regulation of liver metabolism.
Liver has been shown to participate in the regulation of calcium metabolism through hepatic bile system in rats, and bile calcium excretion is increased by hormonal stimulation [5, 6]. On the basis of this finding, it was found that a novel calcium-binding protein, which differs from calmodulin and other calcium-related proteins, was present in the hepatic cytoplasm of rats [7–9]. The name regucalcin was proposed for this calcium-binding protein, which regulates various Ca2+- or Ca2+/calmodulin-dependent enzyme activations in liver cells [10–15].
Regucalcin and its gene (RGN) are identified in over 15 species consisting of regucalcin family [16–21]. Comparison of the nucleotide sequences of regucalcin from vertebrate species is highly conserved in their coding region with throughout evolution. The regucalcin gene is localized on the chromosome X, and the organization of the regucalcin gene consists of seven exons and six introns [21–23].
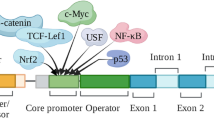
AP-1, NF1-A1, and RGPR-p117, which is a transcription factor, bind to the promoter region of the regucalcin gene and enhance transcription activity of regucalcin gene expression that is mediated through calcium and other signallings [21]. Regucalcin mRNA expression and its protein content are pronounced in the liver and kidney cortex of rats, although it is present only slightly in other tissues (including the duodenum, testis, spleen, lung, smooth muscle, heart, and brain) [17, 24–29]. The role of regucalcin is investigated in the liver and kidney cells in detail [reviewed in Ref. 30–34]. After finding of regucalcin, the identical protein to regucalcin was also reported as senesence marker protein-30 (SMP30) [35, 36].
There are growing evidences that regucalcin plays an important role as a regulatory protein for calcium signaling from the cytoplasm to the nuclei in liver cells [37]. Overexpression of regucalcin has been demonstrated to inhibit cell apoptosis and cell proliferation induced by various signaling factors. This review has been written to outline the recent advances that have been made concerning the role of regucalcin as a regulatory protein in cell signaling in liver, kidney, and other tissues: its role in regulation of intracellular Ca2+ homeostasis, inhibition of Ca2+-dependent enzyme activations, regulation of nuclear calcium signaling, and inhibitory effects in cell apoptosis and cell proliferation induced by various signaling factors.
Properties of calcium-binding in regucalcin
The molecular weight of rat regucalcin is estimated as 33,388 Da, composing of 299 amino acid residues from the cloning of rat regucalcin cDNA [16]. The regucalcin molecule does not contain the EF-hand motif as a calcium-binding domain [16]. The isoelectric point of regucalcin is 5.20 [16].
From the experimental data by Scatchard plot, the apparent association constant (Kf) for calcium ion (Ca2+) of regucalcin is found to be 4.19 × 105 M−1 by equilibrium dialysis with a correlation coefficient of 0.99, and there are 6.52 high-affinity sites per molecule of protein [7–9]. Regucalcin appears to have six or seven high-affinity binding sites for Ca2+ per molecule of protein [8]. Extrapolation of the regression line to infinite Ca2+ concentration indicated a maximal Ca2+-binding of 2.28 × 10−4 mol of Ca2+ per gram of protein. It is known that calmodulin exists as a monomer with a molecular weight of 17,000 and contains four Ca2+-binding sites [1].
The conformational changes induced by binding of Ca2+ to regucalcin have been investigated by means of the ultraviolet (UV) absorption spectrum [9]. UV of regucalcin showed a maximum at 278 nm in the range from 240 to 330 nm. In the presence of Ca2+ (0.1 and 1.0 mM), a decrease in absorption at 278 nm was observed [9]. Such a negative UV difference is similar to the Ca2+-induced absorption changes in calmodulin. The spectrum can be attributed to charges in both tyrosine and tryptophan residues. Changes in the environment of both aromatic amino acids occur upon Ca2+-binding [9].
Fluorescence spectroscopy is used to study the effect of Ca2+ on the conformation of regucalcin [9]. The spectral emission was quenched after the addition of 1.0 mM Ca2+. Known fluorescence emission properties of isolated tyrosine and tryptophan residues suggest, as indicated above, that changes in the environment of these two aromatic amino acids occur upon Ca2+-binding. These observations demonstrate that Ca2+-binding induces conformational changes in regucalcin. These changes may result in increasing the hydrophobicity of regucalcin [9, 16].
The conformation of the polypeptide backbone of regucalcin has been studied by circular dichroism (CD) spectroscopy [9]. The presence of 1.0 mM Ca2+ caused clear alterations in the CD spectrum. The apparent α-helical content of regucalcin in Ca2+-free buffer was estimated to be 34%, and the presence of 1.0 mM Ca2+ decreased this by 4.5%. Thus, conformational changes are induced by Ca2+ binding to regucalcin. This binding also loosens the conformation of regucalcin. The apparent α-helical content of calmodulin is 30%, and it is increased after Ca2+ binding and its conformation is tightened [9, 16]. The increase in hydrophobicity after Ca2+ binding represents the mechanism that calmodulin activates its target proteins. However, regucalcin reverses the activation of many enzymes by Ca2+/calmodulin. The role of regucalcin may be different from that of calmodulin in cells.
Regucalcin differs entirely from other calcium-binding proteins of the EF-hand type: calmodulin, calcineurin, parvalbumin, S-l00a, S-100b proteins, caligulin, calregulin, calbindin, calreticulin, and annexins. This is supported from the results of the molecular cloning and sequencing of the cDNA coding for a regucalcin from various mammalian livers [16, 20]. The nucleotide and amino acid sequences of regucalcin does not have statistically significant homology, as compared with the registered sequences which are found in the EMBL and GenBank detabases (D14327 and D86217) [16].
The hydropathy profile of regucalcin shows that there is a hydrophobic sequence in both N-terminal and C-terminal regions of the regucalcin molecules [16]. Regucalcin shows a hydrophilic character as molecule [16, 20]. The most common EF-hand is composed of the helix-loop-helix-domain. The prototype loop consists of 12 amino acids, of which five have a carboxyl (or a hydroxyl group) in their side chain, precisely spaced so as to coordinate the Ca2+. Analysis of the structure of the EF-hand from the regucalcin sequence did not give the expected pattern of amino acids conforming to the typical EF-hand structure of a calcium-binding site.
Amino acid analysis shows that regucalcin has a relatively high content of glycine and much lower amounts of glutamic acid, valine, aspartic acid, and lysine [16, 20]. Regucalcin contains about 20% (mol%) glutamic and aspartic acids and about 17% amide residues (lysine, histidine, and arginine) and thus a high proportion of charged residues: regucalcin molecule contains aspartic acid (24 residues) and glutamic acid (16 residues) [16]. Acidic amino acids comprise approximately one-fifth of the regucalcin molecule, a prevalence that appears to be characteristic of the composition of all Ca2+-binding proteins. The significance of the high di-carboxylic acid content is that it permits binding of Ca2+ to protein. These amino acids may be related to Ca2+ binding.
The result of crystal structure with X-ray diffraction data shows that regucalcin contains the metal site bound with either a Ca2+ or a Zn2+ atom, suggesting that the Ca2+-bound form may be physiologically relevant for stressed cells with an elevated Ca2+ level [38]. This supports our finding for regucalcin as a calcium-binding protein.
Regulation of regucalcin gene expression
The rat regucalcin gene is localized on the proximal end of the rat chromosome Xq1l.1-12 [22], and the gene is demonstrated in human, mouse, cow, monkey, dog, rabbit, and chicken but not in yeast [17]. The amino acid sequence of mouse regucalcin had 94% homology as compared with that of rat regucalcin [20]. Regucalcin may be a protein that is highly differentiated. The organization of the rat regucalcin gene seems to be about 18 kb in size, and consisted of seven exons and six introns [39]. There are many regulatory elements (AP-1, NF1-A1, RGPR-p117, β-catenin, and NF-κB) in the 5′-flanking region [21, 40–48]. The promoter activity of the rat regucalcin gene is enhanced by treatment with Bay K 8644, dibutyryl cyclic AMP, phorbol esters, insulin, and dexamethasone [42]. Using gel mobility shift assays, it is found that nuclear proteins from rat liver cells and rat hepatoma H4-II-E cells specifically bind to the 5′-flanking region of the rat regucalcin gene [40–42]. Treatment with Bay K 8644, dibutyryl cyclic AMP, phorbol esters, and insulin stimulates the binding of nuclear factors to the 5′-flanking region of the rat regucalcin gene in H4-II-E cells. These factor-inducible nuclear proteins are related to enhance promoter activity of the regucalcin gene [42].
Regucalcin mRNA expression has been demonstrated to mediate through signaling pathway of Ca2+/calmodulin-dependent protein kinase, protein kinase C, and tyrosine kinase in the cells [49–51]. AP-1 factor binds to the 5′-flanking region of the rat regucalcin gene that is mediated through the Ca2+ response [41]. AP-1 factor is complex of c-fos/c-jun that is phosphorylated by protein kinases [52, 53]. Calcium signaling system is an important pathway in the stimulation of regucalcin mRNA expression.
Regucalcin mRNA is mainly present in liver and renal cortex with a size of 1.8 kb [24]. The expression of regucalcin mRNA in the liver and renal cortex is clearly stimulated through an increase in the cellular Ca2+ levels following an oral administration of calcium chloride in rats in vivo [54–56]. Hepatic regucalcin mRNA expression is increased with fetal development and its expression is stimulated after the intake of dietary calcium to maternal rats in vivo [57]. Liver regucalcin concentration is increased after an oral administration of calcium in rats [55].
Hepatic regucalcin mRNA expression is also stimulated after a single subcutaneous administration of calcitonin [58], insulin [59], and estrogen [60], suggesting that the expression of regucalcin mRNA is enhanced through various hormonal stimulation. Regucalcin mRNA expression is increased in regenerating rat liver, suggesting its role in the proliferation of liver cells [61]. Aging has been shown to decrease liver regucalcin mRNA expression [62].
Rat regucalcin immunoreactivity is most pronounced in the liver of rats; it is not seen in the duodenum, testicle, spleen, lung, and smooth muscle (bladder) and is barely visible in the kidney, heart, and brain [25, 26]. Regucalcin is primarily located in the rat liver. Thus, the tissue specific distribution of regucalcin is demonstrated by northern blotting analysis or enzyme immunoassay.
Role of regucalcin in intracellular Ca2+ homeostasis
Intracellular Ca2+ homeostasis is regulated through plasma membrane (Ca2+–Mg2+)-adenosine 5′-triphosphatease (ATPase), microsomal Ca2+-ATPase, mitochondrial Ca2+ uptake, and nuclear Ca2+ transport in the cells. Regucalcin has been demonstrated to regulate Ca2+-transporting systems in the liver, renal cortex cells, heart, and brain tissues, suggesting its role in the regulation of intracellular Ca2+ homeostasis.
Role of regucalcin in Ca2+ homeostasis in liver cell
The high-affinity (Ca2+–Mg2+)-ATPase is located on the plasma membranes of liver cells [63, 64]. This enzyme acts as a Ca2+ pump to exclude the metal ion from the cytoplasm of liver cells. Addition of regucalcin into the reaction mixture in vitro caused an increase in (Ca2+–Mg2+)-ATPase activity in the plasma membranes isolated from rat liver, suggesting a role in the regulation of Ca2+ pump activity [65]. Regucalcin directly activates (Ca2+–Mg2+)-ATPase independently of Ca2+-stimulated phosphorylation of the enzyme [65–67], and it has been shown to stimulate ATP-dependent calcium transport across the plasma membrane vesicles of rat liver after addition of 45Ca2+ into the reaction mixture in vitro [68]. Regucalcin-enhanced ATP-dependent 45Ca2+ uptake in the plasma membrane vesicles is completely inhibited in the presence of N-ethylmaleimide or digitonin [67]. Regucalcin has been shown to bind the lipid components of liver plasma membrane, and it acts on the sulfhydryl (SH) groups that are an active site of (Ca2+–Mg2+)-ATPase [67]. The mechanism of regucalcin in activating (Ca2+–Mg2+)-ATPase may be not involved on GTP-binding protein that modulates the receptor-mediated hormonal effect (including calcitonin, epinephrine, phenylephrine, and insulin) in liver plasma membranes [69].
The effect of hormonal signaling factors (inositol-glycan, dibutyryl cyclic AMP, and inositol 1,4,5-trisphosphate) on the regucalcin-increased (Ca2+-Mg2+)-ATPase activity in rat liver plasma membranes is examined [70]. Inositol-glycan, which is generated by insulin [70], can directly activate the plasma membrane (Ca2+–Mg2+)-ATPase, and its effect is modulated by regucalcin. Cross talk with signaling factors may be seen in the regulation of Ca2+ pump activity in the plasma membranes of liver cells.
The physiological role of regucalcin in the regulation of (Ca2+–Mg2+)-ATPase activity in liver plasma membranes is examined after an oral administration of calcium chloride solution in rats [71]. Calcium administration caused an increase in (Ca2+–Mg2+)-ATPase activity in liver plasma membranes [71]. This increase was abolished in the presence of anti-regucalcin antibody, suggesting an involvement of endogenous regucalcin that is distributed in the cytoplasm. Regenerating rat liver with a proliferative cells significantly increased liver calcium content and plasma membrane (Ca2+–Mg2+)-ATPase activity between 12 and 48 h after partial hepatectomy [72]. This increase was completely abolished in the presence of anti-regucalcin antibody, indicating an involvement of endogenous regucalcin [72]. Activatory effect of regucalcin on hepatic plasma membrane (Ca2+–Mg2+)-ATPase was impaired in liver injury with carbon tetrachloride administration in rats [73]. Regucalcin plays a role as an activator protein for Ca2+ pump enzyme in the hepatic plasma membranes.
Regucalcin binds Ca2+ in the cytoplasm of liver cells, and the metal is subsequently transported into the organelle dependent on ATP [74]. Regucalcin may not tightly bind cytosolic Ca2+ of lower levels, since its calcium-binding constant is 4.19 × l05 M−1 [8]. Regucalcin may regulate cytoplasmic Ca2+ levels by activating Ca2+ pump enzyme in the plasma membranes of liver cells.
Regucalcin can stimulate the uptake of Ca2+ by rat liver mitochondria [75, 76]. The effect of regucalcin on mitochondrial Ca2+ uptake is inhibited in the presence of ruthenium red or lanthanum chloride [75], which is an inhibitor of mitochondrial Ca2+ transport. Regucalcin may have a role in the reduction of cytoplasmic Ca2+ levels due to activating mitochondrial Ca2+ uptake.
Regucalcin has also been demonstrated to activate Ca2+ pump enzymes (Ca2+-ATPase) and to stimulate ATP-dependent 45Ca2+ uptake by liver microsomes [77, 78], suggesting a role in the regulation of cytoplasmic Ca2+ levels. The effect of regucalcin in increasing Ca2+-ATPase activity in the microsomes is inhibited in the presence of thapsigargin, a specific inhibitor of microsomal Ca2+ pump enzyme. Regucalcin acts on the SH groups of microsomal Ca2+-ATPase due to the binding on the membranous lipids [78].
The components of Ca2+ uptake (Ca2+-ATPase) and Ca2+ release (Ca2+-channels) are located at separate sites on liver microsomes. Interestingly, regucalcin has been found to stimulate Ca2+ release from rat liver microsomes [79]. The mechanism is related to the inositol 1,4,5-triphosphate (IP3)-induced Ca2+ release [80]. Regucalcin may bind to IP3 receptors on the microsomes. This cell physiological significance of regucalcin is unknown. Presumably, regucalcin stimulates microsomal Ca2+ uptake when cytosolic Ca2+ concentration is raised. Also, regucalcin regulates Ca2+ storage in the endoplasmic reticulum of liver cells: it stimulates Ca2+ release from the microsomes to restore the microsomal calcium accumulation to regulate Ca2+-related microsomal functions. Regucalcin has been shown to have the reversible effect on liver microsomal glucose-6-phosphatase activity increased by Ca2+ addition [11].
The existence of an ATP-stimulated Ca2+-sequestration system is also found in liver nuclei, and it generates a net increase in nuclear matrix free Ca2+ concentration [81]. This system may play an important role in the regulation of intranuclear Ca2+-dependent processes [82]. ATPase, which is stimulated by Ca2+ in the presence of Mg2+, exists in the nuclei of rat liver, and the Ca2+-stimulated ATPase activity is involved in the nuclear Ca2+ uptake [83]. Regucalcin increases Ca2+-ATPase activity in rat liver nuclei [84]. Regucalcin has also been shown to stimulate Ca2+ release from liver nuclei [85]. Presumably, regucalcin has a role in the regulation of liver nuclear function through the effect on Ca2+ transporting system in the nuclei.
Regucalcin (SMP30) has been shown to lower intracellular Ca2+ levels by modulating plasma membrane Ca2+-pumping activity in the cloned human hepatoma HepG2 cells that overexpress regucalcin [86].
Role of regucalcin in Ca2+ homeostasis in kidney cells
Regucalcin is largely present in the kidney cortex of rats [26]. Kidney plays a physiological role in the regulation of calcium homeostasis in blood through re-absorption of urinary calcium [87, 88]. Renal cortex cells play a role in the re-absorption of urinary calcium.
Regucalcin mRNA is expressed in the kidney cortex but not in the medulla of rats [56], and its expression is stimulated after calcium administration in vivo [56]. The binding of kidney nuclear proteins to the 5′-flanking region of the rat gene for regucalcin has been shown to be enhanced through Ca2+/calmodulin signaling [89, 90]. Regucalcin mRNA expression is stimulated after the administration of dexamethasone in rats [91], and its expression is suppressed in hypertensive state in rats [92–94]. The specific nuclear factor binds to the NF1-like sequence in the promoter region of regucalcin gene in the kidney cortex of rats [90], and the nuclear factor binding and regucalcin mRNA expression are suppressed after administration of cisplatin that induces kidney damage [90, 95].
Ca2+-ATPase system has been shown to exceed the capacity of the Na+/Ca2+ exchanger, and it plays a primary role in Ca2+ homeostasis of rat kidney cortex cells [96, 97]. Regucalcin has been demonstrated to play a role as an activator of the ATP-dependent Ca2+ pumps in the basolateral membranes isolated from rat kidney cortex [98]. The effect of regucalcin in increasing Ca2+ pump enzyme (Ca2+-ATPase) activity in the basolateral membranes is completely inhibited in the presence of N-ethylmaleimide, indicating that regucalcin may act on the SH groups of Ca2+-ATPase [98].
Regucalcin has also been shown to increase Ca2+-ATPase activity and ATP-dependent calcium uptake in the microsomes of rat kidney cortex [99]. These increases are clearly decreased in the presence of N-ethylmaleimide, suggesting that regucalcin acts on the SH groups of Ca2+-ATPase in the microsomes [97].
The finding, that regucalcin increases Ca2+-ATPase activity in the basolateral membranes and microsomes of rat renal cortex, suggests a physiological role of regucalcin in the regulation of the Ca2+ homeostasis in renal cells. Regucalcin may be responsible for ATP-dependent transcellular Ca2+ transport, and it participates in the promotion of Ca2+ re-absorption in the nephron tubule of kidney cortex. Regucalcin may play a physiological role in the regulation of calcium metabolism in blood through re-absorption of urinary calcium in kidney.
Role of regucalcin in Ca2+ homeostasis in heart cells
The Ca2+ current is one of the most important components in cardiac excitation–contraction coupling [100]. This coupling mechanism is based on the regulation of intracellular Ca2+ concentration by Ca2+ pump in the sarcoplasmic reticulum of heart muscle [100]. The role of regucalcin in the regulation of heart muscle function is shown. Regucalcin mRNA is expressed in rat heart [27]. The result with western blot analysis indicates that regucalcin is present in the cytoplasm of heart muscle cells [27]. Regucalcin concentration in the heart muscle tissues has been shown to be about 3.86 × 10−8 M [26]. Regucalcin mRNA expression in the hearts of rats is decreased with increasing age [101], and free radical stress has a suppressive effect on its gene expression [101]. Overexpression of regucalcin in transgenic rats has been found to accelerate free radical stress-induced death of rats [101].
Regucalcin has been found to increase Ca2+-ATPase activity and ATP-dependent Ca2+ uptake, which regulates intracellular Ca2+ concentration related to cardiac excitation–contraction coupling, in rat heart microsomes, suggesting its role in the regulation of heart muscle function [27]. The effect of regucalcin in increasing heart microsomal Ca2+-ATPase activity is inhibited in the presence of thapsigargin [27], a specific inhibitor of the sarcoplasmic reticulum Ca2+ pump enzyme (Ca2+-ATPase) [102], indicating that regucalcin activates Ca2+ pump enzyme in the sarcoplasmic reticulum. It is suggested that regucalcin binds to the lipids at the close site of Ca2+-ATPase in heart microsomes, and that it acts on the SH group which may be an active site of the enzyme and stimulates Ca2+-dependent phosphorylation of Ca2+-ATPase [27].
Phospholamban has been known to inhibit Ca2+ pump enzyme (Ca2+-ATPase) in the sarcoplasmic reticulum (microsomes) of heart muscle [103]. Ca2+-ATPase is activated through cAMP-dependent phosphorylation of phospholamban following hormonal stimulation [103]. The endogenous activatory protein of sarcoplasmic reticulum Ca2+-ATPase is unknown. Regucalcin, which is present in the cytoplasm of heart muscle, may play an important role as an endogenous activator in the regulation of sarcoplasmic reticulum Ca2+-ATPase activity in rat heart muscle [100]. Regucalcin may play a physiological role in the regulation of cardiac excitation–contraction coupling.
The role of regucalcin in the regulation of Ca2+-ATPase activity in the heart mitochondria of rats is examined, moreover [104]. Regucalcin is found to be present in the mitochondria of normal rat heart [104], and this localization is increased in the heart of regucalcin transgenic rats as compared with that of normal rats [104]. The addition of regucalcin (10−11 to 10−8 M) in the enzyme reaction mixture caused a significant increase in Ca2+-ATPase activity in the heart mitochondria in the presence of 50 μM CaCl2 [104]. Ca2+-ATPase activity was also increased in the heart mitochondria of regucalcin transgenic rats [104]. Regucalcin has an activating effect on Ca2+-ATPase in rat heart mitochondria, suggesting its role in the regulation of heart mitochondrial function.
Regucalcin may play a role in the regulation of cytoplasmic Ca2+ levels due to activating Ca2+ pump activity in the sarcoplasmic reticulum and mitochondria in heart cells.
Role of regucalcin in Ca2+ homeostasis in brain tissues
Intracellular Ca2+ concentration in the neuronal cells of brain is regulated by various buffering and transport systems such as the membrane Na+–Ca2+ exchanges, the membranous Ca2+-ATPase, Ca2+-binding proteins, and intracellular Ca2+ uptake systems [105–109]. The changes in the neuronal Ca2+ homeostasis with aging may be implicated in age-related disturbance in cognitive functions [109]. There is growing evidence that the alteration in the neuronal Ca2+ regulation is also implicated in the pathology of Alzheimer’s disease [109].
The expression of regucalcin mRNA is demonstrated in brain tissues [28, 110]. Regucalcin concentration in the brain tissues has been shown to be about 5 × 10−9 M as measured using enzyme-linked immunoadsorbent assay, and this level is lowered with aging [26]. Regucalcin is localized in the neurons isolated from rat brain tissues [28], suggesting its role in brain function.
Brain calcium accumulation has been shown to increase after oral administration of calcium in rats, and fasting enhances its accumulation [111]. The supply of glucose may be required in the regulation of brain Ca2+ homeostasis in rats in vivo [111], suggesting a physiological significance of energy-dependent mechanism in brain calcium metabolism. Fasting caused a significant increase in brain calcium content and Ca2+-ATPase activity in the microsomes and mitochondria of brain tissues in young and aged rats, and these increases were restored after the supply of glucose, supporting a physiologic significance of energy-dependent mechanism in the regulation of brain calcium in rats with different ages [112].
Aging causes a decrease in Ca2+-ATPase activity in the brain plasma membranes of rats [113], suggesting that aging enhances the entry of Ca2+ into brain neuronal cells across the plasma membranes. In addition, aging induces an attenuation of Ca2+-sequestrating system in the brain microsomes, supporting the view that a disturbance of the neuronal Ca2+ regulation is brought with increasing age [114]. Protein kinase C activates brain microsomal Ca2+-ATPase in aged rats [115]. It is speculated that aging-induced increase in Ca2+-ATPase activity results from the translocation to the microsomes of protein kinase C in brain cytosol [115]. The development of brain disease with aging may be partly related to the toxicity of brain calcium raised by the increase in microsomal Ca2+-ATPase activity with aging. The disturbance of brain Ca2+ homeostasis may play a pivotal role in the revelation of brain disease.
Regucalcin has been found to have an inhibitory effect on Ca2+-ATPase activity in rat brain microsomes [110], suggesting that regucalcin plays a role in the regulation of microsomal Ca2+-ATPase activity in rat brain. Interestingly, the concentration of regucalcin in the cerebral cortex and hippocampus of brain tissues is decreased with aging [110]. The suppressive effect of regucalcin on brain microsomal Ca2+-ATPase activity was weakened in aged rats [110]. Presumably, the aging-induced elevation of brain microsomal Ca2+-ATPase activity is partly resulted from an attenuation of regucalcin action on the enzyme activity with aging. There may be a possibility that the translocation of protein kinase C to the brain microsomes of aged rats [115] is a cause of the attenuation of regucalcin effect on the enzyme activity. Regucalcin may play a physiological and pathophysiological role in the regulation of intracellular Ca2+ concentration in the brain tissues.
Regucalcin has an activatory role in the regulation of Ca2+-ATPase activity in the mitochondria of brain tissues of rats [116]. The addition of regucalcin (10−10 to 108 M), which is a physiological concentration in rat brain tissues, into the enzyme reaction mixture containing 25 μM calcium chloride caused a significant increase in Ca2+-ATPase activity, while it did not significantly change in Mg2+-ATPase activity [116].
Regucalcin levels are increased in the brain tissues or the mitochondria obtained from regucalcin transgenic rats. The mitochondrial Ca2+-ATPase activity has been found to increase in regucalcin transgenic rats as compared with that of wild-type rats [116]. Endogenous regucalcin plays a role in the regulation of Ca2+-ATPase activity in the brain mitochondria of rats.
Conclusion
Regucalcin plays a cell physiological role as a regulatory protein that is involved in the regulation of Ca2+ homeostasis in various cell types. The low cytoplasmic Ca2+ concentration of living cells is maintained through energy-requiring pumps. These pumps either remove Ca2+ to the extracellular space by transport across the plasma membrane or accumulate it inside of intracellular organelles such as the mitochondria and endoplasmic reticulum (microsomes). Regucalcin stimulates the activity of these pumps to lower the cytoplasmic Ca2+ levels, as shown in Fig. 1. This may provide the cellular mechanism of the inhibitory effect of regucalcin in the regulation of cell functions related to Ca2+ signaling.
Regucalcin has a pivotal role in keeping intracellular Ca2+ homeostasis that is attenuated with various stimulating in cells. Regucalcin increases plasma membrane (Ca2+–Mg2+)-ATPase, mitochondrial Ca2+-ATPase and microsomal Ca2+-ATPase activities in cells. Regucalcin also stimulates Ca2+ release from the microsomes (endoplasmic reticulum). Regucalcin has an inhibitory effect on nuclear Ca2+-ATPase and a stimulatory effect on Ca2+ release from the nucleus. Through thus mechanism, regucalcin plays a part in regulating the rise of cytosolic Ca2+ concentration and nuclear matrix Ca2+ levels in cells that suppresses Ca2+-dependent cellular events
Regucalcin reverses Ca2+ effect in enzyme regulation
Ca2+ and calmodulin systems generally activate various enzymes in many cells. There are many evidences that regucalcin has an inhibitory effect on enzyme activation by Ca2+/calmodulin [24–28]. This section outlines the findings on the inhibitory effect of regucalcin on action of Ca2+ in cell metabolism.
Liver metabolism is regulated through an increase in Ca2+ level in the cytoplasm of liver cells due to hormonal stimulation [3–5]. Regucalcin has an inhibitory effect on Ca2+/calmodulin-dependent enzyme activity in vitro. The hormonal effect on fructose-1,6-diphosphatase, which promotes the conversion from fructose-1,6-diphosphate to glucose-6-phosphate in the hepatic cytoplasm of rats, is mediated through Ca2+. This enzyme activity is activated through Ca2+/calmodulin [10]. The activation of fructose-1,6-diphosphatase by Ca2+/calmodulin was completely reversed after the addition of regucalcin in the enzyme reaction mixture [10].
Phosphorylase a activity in the liver particulate glycogen is increased after addition of Ca2+ (10 μM) [13]. This increase was completely reversed after addition of regucalcin in the enzyme reaction mixture [13]. Regucalcin (1.0 μM) reversed activations of pyruvate kinase [12] and glucose-6-phosphatase [11] after addition of Ca2+ in the enzyme reaction mixture. These findings suggest that regucalcin regulates glycogenolysis and gluconeogenesis that is stimulated by Ca2+ in liver cells.
The reversible effect of regucalcin is also shown in Ca2+-induced inhibition of 5′-nucleotidase activity in liver plasma membranes [15] and deoxyuridine 5′-triphosphatase activity in hepatic cytosol [117].
Thus, regucalcin has a reversible effect on the activation and inhibition of many enzymes by Ca2+.
The controlled use of energy to maintain homeostasis and cellular function is a basic property of all cells. The free energy of ATP is believed to be the major cytosolic intermediate in this process. ATP is produced from the energy obtained by the oxidation of metabolic substrates in glycolysis and oxidative phosphorylation. ATPase produces the energy from ATP in the cell cytosol. The cytosolic factors regulating ATPase activity is important. Regucalcin has been shown to play an inhibitory role in the regulation of ATPase activity in the brain cytosol of young and aged rats [118], suggesting a role in the regulation of energy conversion in brain tissues.
The mechanism of the reversible effect of regucalcin on various enzyme activities, which are regulated through Ca2+, has not been well known. However, action of regucalcin may be partly based on Ca2+ binding, because the protein has 6–7 high-affinity binding sites per molecule (Kf = 4.19 × 105 M−1) [8]. Regucalcin may directly bind to Ca2+ and/or calmodulin. In addition, it is possible that regucalcin may bind to enzymes and that affects enzyme activity.
Regucalcin inhibits Ca2+/calmodulin-dependent enzyme activation
Ca2+/calmodulin-dependent enzymes are localized in many tissues and cells. Regucalcin has been found to have an inhibitory effect on various Ca2+/calmodulin-dependent enzyme activations.
Cyclic adenosine monophosphate (AMP) is a second messenger for hormonal stimulation in many cells. Cyclic AMP is degraded by cyclic AMP phosphodiesterase in liver cytosol [119]. The enzyme activity is increased through Ca2+/calmodulin [1]. Regucalcin has been found to inhibit the activation of cyclic AMP phosphodiesterase by Ca2+/calmodulin in the cytosols of liver and renal cortex [120, 121], suggesting a role of regucalcin in the regulation of cyclic AMP level in the cells.
Nitric oxide (NO) may be important as a signaling factor in many cells [122]. NO, which has an unpaired electron reacts with protein, targets primarily through their thiol or heme groups, and acts as a messenger or modulator molecule in many biological systems. NO is produced from l-arginine with l-citrulline as a coproduct in a reaction catalyzed by NO synthase that requires Ca2+/calmodulin [122].
Regucalcin has been shown to have a suppressive effect in the enhancement of NO synthase activity in the cytosols of liver [123, 124] and kidney cortex [125] of rats. Overexpression of regucalcin did not cause a significant alteration of NO synthase activity in the kidney cortex cytosol of regucalcin transgenic rats as compared with that of wild-type rats [125]. However, the effect of calcium chloride (10 μM) in increasing NO synthase activity in the kidney cortex cytosol of wild-type rats was weakened in regucalcin transgenic rats [125]. The presence of anti-regucalcin monoclonal antibody (25 or 50 ng/ml) in the reaction mixture caused a significant increase in NO synthase activity, and this increase was completely abolished after the addition of regucalcin (10−7 M). Endogenous regucalcin has a suppressive effect on NO synthetase activity in the cytosol of various tissues of rats.
Regucalcin has also been found to suppress Ca2+/calmodulin-dependent NO synthase activity in the heart cytosol of rats [126]. Regucalcin had an inhibitory effect on NO synthase activity in the presence of antagonist for calmodulin [126], indicating a direct effect of regucalcin on the enzyme independent of Ca2+/calmodulin. The physiological significance of regucalcin inhibition of NO synthase in heart muscle cytosol is unknown. However, regucalcin may participate in the regulation of NO production in heart muscle cells. NO acts as a messenger or modulator molecule in heart muscle. NO production may be stimulated through Ca2+ signaling due to hormonal stimulation in heart muscle cells. Regucalcin may have a suppressive effect on over-production of NO due to inhibiting NO synthase in heart muscle cells.
Nitric oxide (NO) acts as a messenger or modulator molecule in brain neurons. Regucalcin has also been shown to reveal a suppressive effect on NO synthase activity in the brain cytosol of young and aged rats, even though regucalcin levels are reduced with increasing age [127]. A remarkable expression of regucalcin protein was seen in the cytosol and nucleus of the brain tissues of regucalcin transgenic rats as compared with that of wild-type rats [128]. NO synthetase activity was decreased in the brain cytosol of transgenic rats, and the presence of anti-regucalcin monoclonal antibody (50 ng/ml) in the enzyme reaction mixture caused a significant increase in cytosolic NO synthase activity in the cytosol of brain tissues of wild-type rats [128]. Endogenous regucalcin plays a suppressive role in the regulation of brain neuronal NO synthase activity in rats.
Thus, regucalcin has been demonstrated to have a suppressive role on Ca2+/calmodulin-dependent NO synthase activity in various tissues. Regucalcin may play a role as a suppressor protein in NO production in many cell types, and it may regulate many cellular events that are involved in NO signaling.
Superoxide dismutase (SOD) plays a role in the prevention of cell death and apoptosis in the heart. The decrease in Mn-SOD activity is associated with increased mitochondrial oxidative damage as demonstrated by a decrease in the activities of iron sulfhydryl proteins sensitive to oxygen stress [129]. Cu/Zn-SOD has been shown to play the role of protector against doxorubicin-induced cardiotoxicity in mice [130]. Meanwhile, NO has a role in the suppression of myocardial O2 consumption in rats [131].
Regucalcin has been found to increase SOD activity in the cytosol of rat liver [132] and heart [133]. Regucalcin has an inhibitory effect on NO synthase activity in the heart cytosol [133]. Production of superoxide radicals is widely accepted as the cause of the cardiodamage. Presumably, regucalcin participates in the control of production of superoxide radicals in rat heart muscle cells.
The multifunctional Ca2+/calmodulin-dependent protein kinases play an important role in the response of the cells to a calcium signal [1, 134]. Regucalcin has been shown to inhibit Ca2+/calmodulin-dependent protein kinase activity in the cytosol of rat liver [135], kidney cortex [136], and brain tissues of rats [137, 138]. An appreciable effect of regucalcin is seen at 0.5 μM, which is a cell physiological concentration. As Ca2+/calmodulin-dependent protein kinase in the cytoplasm is activated through calcium signal, regucalcin may regulate a signal transduction for Ca2+. The mechanism of action of regucalcin may be partly based on its binding to Ca2+/calmodulin and/or enzyme. Regucalcin has been demonstrated to bind on calmodulin in analysis with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using calmodulin-agarose beads [139].
Nishizuka [2] discovered a diacylglycerol-activated Ca2+ and phospholipid-dependent protein kinase (protein kinase C). Protein kinase C is distributed widespread in the body, with amounts in the liver being intermediate between the high levels found in brain and spleen [2]. Protein kinase C is capable of phosphorylating cytoplasmic proteins. It is found that regucalcin inhibits protein kinase C activity in the cytoplasm of rat liver [140], kidney cortex [141], and brain cytosol and brain neurons [137, 138], supporting the view that regucalcin plays a role in the regulation of Ca2+-dependent cellular functions. The presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture caused a significant elevation of protein kinase activity, indicating that the endogenous regucalcin has an inhibitory effect on the enzyme activity [137, 138].
The regulatory effect of regucalcin in rat brain function may be attenuated with aging. Increasing age enhances protein kinase activity in rat brain cytosol [137]. This enhancement may be partly involved in aging-decreased regucalcin in rat brain tissues [110]. It is speculated that the endogenous regucalcin plays a suppressive role in the activation of Ca2+-dependent protein kinase in the brain cytosol, and that aging may weaken the effect of regucalcin. Regucalcin may play a pivotal role in the regulation of phosphorylation of the cytosolic proteins in brain tissues. Interestingly, it has been reported that regucalcin gene is localized on human chromosome X that encompasses the map location for a growing number of diseases with a genetic basis; these include syndromic and non-syndromic forms of X-linked mental retardation and X-linked neuromuscular diseases [23]. Regucalcin may have a pathophysiological role in brain disease with aging.
As mentioned above, regucalcin plays an inhibitory role in signaling pathway that is mediated through cyclic AMP, NO, and Ca2+-dependent protein kinases in many tissues and cell types.
Protein phosphorylation–dephosphorylation is a universal mechanism by which numerous cellular events are regulated [142]. It has become apparent that there may exist many phosphatases that, like the kinases, are just elaborately and rigorously controlled [142, 143]. Protein phosphatase plays an important role in intracellular signal transduction due to hormonal stimulation [142].
Calcineurin, a calmodulin-binding protein, has been shown to possess a Ca2+-dependent and calmodulin-stimulated protein phosphatase activity [144]. Regucalcin has been demonstrated to inhibit calcineurin activity in the cytosol of liver and renal cortex after its binding to calmodulin [145, 146]. Protein phosphatases, which endogenous regucalcin acts in liver cytoplasm, may be insensitive to okadaic acid [147]. Protein phosphatase activity toward phosphotyrosine, phosphoserine, and phosphothreonine in the cytosol of rat liver was elevated in the presence of anti-regucalcin monocional antibody in the enzyme reaction mixture in vitro, suggesting its role of endogenous regucalcin [145]. Regucalcin may be a unique protein, which has inhibitory effects on protein tyrosine phosphatase and protein serine/threonine phosphatase. Regucalcin, which was localized in rat liver nuclei, has been shown to inhibit nuclear protein phosphatase activity [148].
Endogenous regucalcin plays a role in the regulation of protein phosphatase activity in the cytosol and nuclei of rat renal cortex [149–151]. Regucalcin has been found to be present in the cytosol and nuclei of rat kidney cortex using western blot analysis [151]. The addition of regucalcin (50–250 nM) in the enzyme reaction mixture obtained from the cytoplasm and nuclei from rat kidney cortex caused a decrease in protein phosphatase activity toward phosphotyrosin, phosphoserine, and phosphothreonine [149, 150]. The effect of calcium (25 μM) and calmodulin (2.5 μg/ml) in increasing protein phosphatase activity was decreased after the addition of regucalcin. Protein phosphatase activity in the cytosol and nuclei was increased in the presence of anti-regucalcin monoclonal antibody (10–50 ng/ml) in the enzyme reaction mixture [149, 150]. Regucalcin plays a suppressive role in the regulation of protein phosphatase activity in the cytoplasm and nucleus of rat kidney cortex.
Kidney cortex calcium content and the cytosolic and nuclear regucalcin levels were increased at 0.5–5 h after a single intraperitoneal administration of calcium chloride solution (10 mg Ca/100 g body weight) in rats [151]. The cytosolic and nuclear protein phosphatase activity, which is raised in calcium-administered rats, was found to enhance when anti-regucalcin monoclonal antibody was added in the enzyme reaction mixture [151]. The effect of antibody was completely abolished after the addition of regucalcin in the enzyme reaction mixture. Thus, endogenous regucalcin has a suppressive effect on the enhancement of protein phosphatase activity in the cytosol and nucleus of kidney cortex in calcium-administered rats.
Cardiac hypertrophy is induced by calcineurin, which dephosphorylates the transcription factor NF-A3, enabling it to translocate to the nucleus [152]. Transgenic mice, that express activated forms of calcineurin or NF-AT3 in the heart, develops cardiac hypertrophy and heart failure that mimic human heart disease [152], suggesting a hypertrophic signaling pathway. If regucalcin has a suppressive effect on calcineurin activity in the heart cytosol of normal and transgenic rats [153], overexpression of regucalcin may have a pathophysiological role in the prevention of development of cardiac hypertrophy and heart faliure.
Regucalcin has also been shown to have an inhibitory effect on Ca2+/calmodulin-dependent protein phosphatase activity toward phosphotyrosine, phosphoserine, and phosphothreonine in rat brain cytosol [154] and neurons [28]. The presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture caused a significant elevation of protein phosphatase activity in the brain cytosol, indicating that the endogenous regucalcin has a suppressive effect on the cytosolic enzyme activity [154]. Regucalcin has been shown to have an inhibitory role in the regulation of protein phosphatase activity in rat brain cytosol.
Regucalcin is localized in the microsomes of rat brain, and aging causes a decrease in its protein levels [155]. Regucalcin has been also shown to have a suppressive effect on protein tyrosine phosphatase activity in rat brain microsomes [155]. Aging caused an increase in protein tyrosine phosphatase activity in rat brain microsomes and the suppressive effect of regucalcin on the enzyme activity was weakened in aged rats [155], suggesting that the decrease in microsomal regucalcin with aging is partly involved in the enhancement of microsomal protein tyrosine phosphatase activity with aging [155].
Regucalcin has been found to be present in the nucleus of rat brain and the endogenous regucalcin has a suppressive effect on the nuclear protein tyrosine phosphatase activity [156]. Increasing age has also been found to induce a reduction in rat brain nucleus and may lead to attenuation of the suppressive effect of regucalcin on the nuclear protein tyrosine phosphatase activity [156]. Regucalcin may play a pivotal role as a regulatory protein in the regulation of brain function that relates to protein phosphorylation–dephosphorylation.
Thus, regucalcin may play a physiological role in the intracellular control of the hormonal stimulation for phosphorylation and dephosphorylation of many proteins in various cell types.
As mentioned above, regucalcin has been demonstrated to reverse the activity of many Ca2+-activated enzymes (phosphorylase a, glucose-6-phosphatase, fructose-1,6-bisphosphatase, pyruvate kinase, protein kinase C, Ca2+/calmodulin-dependent protein kinase, protein phosphatase, and Ca2+/calmodulin-dependent cyclic AMP phosphodiesterase) and of Ca2+-inhibited enzymes (5′-nucleotidase and dUTPase).
The first action is that regucalcin binds Ca2+ and that inhibits the metal’s effect on many enzymes. The effect of regucalcin, that reverses Ca2+ action on many enzymes, may be based on its binding of Ca2+, since the protein has 6–7 high-affinity binding sites per molecule, and a Ca2+-binding constant of 4.19 × 105 M−1 [8]. The intrinsic significance of regucalcin action may be the binding of Ca2+ by its protein.
The second is that Ca2+-binding regucalcin directly inhibits the function of enzymes and somewhat stimulates enzyme function. The direct action of Ca2+-binding regucalcin or the protein itself may be decided by the protein structure of enzymes. Spectroscopical studies have clearly demonstrated that Ca2+-binding induces conformational changes in regucalcin, which may then result in increased hydrophobicity of the protein, and loosening of the conformation of regucalcin [9].
Regucalcin can directly inhibit the enzymes that are activated through Ca2+-calmodulin [10]; this also results from regucalcin that affects the binding of Ca2+ to calmodulin. Which of the two proteins binds Ca2+ may be decided by their relative concentrations of Ca2+ in hepatic cytosol, since the Ca2+-binding constant of regucalcin is greater than that of calmodulin [8]. Calmodulin exists as a monomer of molecular weight 17,000 and contains four Ca2+-binding sites [1]. In the enzyme assay system of Ca2+/calmodulin-dependent cyclic AMP phosphodiesterase activity, the inhibitory effect of regucalcin on the enzyme activation through Ca2+-calmodulin is completely blocked with the addition with increasing concentrations of Ca2+ [117, 118]. This further supports the view that the mechanism by which regucalcin inhibits Ca2+ action is based on the binding of Ca2+.
Moreover, in the enzyme assay system of protein kinases (protein kinase C and Ca2+/calmodulin-dependent protein kinase), the effect of regucalcin, which inhibits the activation of enzymes by Ca2+, is seen with increasing concentrations of Ca2+. This suggests that regucalcin directly inhibits the enzyme activation in addition to Ca2+-binding. Also, it is possible that Ca2+-binding regucalcin or the protein itself can directly inhibit the enzyme activity.
There may be many enzymes that are regulated by regucalcin and/or Ca2+-binding regucalcin in various cell types. This remains to be elucidated.
Regucalcin regulates protein synthesis and degradation
Regucalcin has been shown to have a regulatory effect on protein synthesis and protein degradation, suggesting that regucalcin plays a role in the regulation of protein turnover in cells.
Role of regucalcin in protein synthesis
Protein synthesis is depressed in a variety of eukaryotic cell types exposed to conditions depleting Ca2+ but not Mg2+ [157]. It has been also proposed that hormones (vasopressin and α-adrenergic agonist), which are known to mobilize sequestered Ca2+ within liver cells, inhibit amino acid incorporation by influencing a Ca2+ requirement associated with protein synthesis [158]. Moreover, vasopressin inhibits the rate of protein synthesis in isolated hepatocytes partially depleted of Ca2+ [159]. These investigations propose the hypothesis that a sequestered pool of intracellular Ca2+ is required for the maintenance of high rates of protein synthesis in liver cells [158, 159]. On the other hand, vasopressin and α-adrenergic agonist cause an increase in the intracellular free Ca2+ concentration of hepatocytes that are not depleted of Ca2+ [160, 161]. Whether the increase in intracellular Ca2+ influences hepatic protein synthesis is well undefined, however, it may be important to clarify the effect of Ca2+ addition on hepatic protein synthesis in vitro.
It has been demonstrated that Ca2+, of various metals, can uniquely inhibit in vitro protein synthesis using the 5,500g supernatant fraction (the microsomes and cytosol) of rat liver homogenate [162]. Its inhibition was seen after addition of 1.0 μM Ca2+. Ca2+ addition caused a remarkable decrease in the activity of aminoacyl (leucyl)-tRNA synthetase, which is a rate-limiting enzyme of protein synthesis at translational process, in hepatic cytosol [162]. Ca2+ may directly inhibit hepatic protein synthesis in subcellular fraction of liver cells. Ca2+ is required for protein synthesis in hepatocytes exposed to conditions depleting the cation [162]. It is not clarified whether protein synthesis in hepatocytes not depleted of Ca2+ requires exogenous Ca2+. The mechanism by which Ca2+ is required in protein synthesis of hepatocytes depleted of Ca2+ may be complex.
Calmodulin, which can amplify Ca2+ effects on enzymes [1], did not have an appreciable effect on in vitro protein synthesis using the 5,500g supernatant fraction of liver homogenate in the presence of Ca2+ (10 μM) [162]. The activity of aminoacyl-tRNA synthetase in hepatic cytosol was not altered through calmodulin [163]. Presumably, the protein synthesis is inhibited by Ca2+, which is not bound to calmodulin.
The role of regucalcin in the regulation of in vitro protein synthesis using the 5,500g supernatant fraction of rat liver homogenate is investigated [162]. Regucalcin caused a remarkable inhibition of hepatic protein synthesis in vitro [162]. Regucalcin could not reverse the Ca2+-induced inhibition of protein synthesis [162], although it has been shown that regucalcin can reverse the Ca2+ effect on many enzymes in liver cells. Since regucalcin can bind to liver cytosolic proteins and the binding is slightly enhanced with the coexistence of 0.1 μM Ca2+ [65], Ca2+-binding regucalcin and/or Ca2+ free regucalcin may be able to inhibit hepatic protein synthesis. In fact, the presence of regucalcin (1 and 2 μM) could fairly decrease hepatic protein synthesis that was reduced after the addition of 10 μM Ca2+[162]. Regucalcin itself may play a role in the regulation of protein synthesis in liver cells.
Regucalcin has been shown to inhibit hepatic aminoacyl-tRNA synthase activity [162]. The inhibitory effect of regucalcin was seen in the presence of Ca2+ (10 μM). The inhibitory effect of regucalcin on hepatic protein synthesis may be partly based on a remarkable decrease of aminoacyl-tRNA synthetase activity caused by regucalcin. Regucalcin may bind aminoacyl (leucyl)-tRNA synthetase in hepatic cytosol, since iodinated regucalcin can bind the proteins in hepatic cytosol [65]. Regucalcin may be able to regulate liver cell function that is not affected by cellular Ca2+.
The role of endogenous regucalcin on protein synthesis is examined using anti-regucalcin monoclonal antibody, moreover [163]. The presence of anti-regucalcin monoclonal antibody in the reaction mixture caused a significant increase in protein synthesis and [3H] leucyl-tRNA synthetase activity in normal rat liver. These increases were completely prevented in the addition of exogenous regucalcin (1.0 μM). Liver cytosol contained about 16 μg of regucalcin per 1 mg of the cytosolic protein; the reaction mixture contained about 0.17–0.19 μM of endogenous regucalcin, because the cytosolic protein in the range 360–390 μg was added into the mixture of 1.0 ml. Endogenous regucalcin may have a suppressive effect on protein synthesis in liver cells.
Hepatic protein synthesis has been shown to enhance regeneration of rat liver, which induces a proliferation of liver cells after partial hepatectomy [163]. This enhancement was remarkable at 24 and 48 h after partial hepatectomy. Hepatic protein synthesis in regenerating liver was further enhanced in the presence of anti-regucalcin monoclonal antibody in the reaction mixture. Endogenous regucalcin has a suppressive role on the enhancement of protein synthesis in regenerating liver.
Role of regucalcin in protein degradation
Evidence for the role of Ca2+-activated protease (calpains) is implicated in signal transduction [164]. Two neutral Ca2+-requiring proteinases, differing in molecular size, have been isolated from rabbit liver cytosol [165]. Both are recovered as inactive proenzymes that can be converted to the active forms by high (0.1–1.0 mM) concentrations of Ca2+ in the absence of substrate or, in the presence of a protein substrate, by low (1–5 μM) concentrations of Ca2+ [165]. The activated proteinases required only 1–5 μM Ca2+ for maximal activity [165].
The addition of regucalcin (0.25–2.0 μM) into the enzyme reaction mixture has been found to induce a remarkable increase of neutral proteinase activity in the presence of 5.0 μM Ca2+ [166]. The effect of regucalcin was seen at 0.25 μM. Regucalcin may activate Ca2+-requiring proteinase in rat liver cytosol. The effect of regucalcin in increasing liver cytosolic proteinase activity is also seen in the absence of Ca2+ [166]. This increase was remarkable as compared with that of Ca2+ addition. Regucalcin may activate Ca2+-not requiring neutral proteinase in rat liver cytosol. Regucalcin has a reversible effect on the activation of various enzymes by Ca2+ and/or calmodulin [120, 121, 147]. The finding, that regucalcin can activate neutral proteinase in rat liver cytosol in the presence or absence of Ca2+, was novel.
The activatory effect of regucalcin on liver cytosolic proteinase activity was not seen in the presence of anti-regucalcin antiserum in the enzyme reaction mixture [166], suggesting a role of endogenous regucalcin in the activation of cytosolic proteinase.
The activatory effect of regucalcin on neutral proteases in the liver cytosol is characterized [167]. Leupeptin is a potent inhibitor of SH-proteinase. The regucalcin-increased proteinase activity was inhibited in the presence of leupeptin in the enzyme reaction mixture [167]. Regucalcin may activate neutral cysteinyl-proteinase in the liver cytosol. The effect of regucalcin in increasing proteolytic activity in rat liver cytosol was not abolished in the presence of diisopropylfluorophosphate (DFP), an inhibitor of serine-protease, although DFP alone had an inhibitory effect on the proteolytic activity [167]. Regucalcin does not act on serine-proteases in liver cytosol. The effect of regucalcin was also seen in the presence of a chelator of metal ions, suggesting that regucalcin does not activate metal-related proteases in liver cytosol.
The proteolytic activity in liver cytosol was markedly increased after the addition of dithiothreitol (DTT), a protecting reagent for SH group, in the enzyme reaction mixture and this increase was completely inhibited in the presence of N-ethylmaleiimide (NEM), a SH group-modifying agent [167]. The effect of regucalcin in increasing proteolytic activity was seen in the presence of DTT in liver cytosol, although it was completely inhibited in the presence of NEM [167]. Regucalcin may act on the SH group of cysteinyl-proteases in liver cytosol.
Two forms of Ca2+-activated neutral proteases (calpains) have been identified in hepatocytes and other cells, m-calpain and μ-calpain [165]. Isolated μ-calpain requires micromolar concentrations of Ca2+ for activation, while m-calpain requires millimolar concentrations [168]. The activation of proteases in rabbit liver cytosol required only 1–5 μM Ca2+ for maximal activity [167]. Ca2+ (10 μM)-increased proteolytic activity in rat liver cytosol was inhibited in the presence of NEM, indicating that neutral proteases (including calpains), which are activated by Ca2+, exist in liver cytoplasm [167].
The activity of calpains, which are thiol protease [169], increases in hepatocytes following addition of ATP [170]. The proteolytic activity in liver cytosol was decreased after the addition of calpastatin, a specific inhibitory of calpains, indicating the existence of calpains in the cytosol [167]. Regucalcin-increased proteolytic activity was abolished in the presence of calpastatin. Regucalcin may be an activator for calpains. m-Calpain isolated from rabbit skeletal muscle was activated by regucalcin independent on Ca2+. This activation was inhibited after the addition of NEM [167]. Regucalcin acts on the SH group in m-calpain. Presumably, regucalcin may be able to activate both m- and μ-calpains.
The role of regucalcin in the regulation of neutral proteolytic activity in rat kidney cortex cytosol is also examined [171, 172]. Regucalcin had an activatory effect on neutral proteolytic activity in the kidney cortex cytosol [172]. This increase was abolished in the presence of anti-regucalcin monoclonal antibody, supporting the view that endogenous regucalcin plays a role as activator of proteases in the renal cortex cortisol [172]. The effect of regucalcin on proteolytic activity was not altered in the presence of calcium chloride (0.01 and 1.0 mM) or EGTA (1.0 mM), indicating that the effect of regucalcin was independent on Ca2+. Regucalcin may activate both calpains and other proteases in the kidney cortex cytosol. Regucalcin has been also shown to activate SH proteases in rat renal cortex cytosol. Presumably, regucalcin acts on the SH groups of protease in rat renal cortex cytosol.
The activatory effect of regucalcin on proteases is seen in the concentrations of 0.01–0.25 μM [171]. The concentration of regucalcin in rat kidney tissue is found to be present at about 5.3 μM. Regucalcin plays a physiological role in the activation of thiol proteases in renal cortex cells.
Regucalcin uniquely activates thiol proteases independent on Ca2+ in the liver cytosol, whereas it has no effect on serine proteases and metalloproteases [166, 167]. Such an effect of regucalcin was also found in the renal cortex cytosol [172]. Regucalcin may be an activator on thiol proteases in liver and kidney cells. Regucalcin also plays a role as an activator in other tissues that express regucalcin.
Calpains are ubiquitous, non-lysosomal, calcium-dependent proteases that may play important roles in Ca2+-mediated intracellular processes [164, 165, 170]. The ability of calpain to alter the limited proteolysis, the activity or function of numerous cytoskeletal proteins, protein kinases, receptors and transcription factors suggests the involvement of the protease in various Ca2+-regulated cellular functions [173]. Calpains may also play an integral role in modulating the activity of protein kinase C, a key protein in many signal transduction processes [174], since they convert the native Ca2+/phospholipids-dependent kinase to a soluble form that does not require Ca2+ or phospholipids for activity [2]. Regucalcin can increase the activity of thiol proteases including calpain in rat liver and renal cortex cytosols. Regucalcin may play a pivotal role in the regulation of cellular functions related to Ca2+ mediated through thiol proteases. Presumably, regucalcin plays an important role in the regulation of signal transduction that is involved in proteases.
Role of regucalcin in the regulation of nuclear function
Mounting evidence suggests that Ca2+ is active in liver nuclear function [81–83]. Calmodulin exists in rat liver nuclei [82]. The existence of an ATP-stimulated Ca2+-sequestration system in rat liver nuclei that requires calmodulin and generates a net increase in nuclear matrix free Ca2+ concentration has been reported [81]. Calmodulin stimulates DNA synthesis in liver cells [175], and the effect of calmodulin is mediated through α-adrenergic stimulation [176, 177]. There are many evidences that regucalcin plays an important role in the regulation of nuclear functions.
Nuclear localization of regucalcin and its binding to nuclear protein or DNA
Regucalcin has been shown to localize in the nuclei of rat liver [37, 148]. Regucalcin can bind on calmodulin-agarose beads [139]. Liver nuclear extract were incubated with calmodulin-agarose beads, and calmodulin-agarose beads were applied to SDS-PAGE. Band that coincides with regucalcin was found on SDS-PAGE [139], suggesting that regucalcin is present in the nucleus [148].
Exogenous regucalcin has been shown to transport into the nucleus isolated from normal rat liver [37]. Endogenous regucalcin is present in the nuclei of rat liver using western blotting analysis [37]. When isolated liver nuclei were incubated in the presence of exogenous regucalcin (50 μg/ml; 1.5 μM), potent band for regucalcin was found in the nucleus [37], supporting that the protein is translocated into the nucleus. This translocation was seemed to be an early event, since potent band for regucalcin was seen with only 10 min of incubation. A part of regucalcin, which is localized in the cytoplasm of liver cells, is translocated to the nucleus [37].
Nuclear regucalcin translocation was not appreciably changed in the presence of ATP (2 mM), guanosine 5′-triphosphate (GTP, 2 mM), and calcium chloride (0.1 mM), suggesting that its translocation is not mediated through nuclear localization signal [37]. ATP and GTP are required for nuclear import of proteins that are localized in the nuclei. ATP or GTP does not regulate the translocation of regucalcin into liver nuclei. Regucalcin is translocated independently of Ca2+.
Nuclear protein transport is blocked in the presence of the lectin wheat germ agglutinin (WGA) [83]. Nuclear regucalcin translocation was not appreciably changed in the presence of WGA in the reaction mixture [37]. This finding suggests that the nuclear translocation of regucalcin is not related to nuclear localization signal that is responsible for selection for intranuclear active transport. Presumably, regucalcin is passively transported to the nucleus through nuclear pore in liver cells, since a molecular weight of regucalcin is about 33 kDa [16].
Regucalcin has been also shown to localize in the nuclei of the cloned normal rat kidney proximal tubular epithelial NRK52E cells with immunocytochemical analysis [178]. The nuclear localization of regucalcin is enhanced through hormonal signaling process that is involved in protein kinase C [178].
Regucalcin has been shown to bind proteins in isolated rat liver nuclei using Far-western blot analysis [179]. The results of the Far-western analysis showed the existence of protein components that bind to regucalcin in the nucleus isolated from rat liver [179]. Regucalcin has been also demonstrated to bind DNA using western blot analysis for regucalcin with DNA cellulose-binding assay [179]. These findings show that regucalcin binds proteins and DNA in liver nucleus. Regucalcin may have a regulatory effect on signaling pathways that modulate transcriptional activity in liver cells.
Regucalcin inhibits Ca2+-stimulated nuclear DNA fragmentation
Isolated rat liver nucleus contains a DNA endonuclease activity dependent upon Ca2+ and Ca2+ results in extensive DNA hydrolysis [180]. The Ca2+ dependence of this endogenous DNA fragmentation process is based on DNA endonuclease activity dependent upon sub-micromolar Ca2+ when the nucleus is reconstituted with NAD+ and ATP [180]. This endogenous endonuclease activity may be responsible for the DNA fragmentation occurring during programmed cell death (apoptosis) and certain forms of chemically induced cell killing [180, 181].
To explore the regulatory role of regucalcin in liver nuclear function, it was first examined whether the protein has an effect on Ca2+-activated DNA fragmentation in isolated rat liver nuclei [182]. Among various metals, Ca2+ has been shown to stimulate uniquely in vitro DNA fragmentation in isolated rat liver nuclei [182]. This increase was seen after the addition of 1.0 μM Ca2+, in agreement with previous work [182]. The presence of regucalcin (0.5–2.0 μM) completely inhibited the activation of liver nuclear DNA fragmentation when 10 μM Ca2+ was added. This inhibition was not seen in the presence of Ca2+ at 25 or 50 μM. Thus, regucalcin had an inhibitory effect on DNA fragmentation when a comparatively lower concentration of Ca2+ (5.0 and 10 μM) was added [182]. The inhibitory effect of regucalcin on DNA fragmentation may be partly based on binding of Ca2+ [9].
DNA fragmentation in rat liver nucleus has been reported to be stimulated through Ca2+-calmodulin [180], which exists in liver nuclei [82]. Addition of calmodulin (10 and 20 μg/ml) did not enhance Ca2+ (10 μM)-activated DNA fragmentation in liver nuclei [182]; however, nuclear endogenous calmodulin may be able to enhance Ca2+-activated DNA fragmentation in the nucleus. Regucalcin has been found to inhibit Ca2+-activated DNA fragmentation after Ca2+ addition [182]. Such an inhibition was also seen in the presence of exogenous calmodulin [182]. Presumably, regucalcin can inhibit Ca2+/calmodulin-dependent DNA fragmentation in liver nuclei, since radio-iodinated regucalcin has been found in the nuclei isolated from rat liver in the absence or presence of 1.0 mM Ca2+ [65].
Several studies have shown that Ca2+ plays an important role in the regulation of nuclear functions [81, 82]. Also, it has been found that a sustained increase in cytosolic Ca2+ level precedes the activation of DNA fragmentation that is characteristic of programmed cell death (apoptosis) and in certain forms of chemically induced cell killing [180, 181]. The finding, that regucalcin inhibits the activation of DNA fragmentation by Ca2+, was the first time for a role of regucalcin in liver nuclear functions.
Regucalcin suppresses nuclear enzyme activity
Smal GTPase Ran (ras-related nuclear protein) is required for protein export from the nucleus and protein import into the nucleus [183]. The role of regucalcin in the regulation of GTPase activity in the nuclei of rat liver is shown [184]. We found the existence of GTPase activity in the nuclei isolated from rat liver [184]. Liver nuclear GTPase activity was increased after calcium addition with a comparatively higher concentration in the enzyme reaction mixture, and this increase was not seen in the presence of TFP, an antagonist of calmodulin [184]. The effect of calcium in increasing nuclear GTPase activity may be related to endogenous calmodulin. Calcium in liver cytoplasm is transported through an energy-dependent mechanism to the nucleus, and the comparatively higher concentration of calcium is found in the nucleus [184]. Calmodulin is shown to be present in liver nucleus [82]. GTPase, which is activated by Ca2+/calmodulin, may also be localized in liver nucleus.
The presence of exogenous regucalcin (0.5 μM) used in the enzyme reaction mixture caused an inhibitory effect on GTPase activity in liver nucleus [184]. This effect was also seen in the presence of EGTA, a chelator of Ca2+. Presumably, the inhibitory effect of regucalcin on liver nuclear GTPase activity is revealed independent of Ca2+/calmodulin in the nucleus. Regucalcin has been shown to have an inhibitory effect on the activation of enzymes by Ca2+/calmodulin due to binding Ca2+ and/or calmodulin [135–138]. Regucalcin can inhibit the activity of various enzymes through the mechanism by which it binds directly to the enzyme [30, 32]. Regucalcin may directly inhibit GTPase activity in liver nucleus.
The physiological significance of the inhibitory effect of regucalcin on GTPase activity in liver nucleus is unknown. However, the presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture caused a significant increase in this activity in liver nucleus [184]. This increase was completely blocked after regucalcin addition, suggesting that endogenous regucalcin has a suppressive effect on GTPase activity in liver nucleus. Endogenous regucalcin, which is localized in liver nucleus, may participate in the regulation of nuclear functions that are related to hydrolysis of GTP.
Regucalcin may be able to regulate a process of signal transduction from the cytoplasm to nucleus in liver cells. This process is mediated through various protein kinases. The role of regucalcin in the regulation of Ca2+-dependent protein kinase and protein tyrosine phosphatase activities in isolated liver nucleus is examined [37, 147, 148]. Nuclear Ca2+-dependent protein kinase and protein tyrosine phosphatase activities were increased in the presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture, and these increases were completely abolished after the addition of regucalcin [37]. The translocation of regucalcin to the nucleus may play a suppressive role in the regulation of protein kinase and protein tyrosine phosphatase in liver nucleus [37].
Endogenous regucalcin has been demonstrated to have a suppressive effect on the enhancement of protein kinase activity with a proliferation of liver cells [185]. Protein kinase activity is enhanced in the cytosol and nucleus of regenerating rat liver [185]. Regucalcin had a suppressive effect on tyrosine kinase, protein kinase C, and Ca2+/calmodulin-dependent protein kinase in the cytoplasm and nucleus of regenerating rat liver [120, 135, 136, 185].
Phosphatase activity toward phosphotyrosine, phosphoserine, and phosphothreonine is found in the liver nucleus [148]. Nuclear phosphotyrosine phosphatase activity was increased after Ca2+ addition in the enzyme reaction mixture, although the enzyme activity was not altered by TFP, an inhibitor of calmodulin, or cyclosporine A, an inhibitor of calcineurin [148]. Nuclear phosphatase activity toward phosphotyrosine may be independent of calmodulin. Meanwhile, nuclear phosphatase activity toward phosphoserine was elevated after the addition of Ca2+, while the enzyme activity was appreciably decreased by TFP and cyclosporine A, suggesting that the enzyme activity is partly involved in Ca2+/calmodulin-dependent protein phosphatase (calcineurin). Nuclear phosphatase activity toward phosphothreonine was not altered after the addition of Ca2+, TFP, and cyclosporine A in the enzyme reaction mixture. Vanadate caused an inhibition of nuclear phosphatase activity toward phosphotyrosine and phosphoserine but not phosphothreonin. Thus, different protein phosphatases toward phosphotyrosine, phosphoserine, and phosphothreonine have been shown to be present in liver nucleus.
The addition of regucalcin in the enzyme reaction mixture caused a significant decrease in phosphatase activity toward phosphotyrosine, phosphoserine, and phosphothreonine in the liver nuclei [148]. Liver nuclear phosphatase activity toward phosphoamino acids was assayed using 5–6 mg of nuclear protein per milliliter of reaction mixture; it contained 275–330 ng of nuclear regucalcin [148]. The concentration of endogenous nuclear regucalcin was estimated to be about 82.4–98.8 nM. Further addition of exogenous regucalcin (0.25 μM) caused a significant decrease in nuclear phosphatase activity, although the effect was saturated with increasing concentrations of regucalcin (0.5 μM) [148].
Nuclear phosphatase activity was elevated in the presence of anti-regucalcin monoclonal antibody (25 and 50 ng/ml of reaction mixture) [148]. This elevation was completely abolished after addition of regucalcin. Endogenous regucalcin may regulate protein phosphatase activity toward phosphotyrosine, phosphoserine, and phosphothreonine in liver nucleus. Regucalcin may have an inhibitory effect on various protein phosphatases in liver nucleus.
Regucalcin suppresses nuclear DNA and RNA synthesis
Regucalcin has been shown to have an inhibitory effect on DNA synthesis activity in the nuclei of normal rat liver [186]. The inhibitory effect of regucalcin was seen in the presence of EGTA, a chelator of Ca2+, in the reaction mixture. Ca2+ is present in liver nucleus [186]. Liver nuclear DNA synthesis activity was increased in the presence of EGTA in the reaction mixture, suggesting that Ca2+ suppresses DNA synthesis activity in the nucleus. The effect of regucalcin in inhibiting nuclear DNA synthesis may be not related to Ca2+ in liver nucleus.
Liver nuclear DNA synthesis has been shown to stimulate in regenerating rat liver [187]. Nuclear DNA synthesis was markedly increased at 1 day after hepatectomy, and this increase was also seen at 3 days [187]. Nuclear DNA synthesis was enhanced in the presence of EGTA (0.4 mM) in the incubation mixture. The presence of Ca2+ (1.0–25 μM) caused a significant decrease in the nuclear DNA synthesis of normal rat liver. Regucalcin (0.25 and 0.5 μM) caused an inhibition of nuclear DNA synthesis of normal rat liver [187]. This inhibition was also seen in the presence of Ca2+ (1.0 μM). The inhibitory effect of regucalcin was remarkable in regenerating rat liver nuclei in comparison with that of normal rat liver. Regucalcin has been shown to have a suppressive effect on nuclear DNA synthesis in regenerating rat liver. Regucalcin may have a suppressive role in the enhancement of nuclear DNA synthesis in liver cell proliferation.
Regucalcin has been also shown to have a suppressive effect on DNA synthesis activity in the nuclei isolated from rat renal cortex [188]. The addition of regucalcin (0.1–0.5 μM) in the reaction mixture containing either EGTA (1 mM) or calcium chloride (50 μM) had an inhibitory effect on nuclear DNA synthesis activity [188]. The presence of anti-regucalcin monoclonal antibody (10–50 ng/ml) in the reaction mixture caused a significant increase in nuclear DNA synthesis activity [188]. This increase was completely abolished in the presence of regucalcin (0.5 μM). Endogenous regucalcin has been found to have a suppressive effect on DNA synthesis in the nuclei of rat renal cortex [188].
Regucalcin has been shown to have an inhibitory effect on RNA synthesis in the nuclei isolated from control rat liver [189] and regenerating rat liver [190]. RNA synthesis in rat liver nuclei was stimulated after Ca2+ addition with a comparatively lower concentration, although the sub-micromolar concentration of Ca2+ evoked an inhibition of nuclear RNA synthesis [189]. The addition of Ca2+ with higher concentrations has been reported to have an inhibitory effect of nuclear RNA synthesis in rat liver cells [191]. Since liver nuclei contain a DNA endonuclease activity dependent upon Ca2+ in the submicromolar range and Ca2+ causes extensive DNA hydrolysis [180, 182], nuclear RNA synthesis may be suppressed with higher Ca2+ concentrations. Inactivation of RNA polymerase III transcription has been shown to be calcium dependent; the changes in Ca2+ concentration, the activation of calpains, and the consequent proteolytic degradation of RNA of transcription factors has been suggested to be involved in the regulation of RNA polymerase III transcription in the presence of 1 mM Ca2+ [192].
The effect of regucalcin in decreasing nuclear RNA synthesis activity in normal rat liver was not seen in the presence of α-amanitin, an inhibitor of RNA polymerase II and III [189], suggesting that the suppressive effect of regucalcin on nuclear RNA synthesis activity is partly resulted from its inhibitory action on RNA polymerase II and III [189, 190]. Meanwhile, it has been reported that Ca2+ has a stimulatory effect on RNA synthesis in liver nucleus [191, 192]. This effect may be partly mediated through Ca2+-dependent protein kinase [191, 192]. The stimulatory effect of Ca2+ on nuclear RNA synthesis activity was completely blocked in the presence of regucalcin [189, 190]. Regucalcin has been shown to inhibit Ca2+-dependent protein kinases in rat liver nucleus [185]. Presumably, the effect of regucalcin in decreasing RNA synthesis activity in liver nucleus is partly involved in its inhibitory action on the activities of both RNA polymerase II and III and Ca2+-dependent protein kinases. Further mechanism remains to be elucidated. Regucalcin has been proposed to have a role as a transcriptional factor in liver nucleus.
Regucalcin has been found to have a suppressive effect on liver nuclear DNA and RNA synthesis [186–190]. The mechanism by which regucalcin inhibits nuclear DNA and RNA synthesis is not well known. It is speculated that regucalcin has an inhibitory effect on DNA and RNA polymerase activity. However, the effect of regucalcin in inhibiting nuclear RNA synthesis activity is observed in the presence of α-amanitin, an inhibitor of RNA polymerase II. Regucalcin can directly bind DNA [179]. Which base pairs of DNA bind regucalcin remains to be elucidated. It is possible that regucalcin binds DNA and it has an inhibitory effect on nuclear DNA and RNA synthesis activity.
Regucalcin suppresses apoptosis mediated through cell signaling
Regucalcin inhibits NO synthase activity that is related to apoptosis
Nitric oxide (NO) may be important as a signaling factor in many cells [122], and it plays a role in apoptosis of hepatoma cells [193]. NO mediates apoptosis by d-galactosamine in a primary culture of rat hepatocytes [194]. Regucalcin has been shown to inhibit NO synthase that is related to cell apoptosis [123], suggesting that regucalcin has a suppressive role in apoptosis [195].
Regucalcin has a suppressive effect on Ca2+/calmodulin-dependent NO synthase in the cloned rat hepatoma H4-II-E cells [123]. The effect of regucalcin in decreasing NO synthase activity was also seen in the presence of TFP or EGTA. Presumably, regucalcin has an inhibitory effect on NO synthase activity due to binding to calmodulin and/or the enzyme independently of Ca2+ in proliferative cells.
Overexpression of regucalcin has been also shown to have a suppressive effect on NO synthase activity in H4-II-E cells (transfectants) [123]. This decrease was completely abolished in the presence of anti-regucalcin monoclonal antibody in the reaction mixture. Moreover, the effect of Ca2+/calmodulin addition in increasing NO synthase activity in H4-II-E cells cells (wild type) was completely prevented in transfectants. Endogenous regucalcin had a suppressive effect on NO synthase activity the cloned rat hepatoma H4-II-E cells.
Nitric oxide (NO) synthase activity was enhanced in H4-II-E cells cultured with 10% FBS as compared with that of 1% FBS [123], suggesting that the enzyme is induced in proliferative cells. The enhancement of NO synthase activity in H4-II-E cells cultured with 10% FBS was abolished in the presence of anti-regucalcin monoclonal antibody [123]. Regucalcin levels were elevated in H4-II-E cells with 10% FBS-culture [123]. Endogenous regucalcin may have a suppressive effect on the enhancement of NO synthase activity with proliferation of H4-II-E cells.
A high concentration of NO, which is produced from inducible NO synthase, has been shown to inhibit cell proliferation [195] and to induce cell apoptosis [196]. It is reported that a low concentration of NO, which is produced from endothelial NO synthase, protects against the cytotoxic effects of reaction oxygen species in cells [197]. Whether endogenous regucalcin suppresses NO production in H4-II-E cells is unknown at present. It is speculated, however, that regucalcin may inhibit NO production in H4-II-E cells, since regucalcin can decrease NO synthase activity in the cells [123]. Endogenous regucalcin may have an inhibitory effect on inducible and endothelial NO synthetases in hepatoma cells. Alternatively, regucalcin may have a physiological role in the regulation of NO-related cell functions.
Regucalcin suppresses various factors-induced cell death and apoptosis in liver cells
Tumor necrosis factor α (TNF-α) and NO mediate apoptosis by d-galctosamine in a primary culture of rat hepatocytes [194, 195]. TNF-α induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation [195]. H4-II-E cells with subconfluent monolayer cells were cultured in a medium without FBS in the presence of TNF-α. TNF-α (0.1–10 ng/ml) caused a significant decrease in the number of H4-II-E cells (wild type), inducing cell death. Overexpressing of regucalcin in H4-II-E cells (transfectants) has been found to prevent the effect of TNF-α in decreasing cell number [198]. Overexpression of regucalcin had a preventive effect on cell death induced with the higher concentration of TNF-α (10 ng/ml). This finding demonstrates that overexpression of regucalcin has a suppressive effect on cell death induced by stimulation of TNF-α [198].
Culture with NAME, an inhibitor of NO synthase, had a significant preventive effect on TNF-α-induced cell death. Regucalcin inhibits Ca2+/calmodulin-dependent NO synthase activity in H4-II-E cells [123]. The suppressive effect of regucalcin on cell death may be partly resulted from the inhibition of NO production, which can induce apoptosis, stimulated after TNF-α stimulation in H4-II-E cells.
The effect of caspase inhibitor on TNF-α-mediated cell death in H4-II-E cells is examined [198]. TNF-α-induced cell death was prevented in culture with caspase inhibitor in wild-type cells and transfectants, suggesting that TNF-α-induced cell death is partly involved activation of caspases in H4-II-E cells. Regucalcin may have an inhibitory effect on activation of caspases in the cells.
Lipopolysaccharide (LPS) has been shown to induce cell apoptosis [199, 200]. H4-II-E cells with the subconfluent monolayer were cultured in a medium without FBS in the presence of LPS. LPS caused a decrease in the number of H4-II-E cells (wild-type), inducing cell death and apoptosis [201]. This decrease was completely prevented in the regucalcin cDNA-transfected hepatoma cells overexpressing regucalcin with culture for 12–48 h [201]. Overexpression of regucalcin has a suppressive effect on LPS-stimulated cell death and apoptosis.
LPS acts to modulate the expression of a large number of genes that favor apoptosis of fibroblastic cells that are dependent upon activation of caspase-8 [200]. There is evidence that LPS-induced cell death is mediated through accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus [200]. Culture with LPS caused a significant decrease in Ca2+/calmodulin-dependent NO synthase activity in H4-II-E (wild type) cells [201]. LPS-induced decrease in NO synthase activity was found to prevent in the transfectants overexpressing regucalcin [201]. LPS-induced cell death may be not result from NO production in hepatoma cells, and the suppressive effect of regucalcin on LPS-induced cell death is not involved in NO in the cells. Moreover, LPS-induced cell death was prevemted in culture with caspase-3 inhibitor [201]. The effect of regucalcin in suppressing LPS-induced cell death is partly related to the inhibitory effect on caspase-3 in hepatoma cells.
An induction of apoptosis is partly mediated through pathway of protein kinase. The death of H4-II-E cells (wild type) has been found to be induced in culture with PD98059, a ERK inhibitor, dibucaine, an inhibitor of Ca2+-dependent protein kinase, or staurosporine, a potent inhibitor of protein serine/threonin kinases (protein kinase C), suggesting that various inhibitors-induced cell deaths are partly involved in the inhibition of protein kinases [201]. Overexpression of regucalcin in H4-II-E cells rescued cell death with PD98059 or dibucaine [201]. Such an effect was not observed with staurosporine. PD98059 induces apoptosis, which is in part due to the inactivation of Bcl-2 by increasing phosphorylated Bcl-2 in human prostate cancer cells [202]. Dibucaine has been shown to activate various caspases, such as caspase-3, -6, -8, and -9 (-like) activities, but not caspase-1 (-like) activity, and to induce mitochondrial membrane depolarization and the release of cytochrome C from mitochondria into the cytosol in leukemia cells (HL-60) [203]. Staurosporine induces apoptosis in Chang liver cells by a mitochondria-caspase-dependent pathway, which is closely correlated with a decrease in Bcl-2 and Bcl-XL levels in cancer cells [204]. Regucalcin may partly inhibit the inactivation of Bcl-2 or the activation of caspases in signaling mechanism that PD98059 or dibucaine induces apoptosis.
Calcium channel blockers, the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin and calcium ionophores are potent to lead several cell types to apoptosis [205, 206]. Thapsigargin is an inhibitor of Ca2+-ATPase in the endoplasmic reticulum (Ca2+ store) in cells, and treatment with thapsigargin causes an elevation of sustained Ca2+ concentration in cells and induces apoptosis in the hepatoma cells [207]. Experiments on nucleus isolated from cells clearly demonstrate the induction of Ca2+-dependent endonuclease activity during triggering apoptosis events [207]. Rises in intracellular Ca2+ concentration are believed to activate this nuclease and to mediate DNA cleavages into oligonucleosome fragments [208]. Regucalcin has been shown to have an inhibitory effect on Ca2+-activated DNA fragmentation in isolated rat liver nucleus [182], suggesting that the protein has an inhibitory effect on apoptosis in liver cells. Thapsigargin induces cell death and apoptosis causing DNA fragmentation [202]. Thapsigargin-induced DNA fragmentation in the hepatoma cells is not altered in culture with caspase inhibitor, suggesting that thapsigargin-mediated apoptosis is independent of activation of caspases [202]. Overexpression of regucalcin in the hepatoma cells has been found to suppress DNA fragmentation induced by thapsigargin [202]. This effect was not further enhanced in culture with caspase inhibitor [202]. Presumably, regucalcin has a suppressive effect on thapsigargin-mediated cell death due to preventing the rise in intracellular Ca2+ concentration in the hepatoma cells, since the protein can keep intracellular Ca2+ homeostasis due to activating Ca2+ pum enzymes in the plasma membranes, mitochondria, and endoplasmic reticulum of rat liver cells [30–33].
Calcium entry into cells induces cell death [202, 209]. Culture with Bay K 8644, an antagonist of Ca2+ entry in cells, caused a significant increase in the death of hepatoma H4-II-E cells (wild-type) [202]. Culture with Bay K 8644 did not induce cell death of transfectants (H4-II-E cells) overexpressing regucalcin [202]. Overexpression of regucalcin in H4-II-E cells was found to suppress DNA fragmentation induced by Bay K 8644. Regucalcin may have a suppressive effect on Ca2+ entry-induced stimulation of apoptosis in the hepatoma cells [202]. Regucalcin has a suppressive effect on Ca2+ entry-mediated cell death due to preventing the rise in intracellular Ca2+ concentration in the hepatoma cells. In addition, regucalcin may suppress the effect of Ca2+ on DNA fragmentation in the nucleus of H4-II-E cells.
The effect of insulin or IGF-I on cell death and apoptosis in H4-II-E cells has not been well known. H4-II-E cells were cultured in a medium containing, either vehicle, insulin, insulin-like growth factor-I (IGF-I), epinephrine, or transforming growth factor-β in the absence of FBS [210]. The number of wild-type cells was decreased in the presence of insulin or IGF-I [210]. Agarose gel electrophoresis showed the presence of low-molecular-weight DNA fragments of adherent wild-type cells cultured with insulin or IGF-I [210]. The effect of insulin or IGF-I in stimulating cell death and DNA fragmentation H4-II-E cells (wild type) was prevented in transfectants overexpressing regucalcin [210].
The effect of insulin in decreasing the number of H4-II-E cells was prevented in the presence of caspase-3 inhibitor [210]. The effect of IGF-I on cell death, however, was also observed in the presence of caspase-3 inhibitor [210]. These observations suggest that the effect of insulin on cell death is involved in activation of caspase-3, and that the effect of IGF-I is not dependent on caspase-3 in H4-II-E cells. The effect of IGF-I in inducing cell death in the presence of caspase-3 inhibitor was completely blocked in transfectants overexpressing regucalcin [210], suggesting that regucalcin inhibits signaling pathway of IGF-I-induced cell death that is not mediated through caspase-3 in H4-II-E cells.
The effect of insulin or IGF-I in inducing cell death and apoptosis of H4-II-E cells was not observed in the presence of NAME, an inhibitor of NO synthase [210], suggesting that insulin- or IGF-induced cell death is partly involved in production of NO in H4-II-E cells. Overexpression of regucalcin has been shown to have a suppressive effect on activation of Ca2+/calmodulin-dependent NO synthase in H4-II-E cells [123].
The effect of IGF-I in inducing cell death of H4-II-E cells was also observed in the presence of Bay K 8644 [210]. Such an effect was not seen in the case of insulin [210]. The mode of IGF-I action differs from that of insulin. It is assumed that insulin induces cell death that is partly mediated through intracellular calcium-dependent signaling pathway in H4-II-E cells, and that IGF-I may not be mediated through calcium-dependent signaling pathway in H4-II-E cells. The effect of IGF-I in inducing cell death in the presence of Bay K 8644 was not observed in transfectants overexpressing regucalcin [210].
Genistein has an inhibitory effect on protein tyrosine kinases and it can produce cell cycle arrest and apoptosis in leukemic cells [211]. Genistein was found to induce cell death of H4-II-E cells, and the effect was not seen in the transfectants overexpressing regucalcin [210]. Genistein-induced cell death is partly mediated through inhibition of protein tyrosine kinase in H4-II-E cells. Regucalcin has an inhibitory effect on protein tyrosine kinase activity in the cytoplasm and nucleus of rat liver [185].
The effect of insulin in inducing cell death of H4-II-E cells was not seen in the presence of genistein [210], although such an effect was seen in the case of IGF-I. The effect of IGF-I on cell death in the presence of genistein was prevented in transfectants overexpressing regucalcin [210]. Regucalcin has a suppressive effect on cell apoptosis that is mediated through signaling pathways with dependent or independent on protein tyrosine kinase.
Vanadate is an inhibitor of protein tyrosine phosphatase in cells [142]. Regucalcin has been shown to have an inhibitory effect on protein tyrosine phosphatase activity in the cytoplasm and nucleus of rat liver [147]. Vanadate was found to induce cell death of H4-II-E cells [210], suggesting that cell death is not caused by mechanism that is mediated through inhibition of protein tyrosine phosphatase activity. Vanadate induced cell death for transfectants overexpressing regucalcin [210], suggesting that the suppressive effect of regucalcin on cell death of H4-II-E cells is independent on protein phosphatase. IGF-I had a stimulatory effect on cell death of H4-II-E cells overexpressing regucalcin in the presence of vanadate [210], suggesting that the effect of IGF-I is not mediated through protein tyrosine phosphatase in the transfectants.
The effect of insulin in inducing cell death may be partly mediated through signaling pathway which is involved in caspase-3, calcium, NO, protein tyrosine kinase, or protein tyrosine phosphatase in H4-II-E cells. The effect of IGF-I on cell death of H4-II-E cells may be mediated through NO and other molecules. Overexpression of regucalcin may have a suppressive effect on signaling mechanism by which insulin or IGF-I induces cell death of H4-II-E cells.
Sulforaphane is an isothiocyanate that is present naturally in widely consumed vegetables and has a particularly high concentration in broccoli. This compound has been shown to block the formation of tumors initiated by chemicals in the rat [212]. Sulforaphane has been shown to induce a cell cycle arrest, followed by cell death in HT29 human colon cancer cells [212]. Sulforaphane increases expression of the pro-apoptotic protein Bax, the release of cytochrome C from the mitochondria to the cytosol, and the proteolytic cleavage of poly (ADP-ribose) polymerase in HT29 human colon cancer cells [212]. In human T-cell leukemia, sulforaphane induces apoptosis due to increased p53 and Bax protein expression, and slightly affected Bcl-2 expression [213]. In cultured PC-3 human prostate cancer cells, moreover, sulforaphane-induced apoptosis is associated with up-regulation of Bax, down-regulation of Bcl-2 and activation of caspase-3, -9, and -8 [214]. Sulforaphane has been found to induce cell death and apoptosis in H4-II-E cells [215]. Caspase-3 inhibitor prevented the effect of sulforaphane, while it was not inhibited by NAME, an inhibitor of NO synthase, in H4-II-E cells [215]. Sulforaphane-induced cell death and apoptosis partly result from activation of caspase-3 in the hepatoma cells.
Overexpression of regucalcin had a suppressive effect on cell death and apoptosis induced by sulforaphane in H4-II-E cells [215]. The suppressive effect of regucalcin on sulforaphane-induced cell death and apoptosis in H4-II-E cells may be partly involved in the molecules of Bax, cytichrome C, caspase, and Bcl-2. In addition, regucalcin may have an inhibitory effect on NO synthase and Ca2+-dependent endonuclease activities in H4-II-E cells [123]. Regucalcin has a suppressive effect on many signaling pathways that mediate apoptotic cell death.
As mentioned above, regucalcin has been shown to play a role in the regulation of cell death and apoptosis in H4-II-E cells [198, 201, 210]. Overexpression of regucalcin has been demonstrated to have a suppressive effect on cell death and apoptosis induced by TNF-α, LPS, thapsigargin, Bay K 8644, dibucaine, or PD98059, an inhibitor of protein thyrosine kinase, insulin, or IGF-I in H4-II-E cells [198, 201, 210]. The signaling mechanisms that TNF-α, LPS, or other factors mediate cell death and apoptosis may be different. The suppressive effect of regucalcin on apoptotic cell death is related to its inhibitory effect on the activities of various protein kinases, NO synthase, caspase-3, or Ca2+-dependent endonuclease, and its activatory effect on Bcl-2. Regucalcin has a suppressive effect on many signaling pathways that mediate cell death and apoptosis, and the protein suppresses cell death and apoptosis mediated through many different signaling pathways in H4-II-E cells.
Regucalcin has a suppressive effect on cell apoptosis in kidney cells
Regucalcin has been shown to express in the cloned normal rat kidney proximal tubular epithelial NRK52E cells and its expression is enhanced after hormonal stimulation [216]. Nuclear localization of regucalcin is enhanced after hormone stimulation in NRK52E cells [178]. The role of regucalcin in cell death and apoptosis is examined using NRK52E cells overexpressing regucalcin [217]. The number of wild-type cells was decreased with culture for 42–72 h in the presence of TNF-α, LPS, Bay K 8644, or thapsigargin [217]. These effects were prevented in transfectants overexpressing regucalcin. DNA fragmentation induced after culture with LPS, Bay K 8644, or thapsigargin were prevented in transfectants overexpressing regucalcin [217]. Thus, overexpression of regucalcin has a suppressive effect on apoptotic cell death induced by TNF-α, LPS, Bay K 8644, or thapsigargin in kidney NRK52E cells. The effect of regucalcin in suppressing apoptotic cell death may be mediated through its action on many intracellular signaling pathways in NRK52E cells.
Bcl-2 is a suppressor in apoptotic cell death [218]. Apaf-1 participates in activation of caspase-3 [219]. Akt-1 involves in survival signaling pathway for cell death [220]. Overexpression of regucalcin caused a remarkable elevation of Bcl-2 mRNA expression in NRK52E cells, and it slightly stimulated Akt-1 mRNA expression in the cells. Apaf-1, caspase-3, or G3PDH mRNA expressions were not significantly altered in transfectants [217]. Presumably, the enhancement of Bcl-2 mRNA expression contributes to the suppression of apoptotic cell death in NRK52E cells overexpressing regucalcin. Regucalcin may play a role in the regulation of Bcl-2 gene expression in NRK52E cells.
TNF-α enhanced the expression of caspase-3 mRNA in NRK52E cells [217]. This enhancement was found to suppress in transfectants [217], suggesting that the mechanism by which regucalcin suppresses TNF-α-induced cell death is partly related to the decrease in caspase-3 mRNA expression in transfectants.
The presence of LPS caused a significant decrease in Bcl-2 mRNA levels in NRK52E cells, suggesting that this decrease is partly related to LPS-induced cell death [217]. The enhancement of Bcl-2 mRNA expression induced by overexpression of regucalcin was also seen in the presence of LPS [217]. LPS-stimulated expression of Apaf-1 mRNA was suppressed after overexpression of regucalcin [217]. This may partly involve in the suppression of LPS-induced cell death in NRK52E cells overexpressing regucalcin.
Culture with Bay K 8644 or thapsigargin was found to cause a significant increase in caspase-3 mRNA levels in wild-type cells, indicating that the increased gene expression partly contributes to inducing apoptotic cell death [217]. This increase was completely prevented in transfectants. Regucalcin may have a suppressive effect on caspase-3 mRNA expression enhanced by Bay K 8644 or thapsigargin in NRK52E cells. Thus, regucalcin was found to regulate the expression of Bcl-2, caspase-3, and Akt-1 mRNAs in the cloned normal rat kidney NRK52E cells. The change in protein levels, however, remains to be elucidated.
Toxic factors have been reported to induce renal failure due to stimulating apoptotic cell death [221]. Overexpression of regucalcin was found to have a suppressive effect on apoptotic cell death induced by various factors (including TNF-α, LPS, Bay K 8644, or thapsigargin) in NRK52E cells. Regucalcin may have a role as a suppressor in the development of apoptotic cell death in kidney proximal tubular epithelial cells. Presumably, regucalcin plays a physiological role in the maintenance of homeostasis of cellular response for cell stimulation.
Conclusion
Overexpression of regucalcin rescues cell death and apoptosis induced in culture with various factors in the hepatoma cells and normal kidney cells. The cell signaling mechanisms, that these factors mediate cell death and apoptosis, may be different. Regucalcin may have a suppressive effect on many signaling pathways that mediate cell death and apoptosis, as is summarized in Fig. 2. The suppressive effect of regucalcin on cell death and apoptosis may be related to the inhibitory effect on the activities of NO synthase, caspase-3, or Ca2+-dependent endonuclease and its activatory effect on Bcl-2. Moreover, regucalcin has regulatory effects on gene expression of many molecules that are related to cell apoptosis. Regucalcin plays an important role as a regulatory protein in intracellular signaling pathway which is related to cell death and apoptosis.
Regucalcin has a role as suppressor in cell death and apoptosis induced by various factors. Regucalcin suppresses cell death induced by various factors (including TNF-α, insulin, IGF-I, LPS, PD98059, dibucaine, thapsigargin, Bay K 8644, or sulforaphane). The suppressive effect of regucalcin on cell death and apoptosis is mediated due to inhibiting the activities of NO synthase, caspase-3, and Ca2+-dependent endonuclease and activating Bcl-2 in the cells
Regucalcin suppresses cell proliferation
Regucalcin mRNA and its protein are expressed in the cloned rat hepatoma H4-II-E cells, although these expressions show low levels as compared to that in the cytosol of normal rat liver [50, 51]. The expression of regucalcin mRNA is stimulated in H4-II-E cells after culture with addition of serum (10% FBS) [50, 51], suggesting that regucalcin plays a role in the proliferation of cells. Regucalcin has been demonstrated to have a role as suppressor in the enhancement of proliferation of liver cells in vitro. This section describes the mechanism by which regucalcin has a suppressive effect on cell proliferation using H4-II-E cells.
Regucalcin suppresses the enhancement of protein kinase activity in cell proliferation
The role of endogenous regucalcin in the regulation of protein kinase activity in the proliferation of H4-II-E cells is examined [222]. H4-II-E cells were cultured for 6–72 h in the presence of FBS (1 or 10%). The number of cells and protein kinase activity in the 5,500g supernatant of cell homogenate was increased 24 and 48 h after the culture with FBS (1 or 10%); the culture with 10% FBS had potent effect as compared with that of 1% FBS [222]. The culture with FBS produced an increase in protein kinase activity and a corresponding elevation of cell number in H4-II-E cells [222]. The increase in protein kinase activity of the 5,500g supernatant of cell homogenate preceded a significant elevation of cell number, suggesting that serum factors (including growth factors and hormones) stimulate cell proliferation that is partly mediated through cascades of protein kinases.
Serum-stimulated protein kinase activity in H4-II-E cells was further enhanced in the presence of calmodulin or dioctanoylglycerol in the presence of calcium chloride, and this increase was inhibited in the presence of trifluoperazine, staurosporine, or genistein in the enzyme reaction mixture [222], indicating that Ca2+/calmodulin-dependent protein kinase, protein kinase C, and protein tyrosine kinase are present in H4-II-E cells [222]. Various protein kinases may be involved in the enhancement of cell proliferation with serum stimulation.
The presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture containing the 5,500g supernatant of cell homogenate of H4-II-E cells with FBS caused a significant increase in protein kinase activity [222]. This effect was completely abolished after the addition of exogenous regucalcin, which has an inhibitory effect on the enzyme activity [222]. This finding indicates that endogenous regucalcin plays a suppressive role in the enhancement of protein kinase activity in the cytoplasm in H4-II-E cells with cell proliferation. The anti-regucalcin monoclonal antibody-increased protein kinase activity in H4-II-E cells was inhibited in the presence of trifluoperzine, staurosporine, or genistein, suggesting that endogenous regucalcin inhibits Ca2+/calmodulin-dependent protein kinase, protein kinase C, or protein tyrosine kinase activities [222].
Serum stimulation may lead to an increase in cell proliferation that is partly mediated through cascade for various protein kinases in H4-II-E cells [222]. Regucalcin may have a suppressive effect for overexpression of cell proliferation due to inhibiting various protein kinases in the cytoplasm and nucleus of H4-II-E cells. Presumably, regucalcin plays a role as suppressor protein in cell proliferation of normal liver cells and hepatoma cells.
Regucalcin suppresses the enhancement of protein phosphatase activity in cell proliferation
Regucalcin has been shown to have an inhibitory effect on protein phosphatase activity in the cytoplasm of rat liver [147, 148]. H4-II-E cells were cultured for 3 days in a medium containing serum (10% FBS). After subconfluency, the cells were used for the assay of protein phosphatase activity toward phosphotyrosine. Ca2+/calmodulin-dependent protein tyrosine phosphatases were present in H4-II-E cells [223]. Regucalcin had an inhibitory effect on Ca2+/calmodulin-dependent protein tyrosine phosphatase activity in the cells [223]. The culture with Bay K 8644, an agonist of Ca2+ channels, caused an elevation of protein tyrosine phosphatase in H4-II-E cells, whereas dibutyryl cyclic AMP had no effect [223]. Bay K 8644-induced increase in protein phosphatase activity was inhibited in the presence of TFP, indicating that this increase is mediated through Ca2+/calmodulin in hepatoma cells [223]. The effect of antibody was enhanced in the presence of TFP [223]. This enhancement may result from an increase in endogenous regucalcin in H4-II-E cells, since the expression of regucalcin mRNA in H4-II-E cells is stimulated after the culture with Bay K 8644 [223]. This finding may support the view that endogenous regucalcin, which is enhanced through Ca2+ signaling, has a suppressive effect on Ca2+/calmodulin-activated protein phosphatase activity in proliferative cells.
The presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture caused a remarkable elevation of protein tyrosine phosphatase activity in the cell homogenate (5,500g supernatant) of H4-II-E cells cultured with serum addition (1 or 10% FBS) [224]. This elevation was completely prevented after the addition of regucalcin [224]. Endogenous regucalcin may have a suppressive effect on the enhancement of protein tyrosine phosphatase activity in the proliferative cells.
There may be many protein phosphatases in hepatoma H4-II-E cells. The antibody-increased protein tyrosine phosphatase activity was also inhibited by okadaic acid or vanadate, which is an inhibitor of protein phosphatases [142], although cyclosporine A, an inhibitor of calcineurin (protein phosphatase) [225], had no effect [224]. Endogenous regucalcin may also act on okadaic acid or vanadate-sensitive protein tyrosine phosphatases in H4-II-E cells.
The culture with serum addition (10% FBS) caused an increase in proliferation of H4-II-E cells and a corresponding elevation of protein tyrosine phosphatase activity in the cells [224]. This finding suggests that the augmentation of protein tyrosine phosphatase activity is partly involved in the proliferation of H4-II-E cells, although protein phosphatase activity toward phosphoserine and phosphothreonine was not raised in the proliferative cells cultured with serum addition [224].
Processes that are reversibly controlled by protein phosphorylation require not only a protein kinase but also a protein phosphatase [142, 226]. Target proteins are phosphorylated at specific sites by one or more protein kinases and these phosphoproteins are removed by specific protein phosphatase [225].
As mentioned above, regucalcin may play an important role as a suppressor for the enhancement of cell proliferation due to inhibiting various protein kinases and protein phosphatases activities that are raised in the proliferation of H4-II-E cells.
Regucalcin suppresses the enhancement of DNA synthesis in cell proliferation
Endogenous regucalcin has a suppressive effect on the enhancement of DNA synthesis in the nuclei of H4-II-E with cell proliferation [227]. Cells were cultured for 6–96 h in medium containing FBS (1 or 10%). Cell number was increased between 24 and 96 h; cell proliferation was markedly stimulated after culture with 10% FBS as compared with that of 1% FBS [227]. Nuclear DNA synthesis activity in vitro was elevated 6 h after culture with 10% FBS and its elevation was remarkable at 12 and 24 h after the culture [227]. The increase in nuclear DNA synthesis activity preceded an elevation of the number of the cloned rat hepatoma cells H4-II-E cells cultured with FBS (1 or 10%) [227].
The increase in nuclear DNA synthesis activity at 12 and 24 h after culture with FBS was inhibited in the presence of PD98059, an inhibitor of MAP kinase, staurosporine, an inhibitor of protein kinase C, and TFP, an antagonist of Ca2+/calmodulin-dependent protein kinase, in the reaction mixture [227]. The increase in nuclear DNA synthesis activity after serum stimulation may be partly mediated through action of various protein kinases in the nuclei of H4-II-E cells. However, serum-stimulated increase in nuclear DNA synthesis activity was not related to various protein phosphatases [227].
Regucalcin has been shown to transport in the nucleus of rat liver [37, 148], and it can inhibit nuclear DNA synthesis of normal rat liver [186, 187]. The presence of regucalcin in the reaction mixture caused a decrease in DNA synthesis activity in the nuclei of H4-II-E cells cultured with FBS [227]. This effect was not altered in the presence of various protein kinase inhibitions. Regucalcin has been demonstrated to inhibit the activity of various protein kinases in the nuclei of liver cells [185, 222]. The effect of regucalcin in decreasing nuclear DNA synthesis activity may be partly mediated through the pathway of various protein kinases in H4-II-E cells.
Nuclear DNA synthesis activity was increased in the presence of anti-regucalcin monoclonal antibody in the reaction mixture containing the nucleus of H4-II-E cells cultured for 24 h with 10% FBS [227]. This elevation was inhibited after addition of various protein kinase inhibitors in the reaction mixture [227]. These findings support the view that endogenous regucalcin suppresses DNA synthesis activity through mechanism by which it inhibits nuclear protein kinases, and that it has a suppressive effect on DNA synthesis activity in the nuclei of H4-II-E cells with proliferation.
The transcriptional activity for regucalcin gene has been shown to enhance in H4-II-E cells cultured with Bay K 8644, an agonist of Ca2+ entry in cells, in the presence of 10% FBS [44, 51]. Culture with Bay K 8644 caused an increase in regucalcin levels in H4-II-E cells that were cultured in the presence of FBS (1 and 10%) [227]. In this case, nuclear DNA synthesis activity was not changed after culture with Bay K 8644 [227]. The presence of anti-regucalcin monoclonal antibody in the reaction mixture containing the nuclei of H4-II-E cells cultured with Bay K 8644 resulted in an elevation of nuclear DNA synthesis activity [227], suggesting that nuclear DNA synthesis activity is suppressed through endogenous regucalcin that is increased in the nuclei of H4-II-E cells cultured with Bay K 8644.
As mentioned above, regucalcin may have a suppressive effect on the enhancement of nuclear DNA synthesis activity in proliferative cells, and it may play a suppressive role for overexpression of cell proliferation. This was further supported in H4-II-E cells overexpressing regucalcin stably [228].
The regucalcin content of regucalcin/pCXN2-transfected cells used in this study was 19.7-fold as compared with that of the parental wild-type H4-II-E cells and pCXN2 vector-transfected cells (mock type) [228]. Regucalcin/pCXN2 vector-transfected cells (transfectants) were cultured for 72 h in the presence of FBS (10%) [228]. Cell numbers and DNA synthesis activity in the transfectants were found to suppress as compared with those of wild and mock type, suggesting that overexpression of regucalcin has a suppressive effect on cell proliferation [228].
The presence of anti-regucalcin monoclonal antibody in the reaction mixture caused an increase in DNA synthesis activity in the nuclei obtained from wild-type H4-II-E cells, mock-type cells, and transfectants with overexpression of regucalcin [228]. However, the augmentation of nuclear DNA synthesis activity was remarkable in the transfectants [228]. This may support the view that endogenous regucalcin has a great suppressive effect on nuclear DNA synthesis activity.
The expression of regucalcin mRNA has been shown to stimulate through a Ca2+-signaling mechanism in H4-II-E cells [41, 51]. Regucalcin is translocated to the nucleus of liver cells [37, 149]. Regucalcin inhibits nuclear protein kinase and protein phosphatase activities [222, 223], which are involved in signal transduction to the nucleus, and it causes an inhibition in nuclear DNA synthesis in proliferative liver cells [227]. Regucalcin may play a suppressive role for the overexpression of proliferation of liver cells.
Regucalcin suppresses cell cycle-related genes in proliferative cells
Regucalcin has a suppressive effect on liver cell proliferation [222–224]. Whether regucalcin suppress cell cycle-related genes is examined in proliferative cells [229]. H4-II-E cells (wild type) and stable regucalcin/pCXN2 transfectants were cultured for 72 h in a medium containing 10% FBS to obtain subconfluent monolayers. The proliferation of cells was suppressed in transfectants cultured for 24–72 h. The proliferation of wild-type cells was inhibited when the cells were cultured for 72 h in a medium containing an inhibitor of transcriptional activity or protein synthesis. Such an effect was not seen in transfectants [229]. Regucalcin has a suppressive effect on cytosolic protein synthesis [162, 163] and nuclear RNA synthesis [189, 190] in rat liver. If overexpression of regucalcin inhibits protein and RNA synthesis in transfectants, the inhibitors of transcriptional activity or protein synthesis may not have additional effect. This suggests that the effect of regucalcin in suppressing cell proliferation is partly mediated through its suppressive effect on protein and RNA synthesis in the cells.
Regucalcin has an inhibitory effect on various protein kinases in rat liver cytosol and nucleus [135, 140, 185, 222]. The proliferation of H4-II-E cells (wild type and transfectants) was inhibited in the presence of PD98059, dibucaine, staurosporine, or genistein, which is an inhibitor of various protein kinases [229]. The effect of regucalcin in suppressing cell proliferation may be partly related to its inhibitory effect on MAP kinase, Ca2+/calmodulin-dependent kinase, and protein tyrosine kinase in H4-II-E cells.
Wortmannin is known to have an inhibitory effect on PI3-kinase. The proliferation of H4-II-E cells (wild type) was inhibited in the presence of wortmannin, an inhibitor of PI3-kinase, or vanadate, an inhibitor of protein tyrosine phosphatase [229]. These effects were not observed in transfectants [230]. Regucalcin may inhibit PI3-kinase and protein tyrosine phosphatase activities, and those inhibitory effects may partly contribute to the suppression of proliferation in H4-II-E cells.
Bay K 8644 is an agonist of calcium entry into cells. The proliferation of H4-II-E (wild type) was inhibited in the presence of Bay K 8644 [229]. This effect of Bay K 8644 was not seen in transfectants [229], because regucalcin has a role in the maintenance of intracellular calcium homeostasis in many cell types [Review in Ref. 230].
Overexpression of regucalcin has been found to suppress the inhibitory effect of various factors, which induce cell cycle arrest, on the proliferation of H4-II-E cells (wild type) [229]. The effect of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinase cdc2, cdk2m, and cdk5 [231], or sulforaphane, which can induce G2/M phase cell cycle arrest [232], in inhibiting the proliferation of wild-type cells was not observed in transfectants [229]. Sulforaphane with a higher concentration caused a decrease in cell number of transfectants, suggesting that the chemical induces cell death and apoptosis (unpublished data). Butyrate induced an inhibition of the proliferation of wild-type cells and transfectants. Roscovitine can arrest in G1 and accumulate in G2 of cell cycle [231]. Butyrate induces an inhibition of G1 progression [233]. The inhibitory effect of roscovitine or sulforaphane on cell proliferation may not be seen in transfectants, if regucalcin has a suppressive effect on the same pathway which roscovitine or sulforaphane has an inhibitory effect on cell proliferation. Presumably, regucalcin induces G1 and G2/M phase cell cycle arrest in H4-II-E cells.
The expression of p21 mRNA has been found to enhance in transfectants overexpressing regucalcin, although cdc2a and chk2 (checkpoint-kinase 2) mRNA levels are not changed in transfectants [230]. p21 is an inhibitor of cyclin-dependent kinases (cdk) [234]. Regucalcin may enhance p21 expression and it inhibits G1 progression in H4-II-E cells. It cannot exclude the possibility, however, that regucalcin directly inhibits cdk activity in the cells.
Overexpression of regucalcin suppressed the expression of IGF-I mRNA in H4-II-E cells [229]. IGF-I is a growth factor in cell proliferation. Regucalcin may have a suppressive effect on IGF-I expression in H4-II-E cells, and this suppression of IGF-I expression leads to retardation of cell proliferation.
Regucalcin modulates tumor-related gene expression in proliferative cells
It is known that c-myc, c-fos, c-jun, and Ha-ras are tumor stimulator genes [235]. p53 and Rb are tumor suppressor genes and c-src is oncogene [233]. The expression of c-myc, Ha-ras, or c-src mRNAs was found to suppress transfectants overexpressing regucalcin [179, 236]. The expression of p53 and Rb mRNAs was markedly enhanced in transfectants overexpressing regucalcin [179]. Presumably, the suppression of c-myc, Ha-ras, and c-src mRNAs expressions and the enhancement of p53 and Rb mRNAs expression in transfectants overexpressing regucalcin is partly involved in the retardation of proliferation of hepatoma H4-II-E cells.
The mechanism by which regucalcin regulates the expression of genes related to tumor is unknown. Regucalcin has been shown to translocate into the nucleus of rat liver [37, 148], and the protein has an inhibitory effect on RNA synthesis in isolated rat liver nucleus [189, 190]. Regucalcin can bind DNA and modulates nuclear transcriptional activity [179]. Regucalcin may bind to promoter region of tumor-related genes and it may suppress the expression of tumor stimulator gene or stimulate the expression of tumor suppressor gene in H4-II-E cells overexpressing regucalcin. As a result, the proliferation of hepatoma cells overexpressing regucalcin may be suppressed.
The expression of regucalcin is reduced in the cloned rat hepatica H4-II-E cells as compared with that of normal rat liver interestingly [222], suggesting an involvement of regucalcin in the suppression of carcinogenesis. The down-regulation of regucalcin expression in liver cells may lead to stimulation of cell proliferation with alteration of various tumor-related gene expressions. Regucalcin may play an important role as suppressor in cell proliferation and tumorigenesis of liver cells.
Regucalcin suppresses cell proliferation in normal kidney cells
Regucalcin has been also shown to have a role in the regulation of the proliferation of rat kidney proximal tubular epithelial NRK52E cells [237]. The regucalcin content of regucalcin/pCXN2-transfected cells was about 21-fold as compared with that of the parental wild-type cells. The enhancement in cell proliferation was suppressed in the transfectants overexpressing regucalcin [237]. The decrease in cell number of NRK52E (wild type) cells after culture with butyrate, rescovitine, and sulforaphane, which is an inhibitor of cell cycle, was not observed in transfectants, suggesting that regucalcin induces G1 and G2/M phase cell cycle arrest in NRK52E cells [237].
The inhibition of the proliferation of NRK52E cells induced by PD98059, staurosporine, or dibucaine that is an inhibitor of various protein kinase inhibitors was not seen in the transfectants [238–240]. The suppressive effect of regucalcin on cell proliferation may result from the inhibitory effect of regucalcin on various protein kinases that are involved in stimulation of cell proliferation. The inhibition in proliferation of NRK52E cells by wortmannin, an inhibitor of PI3-kinase, was not observed in transfectants [241], suggesting that regucalcin inhibits PI3-kinase and that it partly contributes suppression of cell proliferation in NRK52E cells.
Bay K 8644 is an agonist of calcium entry into cells. The proliferation of NRK52E cells (wild type) was inhibited in the presence of Bay K 8644. Overexpression of regucalcin had a preventive effect on Bay K 8644-induced inhibition of cell proliferation [237]. Regucalcin has a role in the maintenance of intracellular calcium homeostasis in many cell types.
The effect of regucalcin on the gene expression of proteins that are related to cell proliferation and cell cycle is examined. The expression of c-jun and chk2 (checkpoint-kinase 2) mRNAs was found to suppress in the transfectants [237]. The expression of p53 mRNA was enhanced in transfectants, while the expression of c-myc, c-fos, cdc2, and p21mRNA was not changed in the transfectants [237]. The decrease in c-jun and chk2 mRNA expressions may partly contribute to suppress cell proliferation induced in NRK52E cells overexpressing regucalcin. The expression of the tumor suppressor gene p53 mRNA, which was enhanced with overexpression of regucalcin, may have a partial role in the retardation of proliferation of NRK52E cells. Regucalcin has been shown to localize in the nucleus of NRK52E cells [242]. Regucalcin may have a suppressive effect on cell proliferation due to regulating many gene expressions that is related to cell proliferation in normal kidney NRK52E cells.
Regucalcin, moreover, has been shown to have a regulatory effect on gene expression of proteins that regulate calcium transport system in kidney cells. Overexpression of regucalcin has been found to have suppressive effects on the gene expression of L-type Ca2+ channel and calcium-sensing receptor (CaR), which regulate intracellular Ca2+ signaling in NRK52E cells [242]. Overexpression of regucalcin caused an increase in rat outer medullary K+ channel (ROMK) mRNA expression in NRK52E cells, while it did not have an effect on Na, K-ATPase, and epithelial sodium channel (ENaC) mRNA expressions [242]. The expression of Type II Na-Pi cotransporter (NaPi-IIa) and angiotensinogen mRNAs was not changed in NRK52E cells overexpressing regucalcin [242], suggesting that regucalcin does not have effects on NaPi-IIa and angiotensinogen mRNA expressions in kidney NRK52E cells.
The blockade of calcium influx through L-type calcium channels has been shown to attenuate mitochondrial injury and apoptosis in hypoxia renal tubular cells [243]. The entry of calcium through L-type Ca2+ channels induces mitochondrial disruption and cell death [243]. CaR participates in the regulation of renal Ca2+ transport [244]. It is speculated that regucalcin regulates intracellular Ca2+-signaling pathway through its suppressive effect on L-type Ca2+ channel or CaR mRNA expression in the kidney proximal tubular epithelial cells.
The expression of regucalcin mRNA in NRK52E cells has been shown to enhance after the treatment of PTH [216], suggesting that regucalcin partly mediates cellular response for PTH in kidney cells. Overexpression of regucalcin did not attenuate the expression of L-type Ca2+ channel or CaR mRNAs, which is decreased after PTH treatment, in NRK52E cells. Regucalcin decreased L-type Ca2+ channel or CaR mRNA expressions in NRK52E cells. Regucalcin may partly contribute as a mediator in cellular response for stimulation of PTH in NRK52E cells.
Whether handling of calcium in NRK52E cells is changed in transfectants overexpressing regucalcin is unknown. Overexpression of regucalcin has been shown to have suppressive effects on apoptosis with culture of Bay K 8644 in NRK52E cells [217]. Presumably, the effects of regucalcin on gene expression are not mediated through change in calcium handling in transfectants. Regucalcin has been shown to play a role in the regulation of intracellular Ca2+ transport; the protein activates Ca2+-pumping enzymes (Ca2+-ATPase) in the basolateral membranes, mitochondria, and microsomes in rat kidney cortex [98, 99, 245]. Regucalcin may regulate intracellular Ca2+ homeostasis in kidney proximal tubular epithelial cells so that Ca2+ is passed through transcellular transport. Moreover, regucalcin was found to suppress the expression of L-type Ca2+ channel or CaR mRNAs in NRK52E cells, supporting the view that regucalcin plays a physiological role in the regulation of intracellular Ca2+ homeostasis in kidney proximal tubular epithelial cells.
Overexpression of regucalcin has a suppressive effect on cell responses that are mediated through signaling process following stimulation with TNF-α or transforming growth factor-β1 (TGF-β1) in NRK52E cells [246]. Overexpression of regucalcin had a suppressive effect on apoptotic cell death induced by TNF-α or TGF-β1 that is mediated through caspase-3 in NRK52E cells [246]. Culture with TNF-α or TGF-β1 caused a remarkable increase in α-smooth muscle actin level in NRK52E cells. Such an increase was not seen in transfectants. In addition, the expression of α-smooth muscle actin was markedly suppressed in transfectants cultured without TNF-α or TGF-β1. These findings demonstrate that overexpression of regucalcin has a suppressive effect on the expression of α-smooth muscle actin in NRK52E cells cultured with TNF-α or TGF-β1, and that regucalcin regulates signaling pathway that is mediated through TNF-α or TGF-β1 to stimulate the expression of α-smooth muscle actin in NRK52E cells. TGF-β1 is a key mediator that regulates transdifferentiation of NRK52E cells into myofibroblasts expressing α-smooth muscle actin [247]. This may contribute to renal fibrosis associated with overexpression of TGF-β1 within the diseased kidney [247]. Regucalcin may regulate transdifferentiation to renal fibrosis in NRK52E cells with TGF-β1 or TNF-α.
Overexpression of regucalcin caused a remarkable increase in the expression of mRNA of Smad 2, which is involved in signal transduction of TGF-β1 [248], or NF-κB, which is related to signaling of TNF-α [249], in NRK52E cells. Such an increase was not seen in Smad 3 mRNA expression in transfectants. This finding suggests that regucalcin stimulates the gene expression of Smad 2 or NF-κB, which is related to signaling mechanism of TNF-α or TGF-β1. Regucalcin may have a suppressive effect on signaling pathway by which TNF-α or TGF-β1 stimulates gene expression of NF-κB or Smad 2 in NRK52E cells. These cytokines may not have enhancing effects on gene expression of NF-κB or Smad 2 in transfectants. Presumably, the suppressive effects of regucalcin on apoptotic cell death and α-smooth muscle actin expression may be not involved in the expressions of NF-κB or Smad 2 that is stimulated by TNF-α or TGF-β1 in NRK52E cells.
Conclusion
As mentioned above, regucalcin has been demonstrated to have a suppressive effect on hepatoma H4-II-E cella and normal kidney NRK52E cells. The suppressive effect of regucalcin on cell proliferation is related to its inhibitory effect on the activities of various protein kinases and protein phosphatases, calcium-dependent signaling factors, protein synthesis, nuclear DNA and RNA synthesis, and IGF-I expression and its activatory effect on p21, an inhibitor of cell cycle-related protein kinases. Moreover, regucalcin has been shown to suppress the expressions of c-myc, Ha-ras, and c-src mRNAs and to enhance the expressions of of p53 and Rb mRNAs, which are related to tumorigenesis of liver cells. p53 is also known to stimulate p21 mRNA expression to induce cell cycle arrest. Regucalcin has a suppressive effect on many intracellular signaling pathways that is related to cell proliferation due to hormonal stimulation, as summarized in Fig. 3. The suppressive effect of regucalcin on cell apoptosis and cell proliferation is based on the mechanism by which the protein inhibits cellular events that are mediated through many intracellular signaling factors. Regucalcin may play an important role as suppressor protein in the maintenance of homeostasis of cellular response for cell stimulation.
Regucalcin has a suppressive effect on the enhancement of cell proliferation. Regucalcin mRNA expression is stimulated through the pathway of signaling mechanism concerning Ca2+/calmodulin (CaM)-dependent protein kinase (CaM kinase), protein kinase C (C kinase), protein kinase A (A kinase), and thyrosine kinase due to hormal stimulation. Regucalcin inhibits the activities of various protein kinases and protein phosphatases in the cytoplasm and nucleus of cells, and it also can inhibit Ca2+/calmodulin-dependent enzyme activity (including cyclic AMP phosphodiesterase, NO synthase, superoxide dismutase (SOD), and others. Cytoplasmic regucalcin translocates into nucleus. Regucalcin inhibits nuclear DNA and RNA synthesis. Regucalcin has an inhibitory effect on the expression of c-myc, Ha-ras, and c-src mRNAs, which are tumor stimulator genes. Regucalcin also stimulates the expression of p53 and Rb mRNAs that are tumor suppressor genes. Moreover, regucalcin can inhibit protein synthesis and it can stimulate protein degradation. Regucalcin induces G1 and G2/M phase cell cycle arrest in cells. The suppressive effect of regucalcin on cell proliferation is mediated through regulating many signaling systems
Regucalcin has been demonstrated to have a suppressive effect on cell death and apoptosis and cell proliferation induced by stimulation of various factors in hepatoma H4-II-E cells and normal kidney NRK52E cells. Presumably, regucalcin plays a physiological role in maintaining cell homeostasis of cellular response for various stimulating factors. Regucalcin may be a key molecule as a suppressor protein in cell regulation.
Role of regucalcin in regenerating liver in vivo
Adult rat hepatocytes are normally quiescent in vivo. However, 20–30 h after partial hepatectomy (about 70%) they undergo a synchronous wave of DNA synthesis and cell division and continue to divide until the original mass of the liver is regenerated 3–7 days late [250]. The liver weight at 1 day after hepatectomy is increased about 50% of that of sham-operated rats, and it reached to the same levels as sham operated at 3 days after hepatectomy, indicating that the increase in cell proliferation is induced at 1 day after hepatectomy [251]. Regenerating liver is a good model of liver cell proliferation in vivo.
Hepatocyte growth factor, which can promote liver regenerating after partial hepatectomy [248], has been shown to increase calcium concentration in rat hepatocytes [249]. Calmodulin synthesis in regenerating rat liver is increased at 8 h after hepatectomy [252].
Regenerating rat liver has been shown to enhance Ca2+-ATPase activity in the nuclei between 1 and 5 days after hepatectomy [251]. Liver nuclear Ca2+-ATPase is related to Ca2+ uptake by the nuclei and the metal accumulates nuclear matrix [81, 84, 85]. The increase in the nuclear Ca2+-ATPase activity in regenerating liver caused a corresponding augmentation of the nuclear calcium content [251]. Liver calcium content was increased between 1 and 5 days after hepatectomy, although the nuclear calcium content was a slight increase at 1 day after hepatectomy [251]. Calcium, which increased in the cytoplasm of regenerating liver, may be transported into the nuclei with the enhancement of nuclear Ca2+-ATPase activity.
Protein kinase C and Ca2+/calmodulin-dependent protein kinase exists in liver nuclei [186]. Nuclear Ca2+-ATPase activity of regenerating rat liver was decreased after the addition of protein kinase inhibitor into the enzyme reaction mixture [251]. The increase in nuclear Ca2+-ATPase activity in regenerating liver may be partly activated through Ca2+-dependent kinases. DNA content in the nuclei of regenerating rat liver was increased from 1 day after hepatectomy, while the nuclear DNA fragmentation activity was decreased in regenerating liver [251]. The nuclear DNA fragmentation activity in regenerating liver was not altered in the presence of Ca2+ chelator (EGTA) [251]. Presumably, the increased nuclear calcium in regenerating liver is involved in the regulation of nuclear DNA synthesis rather than the activation of nuclear DNA fragmentation.
Liver regucalcin mRNA expression has been shown to enhance 1–5 days after hepatectomy as compared with that of sham-operated rats, which is at the time point during activation of liver proliferation with a longer time (5 days) [62]. The expression of regucalcin mRNA in regenerating liver has been suggested to mediate through Ca2+/calmodulin in rat liver, since hepatocyte growth factor, which promotes liver regenerating after partial hepatectomy, has been shown to increase calcium concentration in rat hepatocytes [252]. The increase in regucalcin mRNA expression in regenerating liver was independent on the decomposition of mRNA [252]. The process that transcripts to regucalcin mRNA may be stimulated in regenerating liver. The increase in regucalcin may be related to the suppression of cell proliferation in regenerating rat liver.
The role of regucalcin in the regulation of the nuclear function of regenerating rat livers is examined. Protein kinase activity was elevated in the liver nuclei obtained at 6–48 h after partial hepatectomy [185], suggesting that protein kinases, which are involved in Ca2+ signaling, participate in the enhancement of nuclear phosphorylation in regenerating liver with proliferative liver cells. Regucalcin was found to have a suppressive effect on the enhancement of Ca2+/calmodulin-dependent protein kinase and protein kinase C activities in the nuclei of regenerating rat livers [185].
The phosphorylation in the nuclei of regenerating rat livers was increased in the presence of anti-regucalcin antibody in the reaction mixture [185]. Such stimulation was completely abolished in the presence of exogenous regucalcin [185], suggesting that endogenous regucalcin is involved in the suppression of protein kinase activity in the nuclei. The net increase in protein kinase activity after anti-regucalcin antibody addition was about two-fold in the nuclei of regenerating rat liver as compared with that of normal rat liver [185]. Endogenous regucalcin, which is enhanced in regenerating liver, may be translocated to the nucleus in vivo.
Protein phosphatase activity toward phosphotyrosine is also found in the liver nuclei, whereas the nuclear enzyme activity toward phosphoserine and phosphothreonine is only slight [147, 148]. Protein phosphotyrosine phosphatase activity in the cytoplasm and nuclei of liver cells was increased in regenerating liver in rats [147]. This increase was enhanced in the presence of anti-regucalcin monoclonal antibody in the enzyme reaction mixture from regenerating liver [147].
Regucalcin has an inhibitory effect on Ca2+/calmodulin-dependent protein kinases, protein kinase C, and protein phosphatases [139, 140]. Presumably, regucalcin participates in the regulation of cellular function due to inhibiting processes of phosphorylation and dephosphorylation of various proteins in proliferative liver cells. This role may be important intensively in the suppression of over-proliferation of liver cells in vivo.
The suppressive effect of regucalcin on protein synthesis in regenerating rat liver is also shown [163]. Regenerating liver induced an increase in protein synthesis. This increase was enhanced in the presence of anti-regucalcin monoclonal antibody [163]. This elevation was completely prevented after the addition of regucalcin. Endogenous regucalcin has a suppressive effect on the enhancement of protein synthesis in regenerating rat liver with proliferative cells [163]. Regucalcin may have a role as an inhibitor in protein synthesis in proliferative liver cells in vivo.
Liver nuclear DNA synthesis has been shown to stimulate in regenerating rat liver [186, 187]. Liver nuclear DNA synthesis was markedly enhanced 1 and 3 days after partial hepatectomy [186]. Regucalcin was found to have an inhibitory effect on nuclear DNA synthesis in regenerating rat liver [187]. This effect was not resulted from nuclear DNA hydrolysis, because regucalcin can inhibit Ca2+ activated DNA fragmentation in rat liver nuclei [187]. DNA synthesis activity in the nucleus of regenerating rat liver was enhanced in the presence of anti-regucalcin monoclonal antibody in the reaction mixture [187]. This increase was completely abolished after the addition of regucalcin [187]. The endogenous regucalcin in liver nucleus may play a suppressive role in the enhancement of nuclear DNA synthesis activity of regenerating liver.
Nuclear functions in regenerating liver may be stimulated through many signaling factors. The increase in nuclear DNA synthesis activity in regenerating rat liver was prevented in the presence of strurosporine, TFP, and PD98059, which are various protein kinase inhibitors, in the reaction mixture [187]. This suggests that the nuclear DNA synthesis activity in regenerating liver with proliferative cells is partly stimulated through signaling pathway, which is related to protein kinase C, Ca2+/calmodulin-dependent protein kinase, and MAPK kinase in the nucleus. The effect of anti-regucalcin monoclonal antibody in enhancing nuclear DNA synthesis in regenerating liver was blocked in the presence of strurosporine, TFP, and PD98059 [187]. This finding suggests that the suppressive effect of regucalcin on nuclear DNA synthesis activity in regenerating liver may be partly mediated through the inhibitory action of regucalcin on various protein kinases. In addition, regucalcin may directly inhibit nuclear DNA synthesis activity in liver cells.
Regucalcin has also been shown to inhibit RNA synthesis in the nuclei isolated from control rat liver [189] and from regenerating rat liver [190]. The presence of anti-regucalcin monoclonal antibody in the reaction mixture caused a significant increase in RNA synthesis activity in the nuclei of normal rat liver [190]. This increase was completely abolished after the addition of regucalcin. The effect of anti-regucalcin monoclonal antibody in increasing nuclear RNA synthesis activity in regenerating rat liver was enhanced in the presence of the antibody in the reaction mixture [190].
The enhancement of nuclear RNA synthesis activity in regenerating rat liver is partly dependent on the activation of protein kinases and protein phosphatases, which are involved intracellular signaling pathway of hormone and growth factors [190]. The effect of anti-regucalcin monoclonal antibody in enhancing RNA synthesis activity in the nuclei of regenerating rat liver was inhibited in the presence of α-amanitin, PD98059, staurosporine, or vanadate in the reaction mixture [190]. The enhancement of nuclear RNA synthesis activity in regenerating liver is partly suppressed through signaling pathways that endogenous regucalcin inhibits RNA polymerase II and III, MAPK kinase, protein kinase C, and protein phosphatases in the liver nucleus.
Regucalcin could inhibit RNA synthesis activity in the nuclei of normal and regenerating rat livers. Regucalcin may have a role as the transcription-related factor in liver nucleus. Whether regucalcin can directly bind to the promoter region in the gene is unknown, however.
As mentioned above, regucalcin mRNA expression in liver cells is stimulated through the pathway of signaling mechanism concerning protein kinases and protein phosphatases that are increased in regenerating liver with proliferative cells in vivo, and endogenous regucalcin has a suppressive effect on protein, DNA, and RNA synthesis, which is mediated through calcium signaling, in the cells. Presumably, regucalcin plays a suppressive role in the overexpression of cell proliferation in vivo. Regucalcin may have an important role as a suppressor protein in the differentiation and proliferation of liver cells.
Interestingly, new markers for the liver pre-neoplastic foci are investigated in rats treated with diethylnitrosamine and then 2-acetylaminofluorene combined with partial hepatectomy [253]. Transaldolase, rat aflatoxin B1 aldehyde reductase, and gamma-glutamylcysteine synthetase are found as up-regulated gene and regucalcin is found as a down-regulated gene, in line with findings for hepatocellular carcinomas [253]. The suppression of regucalcin expression may play a role in the development of carcinogenesis in liver cells. If the chemical feeding-induced suppression of regucalcin expression influences on Ca2+-signaling regulation in liver cells, it may generate a possible circumstance for carcinogenesis. In this aspect, the pathophysiological role of regucalcin in carcinogenesis with chemical feeding may be significant in vivo.
Role of endogenous regucalcin in animals with transgene
The role of endogenous regucalcin in cellular regulation is shown using regucalcin transgenic (TG) rats in vivo. Regucalcin homozygote male and female rats express a prominent regucalcin protein in the tissues [254]. The expression of regucalcin was remarkable in many tissues of TG female rats: its expression of male rats was seen in the liver, kidney cortex, heart, lung, and stomach [254].
Exogenous regucalcin has been shown to have the regulatory effect on liver nuclear function in vitro [185–188]. Regucalcin levels were increased in the liver nuclei of regucalcin TG male and female rats [255]. The effect of endogenous regucalcin in suppressing protein tyrosine phosphatase and RNA synthesis activities, which are inhibited by exogenous regucalcin in normal rat liver nucleus in vitro [256], is also demonstrated in regucalcin TG rats in vivo using anti-regucalcin monoclonal antibody. Nuclear protein tyrosine phosphatase activity was elevated in the presence of anti-regucalcin monoclonal antibody in the reaction mixture containing liver nuclear protein obtained from normal (wild type) rats [256]. The effect of the antibody was not seen in the liver nuclei of regucalcin TG rats [256]. Moreover, RNA synthesis was suppressed in the liver nuclei of regucalcin TG rats as compared with that of normal rats [256]. The effect of anti-regucalcin monoclonal antibody in increasing RNA synthesis was blocked in the liver nuclei of the TG rats [256]. The enhancement of endogenous regucalcin expression plays an important role in the suppression of RNA synthesis in the liver nucleus in vivo, supporting the view that regucalcin may have a role as a negative transcriptional factor in liver nucleus. Endogenous regucalcin plays a suppressive role in the regulation of liver nuclear function in rats in vivo.
Regucalcin has been shown to inhibit protein phosphatase activity in the cytoplasm [145] and nucleus [148, 149] in rat liver cells. Regucalcin has been found to have a suppressive effect on protein tyrosine phosphatase activity in rat liver microsomes in the range of its physiological concentration of liver cytoplasm [257]. The increase in endogenous regucalcin has been also shown to inhibit liver microsomal protein tyrosine phosphatase activity using regucalcin TG rats [257].
Protein tyrosine phosphatases have been postulated to play a key role in the regulation of the insulin action pathway. The inhibition of protein tyrosine phosphatase activity mimics insulin action [258]. It has been shown that the increase in protein tyrosine phosphatase activity in skeletal muscle, adipocytes, and liver is associated with insulin resistance and diabetic state [255, 259, 260]. The expression of regucalcin mRNA in rat liver has been shown to enhance after feeding and insulin treatment [59]. Regucalcin, which can inhibit protein tyrosine phosphatase activity in rat liver microsomes, may have a pathophysiological role in hepatic insulin resistance. Overexpression of regucalcin has been demonstrated to enhance glucose utilization and lipid production in the cloned rat hepatoma H4-II-E cells in vitro and it is involved in insulin resistance [261], and it suppresses the enhancing effect of insulin or glucose on the gene expression of insulin signaling-related proteins in the H4-II-E cells [262].
Regucalcin has been found to inhibit NO synthase activity in the cytosols of liver [124], kidney cortex [125], heart [126], and brain [127]. NO may act as a messenger or modulator molecule in many cells [122]. NO synthase is activated by Ca2+/calmodulin which is related to Ca2+ signaling. Overproduction of NO may lead to the damage of many cells. Regucalcin may have a suppressive effect on overproduction of NO in the cells, and the protein may have a protective effect on NO-induced damage of cells. Overexpression of regucalcin in TG rats has a suppressive effect on NO synthase activity in the cytosol of liver [124], kidney cortex [125], heart [126], and brain [128]. The presence of anti-regucalcin monoclonal antibody in the reaction mixture containing the cytosolic protein of various tissues obtained from regucalcin TG rats caused an increase in NO synthetase activity, and this increase was completely abolished after the addition of regucalcin. This finding supports the view that endogenous regucalcin has a suppressive effect on NO synthase activity in vivo.
The role of endogenous regucalcin in the regulation of Ca2+-ATPase activity in the mitochondria of brain tissues is examined using regucalcin TG rats [116]. The addition of regucalcin, which is a physiological concentration in rat brain tissues, into the enzyme reaction mixture caused a significant increase in Ca2+-ATPase activity, while it did not have an effect on Mg2+-ATPase activity [116]. Regucalcin was increased in the brain tissues or the mitochondria of regucalcin TG rats [116]. The mitochondrial Ca2+-ATPase activity was increased in the TG rats as compared with that of wild-type rats. Thus, endogenous regucalcin has an enhancing effect on Ca2+-ATPase activity in the mitochondria of rat brain tissues in vivo.
Thus, the increase in endogenous regucalcin has been demonstrated to have a suppressive effect on many functions in various tissues, which the effect of exogenous regucalcin are found in vitro, using regucalcin TG rats in vivo.
Interestingly, osteoporosis and hyperlipidemia are induced in regucalcin TG rats [reviewed in Ref. 263], indicating a pathophysiological role of regucalcin. This may be resulted from the regulatory effect of regucalcin on cell signaling systems in cells that are related to bone cells [264–267] and liver cells [261, 262].
One party, regucalcin (SMP30) deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span [268]. Regucalcin (SMP30) knockout mouse liver is highly susceptible to TNF-α- and Fas-mediated apoptosis [269], supporting the view that regucalcin has a suppressive effect on cell apoptosis in cell culture system [201, 215]. The deficiency of regucalcin (SMP30) induces a decrease in l-ascorbic acid (vitamin C) in mice [270], although this may not be significant because of vitamin C is not synthesized in human.
β-Catenin loss and regucalcin decrease have been shown to be contributing to apoptosis [271]. β-Catenin-null hepatocytes or regucalcin small interfering RNA-transfected HepG2 cells were cultured, and these cells exhibited significant apoptosis that was alleviated by the addition of ascorbic acid, which in turn may be one of the mechanisms contributing to the role of β-catenin in cell survival through regucalcin expression [271].
The exploration of other roles of regucalcin in vivo is expected.
Prospects
Regucalcin, which its gene is localized on the chromosome X in human and rats, is thought as a protein that is highly differentiated because of a great conservation of the regucalcin genes throughout evolution in vertebrates specifies. Regucalcin plays a multifunctional role in cellular regulation; a role in keeping intracellular Ca2+ homeostasis, an inhibitory role on various Ca2+-dependent enzyme activations, protein kinases and protein phosphatases. Regucalcin regulates due to suppressing protein synthesis and stimulating protein degradation, and it has a suppressive effect on DNA and RNA synthesis that are mediated through various signaling systems in the nucleus. Regucalcin suppresses an enhancement of cell proliferation and apoptotic cell death that are induced by various signaling factors. Regucalcin has been proposed to play a pivotal role as a suppressor protein of intracellular signaling system in maintaining cell homeostasis. Regucalcin is the first time finding in protein molecule that has a role as a suppressor protein in cell signaling, although it is well known that many proteins enhance cell signal transduction so far.
There are growing evidences that regucalcin may be a key molecule in metabolic disease. The overexpression of regucalcin gene has been known to induce osteoporosis and hyperlipidemia in the transgenic rats and the deficiency of regucalcin (SMP30) induces a decrease in vitamin C (it is not synthesized in human), which is related to regulation of oxidative stress in mice and to inducing of cell apoptosis. The expression of regucalcin gene is downregulated in the development of carcinogenesis in liver cells, suggesting that its suppression induces promotion of tumor cells.
The gene therapy, that targets the regucalcin gene, may be useful as a therapeutic tool for disease with the attenuation of regucalcin gene expression. Development of drug, which modulates regucalcin molecule, may have a clinical significance in the restoration of metabolic disorder that is implicated to regucalcin. Clinical studies of regucalcin for disease are expected.
References
Cheung WY (1980) Calmodulin plays a pivotal role in cellular regulation. Science 202:19–27
Nishizuka Y (1986) Studies and perspectives of protein kinase C. Science 233:305–331
Williamson JR, Cooper RK, Hoek JB (1981) Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta 639:243–295
Kraus-Friedman N, Feng L (1996) The role of intracellular Ca2+ in the regulation of gluconeogenesis. Metabolism 48:389–403
Yamaguchi M, Takei Y, Yamamoto Y (1975) Effect of thyrocalcitonin on calcium concentration in liver of intact and thyroparathyroidectomized rats. Endocrinology 96:1004–1008
Yamaguchi M, Yoshida H (1985) Participation of Ca2+ channel in liver calcium regulation by calcitonin in rats. Acta Endocrinol 110:239–243
Yamaguchi M, Yamamoto T (1978) Purification of calcium binding substance from soluble fraction of normal rat liver. Chem Pharm Bull 26:1915–1918
Yamaguchi M, Sugii K (1981) Properties of calcium-binding protein isolated from the soluble fraction of normal rat liver. Chem Pharm Bull 29:567–570
Yamaguchi M (1988) Physicochemical properties of calcium-binding protein isolated from rat liver cytosol: Ca2+-induced conformational changes. Chem Pharm Bull 36:286–290
Yamaguchi M, Yoshida H (1985) Regulatory effect of calcium-binding protein isolated from rat liver cytosol on activation of fructose 1,6-diphosphatase by Ca2+-calmodulin. Chem Pharm Bull 33:4489–4493
Yamaguchi M, Mori S, Suketa Y (1989) Effects of Ca2+ and V5+ on glucose-6-phosphatase activity in rat liver microsomes: the Ca2+ effect is reserved by regucalcin. Chem Pharm Bull 37:388–390
Yamaguchi M, Shibano H (1987) Calcium-binding protein isolated from rat liver cytosol reverses activation of pyruvate kinase by Ca2+. Chem Pharm Bull 35:2025–2029
Yamaguchi M, Shibano H (1987) Effect of calcium-binding protein on the activation of phosphorylase a in rat hepatic particulate glycogen by Ca2+. Chem Pharm Bull 35:2581–2584
Yamaguchi M, Shibano H (1987) Reversible effect of calcium-binding protein on the Ca2+-induced activation of succinate dehydrogenase in rat liver mitochondria. Chem Pharm Bull 35:3766–3770
Yamaguchi M, Mori S (1988) Effect of Ca2+ and Zn2+ on 5′-nucleotidase activity in rat liver plasma membranes: hepatic calcium-binding protein (regucalcin) reverses the Ca2+ effect. Chem Pharm Bull 36:321–325
Shimokawa N, Yamaguchi M (1993) Molecular cloning and sequencing of the cDNA coding for a calcium-binding protein regucalcin from rat liver. FEBS Lett 327:251–255
Shimokawa N, Isogai M, Yamaguchi M (1995) Specific species and tissue differences for the gene expression of calcium-binding protein regucalcin. Mol Cell Biochem 143:67–71
Misawa H, Yamaguchi M (2000) Transcript heterogeneity of the human gene for Ca2+-binding protein regucalcin. Int J Mol Med 5:283–287
Murata T, Yamaguchi M (1997) Molecular cloning of the cDNA coding for regucalcin and its mRNA expression in mouse liver: the expression is stimulated by calcium administration. Mol Cell Biochem 173:127–133
Misawa H, Yamaguchi M (2000) The gene of Ca2+-binding protein regucalcin is highly conserved in vertebrate species. Int J Mol Med 6:191–196
Yamaguchi M (2011) The transcriptional regulation of regucalcin gene expression. Mol Cell Biochem 346:147–171
Shimokawa N, Matsuda Y, Yamaguchi M (1995) Genomic cloning and chromosomal assignment of rat regucalcin gene. Mol Cell Biochem 151:157–163
Thiselton DL, McDowall J, Brandau O, Ramser J, d’Esposito F, Bhattacharga SS, Ross MT, Hardcastle AJ, Meindl A (2002) An integrated, functionally annotated gene map of the DXS8026-ELK1 internal on human Xp11.3-Xp11.23: potential hotspot for neurogenetic disorders. Genomics 79:560–572
Shimokawa N, Yamaguchi M (1992) Calcium administration stimulates the expression of calcium-binding protein regucalcin mRNA in rat liver. FEBS Lett 305:151–154
Yamaguchi M, Isogai M, Kato S, Mori S (1991) Immunohistochemical demonstration of calcium-binding protein regucalcin in the tissues of rats: the protein localizes in liver and brain. Chem Pharm Bell 36:1601–1603
Yamaguchi M, Isogai M (1993) Tissue concentration of calcium-binding protein regucalcin in rats by enzyme-linked immunoadsorbent assay. Mol Cell Biochem 122:65–68
Yamaguchi M, Nakajima R (2002) Role of regucalcin as an activator of sarcoplasmic reticulum Ca2+-ATPase activity in rat heart muscle. J Cell Biochem 86:184–193
Yamaguchi M, Hamano T, Misawa H (2000) Expression of Ca2+-binding protein regucalcin in rat brain neurons: inhibitory effect on protein phosphatase activity. Brain Res Bull 52:343–348
Yamaguchi M, Misawa Y, Uchiyama S, Morooka Y, Tsurusaki Y (2002) Role of endogenous regucalcin in bone metabolism: bone loss is induced in regucalcin transgenic rats. Int J Mol Med 10:377–383
Yamaguchi M (1992) A novel Ca2+-binding protein regucalcin and calcium inhibition. Regulatory role in liver cell function. In: Kohama K (ed) Calcium inhibition. Japan Science Society Press and CRC Press, Tokyo, Boca Raton, pp 19–41
Yamaguchi M (1998) Role of calcium-binding protein regucalcin in regenerating rat liver. J Gastroenterol Hepatol 13(Suppl):S106–S112
Yamaguchi M (2000) Role of regucalcin in calcium signaling. Life Sci 66:1769–1780
Yamaguchi M (2000) The role of regucalcin in nuclear regulation of regenerating liver. Biochem Biophys Res Commun 276:1–6
Yamaguchi M (2002) Impact of aging on calcium channels and pumps. In: Mattson MP (ed) Calcium homeostasis and signaling in aging. Elsevier, Amsterdam, pp 47–65
Fujita T, Uchida K, Maruyama N (1992) Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta 1116:122–128
Fujita T, Shirasawa T, Uchida K, Maruyama N (1992) Isolation of cDNA clone encoding rat senescence marker protein-30 (SMP30) and its tissue distribution. Biochim Biophys Acta 1132:297–305
Tsurusaki Y, Misawa H, Yamaguchi M (2000) Translocation of regucalcin to rat liver nucleus: involvement of nuclear protein kinase and protein phosphatase regulation. Int J Mol Med 6:655–660
Chakraborti S, Bahnson BJ (2010) Crystal structure of human senescence marker protein 30: insights linking structural, enzymatic, and physiological functions. Biochemistry 49:3436–3444
Yamaguchi M, Makino R, Shimokawa N (1996) The 5′end sequences and exon organization in rat regucalcin gene. Mol Cell Biochem 165:145–150
Murata T, Yamaguchi M (1998) Tissue-specific binding of nuclear factors to the 5′-flanking region of the rat gene for calcium-binding protein regucalcin. Mol Cell Biochem 178:305–310
Murata T, Yamaguchi M (1998) Ca2+ administration stimulates the binding of AP-1 factor to the 5′-flanking region of the rat gene for the Ca2+-binding protein regucalcin. Biochem J 329:157–163
Murata T, Yamaguchi M (1999) Promoter characterization of the rat gene for Ca2+-binding protein regucalcin. Transcriptional regulation by signaling factors. J Biol Chem 274:1277–1285
Misawa H, Yamaguchi M (2000) Involvement of hepatic nuclear factor I binding motif in transcriptional regulation of Ca2+-binding protein regucalcin gene. Biochem Biophys Res Commun 269:270–278
Misawa H, Yamaguchi M (2000) Intracellular signaling factors-enhanced hepatic nuclear protein binding to TTGGC sequence in the rat regucalcin gene promoter: involvement of protein phosphorylation. Biochem Biophys Res Commun 279:275–281
Misawa H, Yamaguchi M (2002) Identification of transcription factor in the promoter region of rat regucalcin gene: binding of nuclear factor I-A1 to TTGGC motif. J Cell Biochem 84:795–802
Misawa H, Yamaguchi M (2001) Molecular cloning and sequencing of the cDNA coding for a novel regucalcin gene promoter region-related protein in rat, mouse and human liver. Int J Mol Med 8:513–520
Misawa H, Yamaguchi M (2002) Gene expression for a novel protein RGPR-p117 in various species: the stimulation by intracellular signaling factors. J Cell Biochem 87:188–193
Yamaguchi M, Misawa H, Ma ZJ (2003) Novel protein RGPR-p117: the gene expression in physiologic state and the binding activity to regucalcin gene promoter region in rat liver. J Cell Biochem 88:1092–1100
Murata T, Shinya N, Yamaguchi M (1997) Expression of calcium-binding protein regucalcin mRNA in the cloned human hepatoma cells (Hep G2): stimulation by insulin. Mol Cell Biochem 175:163–168
Nakajima M, Murata T, Yamaguchi M (1999) Expression of calcium-binding protein regucalcin mRNA in the cloned rat hepatoma cells (H4-II-E) is stimulated through Ca2+ signaling factors: involvement of protein kinase C. Mol Cell Biochem 198:101–107
Yamaguchi M, Nakajima M (1999) Involvement of intracellular signaling factors in the serum-enhanced Ca2+ binding protein regucalcin mRNA expression in the cloned rat hepatoma cells (H4-II-E). J Cell Biochem 74:81–89
Vinson CR, Sigler PB, McKnight SL (1989) Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science 246:911–916
O’Shea EK, Rutkowski R, Stafford WF III, Kim PS (1989) Preferential heterodimer formation by isolated leucine zippers from fos and jun. Science 245:646–648
Shimokawa N, Yamaguchi M (1993) Expression of hepatic calcium-binding protein regucalcin mRNA is mediated through Ca2+/calmoclulin in rat liver. FEBS Lett 316:79–84
Isogai M, Yamaguchi M (1995) Calcium administration increases calcium-binding protein regucalcin concentration in the liver of rats. Mol Cell Biochem 143:53–58
Yamaguchi M, Kurota H (1995) Expression of calcium-binding protein regucalcin mRNA in the kidney cortex of rats: the stimulation by calcium administration. Mol Cell Biochem 146:71–77
Yamaguchi M, Ueoka S (1998) Expression of calcium-binding protein regucalcin mRNA in fetal rat liver is stimulated by calcium administration. Mol Cell Biochem 178:283–287
Yamaguchi M, Kanayama Y, Shimokawa N (1994) Expression of calcium-binding protein regucalcin mRNA in rat liver is stimulated by calcitonin: the hormonal effect is mediated through calcium. Mol Cell Biochem 136:43–48
Yamaguchi M, Oishi K, Isogai M (1995) Expression of hepatic calcium-binding protein regucalcin mRNA is elevated by refeeding of fasted rats: involvement of glucose, insulin and calcium as stimulating factors. Mol Cell Biochem 142:35–41
Yamaguchi M, Oishi K (1995) 17β-Estradiol stimulates the expression of hepatic calcium-binding protein regucalcin mRNA in rats. Mol Cell Biochem 143:137–141
Yamaguchi M, Kanayama Y (1995) Enhanced expression of calcium-binding protein regucalcin mRNA in regenerating rat liver. J Cell Biochem 57:185–190
Ueoka S, Yamaguchi M (1998) Sexual difference of hepatic calcium-binding protein regucalcin mRNA expression in rats with different ages: effect of ovarian hormone. Biol Pharm Bull 21:405–407
Lotersztajn S, Hanoune J, Pecker F (1981) A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence on an endogenous protein activator distinct from calmodulin. J Biol Chem 256:11209–11215
Chen K-M, Junger KD (1983) Calcium transport and phosphorylated intermediate of (Ca2+–Mg2+)-ATPase in plasma membranes of rat liver. J Biol Chem 258:4404–4410
Yamaguchi M, Mori S, Kato S (1988) Calcium-binding protein regucalcin is an activator of (Ca2+–Mg2+)-adenosine triphosphatase in the plasma membranes of rat liver. Chem Pharm Bull 36:3532–3539
Takahashi H, Yamaguchi M (1993) Regulatory effect of regucalcin on (Ca2+–Mg2+)-ATPase in rat liver plasma membranes: comparison with the activation by Mn2+ and Co2+. Mol Cell Biochem 124:169–174
Takahashi H, Yamaguchi M (1994) Activatory effect of regucalcin on (Ca2+–Mg2+)-ATPase in rat liver plasma membranes: relation to sulfhydryl group. Mol Cell Biochem 136:71–76
Takahashi H, Yamaguchi M (1997) Stimulatory effect of regucalcin on ATP-dependent calcium transport in rat liver plasma membranes. Mol Cell Biochem 168:149–153
Takahashi H, Yamaguchi M (1993) Regucalcin modulates hormonal effect on (Ca2+–Mg2+)-ATPase activity in rat liver plasma membranes. Mol Cell Biochem 125:171–177
Takahashi H, Suzuki S, Yanaguchi M (1995) Stimulatory effect of hormonal signaling factors on (Ca2+–Mg2+)-ATPase activity in rat liver plasma membranes: cross talk with regucalcin. Mol Cell Biochem 151:1–7
Takahashi H, Yamaguchi M (1995) Increase of (Ca2+–Mg2+)-ATPase activity in hepatic plasma membranes of rats administered orally calcium: the endogenous role of regucalcin. Mol Cell Biochem 144:1–6
Takahashi H, Yamaguchi M (1996) Enhancement of plasma membrane (Ca2+–Mg2+)-ATPase activity in regenerating rat liver: involvement of endogenous activating protein regucalcin. Mol Cell Biochem 162:133–138
Takahashi H, Yamaguchi M (1996) Activatory effect of regucalcin on hepatic plasma membrane (Ca2+-Mg2+)-ATPase is impaired by liver injury with carbon tetrachloride administration in rats. Mol Cell Biochem 158:9–16
Yamaguchi M (1985) Mitocondrial uptake of 45Ca2+ bound to calcium- binding protein isolated from rat liver cytosol. Chem Pharm Bull 33:3390–3394
Mori S, Yamaguchi M (1991) Calcium-binding protein regucalcin stimulates the uptake of Ca2+ by rat liver mitochondria. Chem Pharm Bull 39:224–226
Takahashi H, Yamaguchi M (2000) Stimulatory effect of regucalcin on ATP-dependent Ca2+ uptake activity in rat liver mitochondria. J Cell Biochem 78:121–130
Yamaguchi M, Mori S (1989) Activation of hepatic microsomal Ca2+-adenosine triphosphatase by calcium-binding protein regucalcin. Chem Pharm Bull 37:1031–1034
Takahashi H, Yamaguchi M (1999) Role of regucalcin as an activator of Ca2+-ATPase activity in rat liver microsomes. J Cell Biochem 74:663–669
Yamaguchi M, Mori S (1989) Effect of the calcium-binding protein regucalcin on the Ca2+ transport system in rat liver microsomes: the protein stimulates Ca2+ release. Chem Pharm Bull 37:3037–3041
Yamaguchi M, Mori S (1990) Regucalcin-induced Ca2+ release from rat liver microsomes: the effect is inhibited by heparin. Chem Pharm Bull 38:2305–2307
Nicotera P, McConkey DJ, Jones DP, Orrenius S (1989) ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci USA 86:453–457
Bachs O, Carafolli E (1995) Calmodulin and calmodulin-binding proteins in liver cell nuclei. J Biol Chem 262:10786–10790
Csermely P, Schnaider T, Szanto I (1995) Signalling and transport through the nuclear membrane. Biochim Biophys Acta 1241:425–452
Tsurusaki Y, Yamaguchi M (2000) Role of endogenous regucalcin in the regulation of Ca2+-ATPase activity in rat liver nuclei. J Cell Biochem 78:541–549
Yamaguchi M (1992) Effect of calcium-binding protein regucalcin on Ca2+ transport system in rat liver nuclei: stimulation of Ca2+ release. Mol Cell Biochem 113:63–70
Fujita T, Inoue H, Kitamura T, Sato N, Shimosawa T, Maruyama N (1998) Senescence marker protein-30 (SMP30) rescues cell death by enhancing plasma membrane Ca2+-pumping activity in Hep G2 cells. Biochem Biophys Res Commun 250:374–380
Van Os CH (1985) Transcellular calcium transport in intestinal and renal epithelial cells. Biochim Biophys Acta 906:195–222
Taylor CW (1985) Calcium regulation in vertebrates: an overview. Comp Biochem Physiol 82A:249–255
Murata T, Yamaguchi M (1999) Binding of kidney nuclear proteins to the 5′-flanking region of the rat gene for Ca2+-binding protein regucalcin: involvement of Ca2+/calmodulin signaling. Mol Cell Biochem 199:35–40
Misawa H, Yamaguchi M (2001) Involvement of nuclear factor-1 (NF1) binding motif in the regucalcin gene expression of rat kidney cortex: the expression is suppressed by cisplatin administration. Mol Cell Biochem 219:29–37
Kurota H, Yamaguchi M (1996) Steroid hormonal regulation of calcium-binding protein regucalcin mRNA expression in the kidney cortex of rats. Mol Cell Biochem 155:105–111
Shinya N, Kurota H, Yamaguchi M (1996) Calcium-binding protein regucalcin mRNA expression in the kidney cortex is suppressed by saline ingestion in rats. Mol Cell Biochem 162:139–144
Shinya N, Yamaguchi M (1997) Alteration in Ca2+-ATPase activity and calcium-binding protein regucalcin mRNA expression in the kidney cortex of rats with saline ingestion. Mol Cell Biochem 170:17–22
Shinya N, Yamaguchi M (1998) Stimulatory effect of calcium administration on regucalcin mRNA expression is attenuated in the kidney cortex of rats ingested with saline. Mol Cell Biochem 178:275–281
Kurota H, Yamaguchi M (1995) Suppressed expression of calcium-binding protein regucalcin mRNA in the renal cortex of rats with chemically induced kidney damage. Mol Cell Biochem 151:55–60
Agus ZS, Chiu PJS, Goldberg M (1997) Regulation of urinary calcium-excretion in the rat. Am J Physiol 232:F545–F549
Kennedy MB, Bennett MK, Erondu NE, Miller SG (1987) Calcium/calmodulin-dependent protein kinases. In: Cheung WY (ed) Calcium and cell function. Academic Press Inc, New York, pp 61–107
Kurota H, Yamaguchi M (1997) Activatory effect of calcium-binding protein regucalcin on ATP-dependent calcium transport in the basolateral membranes of rat kidney cortex. Mol Cell Biochem 169:149–156
Kurota H, Yamaguchi M (1997) Regucalcin increases Ca2+-ATPase activity and ATP-dependent calcium uptake in the microsomes of rat kidney cortex. Mol Cell Biochem 177:201–207
Langer GA (1992) Calcium and the heart: exchange at the tissue, cell, and organelle levels. FASEB J 6:893–902
Akhter T, Nakagawa T, Kobayashi A, Yamaguchi M (2007) Suppression of regucalcin mRNA expression in the hearts of rats administered with free radical compound: the administration-induced death is accelerated in regucalcin transgenic rats. Int J Mol Med 19:653–658
Thastrup O, Culler PJ, Drbbak BK, Hanley MR, Dawson AP (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA 87:2466–2470
Tada M, Kadoma M (1989) Regulation of the Ca2+ pump ATPase by cAMP-dependent phosphorylation of phospholamban. Bioessays 10:157–163
Akther T, Sawada N, Yamaguchi M (2006) Regucalcin increases Ca2+-ATPase activity in the heart mitochondria of normal and regucalcin transgenic rats. Int J Mol Med 18:171–176
Dahan D, Spanier R, Rahaaminoff H (1991) The modulation of rat brain Na+-Ca2+ exchange by K+. J Biol Chem 266:2067–2075
MacDermott AB, Dale N (1986) Receptors, ion channels and synaptic potential underlying the integrative actions of excitatory amino acids. Trends Neurosci 10:280–284
Cambray-Deakin MA, Burgoyne RD (1992) Intracellular Ca2+ and N-methyl-d-aspartate-stimulated neuritogenesis in rat cerebellar granule cell cultures. Dev Brain Res 66:25–32
Treves S, De Mattei M, Lanfred M, Villa A, Green NM, MacLennan DH, Meldolesi J, Pozzan T (1990) Calreticulin is a candidate for a calsequestrin-like function in Ca2+-storage compartment (calcitosomes) of liver and brain. Biochem J 271:473–480
Hartman H, Eckert A, Muller WE (1994) Disturbances of the neuronal calcium-homeostasis in the aging nervous system. Life Sci 55:2011–2018
Yamaguchi M, Hanahisa Y, Murata T (1999) Expression of calcium-binding protein regucalcin and microsomal Ca2+-ATPase regulation in rat brain: attenuation with increasing age. Mol Cell Biochem 200:43–49
Hanahisa Y, Yamaguchi M (1996) Characterization of calcium accumulation in the brain of rats administered orally calcium: the significance of energy-dependent mechanism. Mol Cell Biochem 158:1–7
Hanahisa Y, Yamaguchi M (1997) Increase in calcium content and Ca2+-ATPase activity in the brain of fasted rats: comparison with different ages. Mol Cell Biochem 171:127–132
Hanahisa Y, Yamaguchi M (2001) Decrease in Ca2+-ATPase activity in the brain plasma membrane of rats with increasing age: involvement of brain calcium accumulation. Int J Mol Med 7:407–411
Hanahisa Y, Yamaguchi M (1999) Brain microsomal calcium accumulation in rats with increasing age: involvement of thapsigargin sensitive Ca2+-ATPase. Int J Mol Med 4:627–631
Hanahisa Y, Yamaguchi M (1998) Increase of Ca2+-ATPase activity in the brain microsomes of rats with increasing ages: involvement of protein kinase C. Brain Res Bull 46:329–332
Yamaguchi M, Takakura Y, Nakagawa T (2008) Regucalcin increases Ca2+-ATPase activity in the mitochondria of brain tissues of normal and transgenic rats. J Cell Biochem 104:795–804
Yamaguchi M, Sakurai T (1992) Reversible effect of calcium-binding protein regucalcin on the Ca2+-induced inhibition of deoxyuridine 5′-triphosphatase activity in rat liver cytosol. Mol Cell Biochem 110:25–29
Hanahisa Y, Yamaguchi M (1999) Effect of calcium-binding protein on adenosine 5′-triphosphatase activity in the brain cytosol of rats of different ages: the inhibitory role of regucalcin. Biol Pharm Bull 22:313–316
Rasmussen J (1970) Cell communication, calcium ion, and cyclic adenosine monophosphate. Science 170:404–412
Yamaguchi M, Tai H (1991) Inhibitory effect of calcium-binding protein regucalcin on Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase activity in rat liver cytosol. Mol Cell Biochem 106:25–30
Yamaguchi M, Kurota H (1997) Inhibitory effect of regucalcin on Ca2+/calmodulin-dependent cyclic AMP phosphodiesterase activity in rat kidney cytosol. Mol Cell Biochem 177:209–214
Lowenstein CJ, Dinerman JL, Snyder SH (1994) Nitric oxide: a physiologic messenger. Ann Intern Med 120:227–237
Izumi T, Tsurusaki Y, Yamaguchi M (2003) Suppressive effect of endogenous regucalcin on nitric oxide synthase activity in cloned rat hepatoma H4-II-E cells overexpressing regucalcin. J Cell Biochem 89:800–807
Yamaguchi M, Takahashi H, Tsurusaki Y (2003) Suppressive role of endogenous regucalcin in the enhancement of nitric oxide synthase activity in liver cytosol of normal and regucalcin transgenic rats. J Cell Biochem 88:1226–1234
Ma ZJ, Yamaguchi M (2003) Regulatory effect of regucalcin on nitric oxide synthase activity in rat kidney cortex cytosol: role of endogenous regucalcin in transgenic rats. Int J Mol Med 12:201–206
Ma ZJ, Yamaguchi M (2002) Suppressive role of endogenous regucalcin in the regulation of nitric oxide synthase activity in heart muscle cytosol of normal and regucalcin transgenic rats. Int J Mol Med 10:761–766
Tobisawa M, Yamaguchi M (2003) Inhibitory role of regucalcin in the regulation of nitric oxide synthase activity in rat brain cytosol: involvement of aging. J Neurol Sci 209:47–54
Tobisawa M, Yamaguchi M (2003) Role of endogenous regucalcin in brain function: suppression of cytosolic nitric oxide synthase and nuclear protein tyrosine phosphatase activities in brain tissue of transgenic rats. Int J Mol Med 12:581–585
Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richaardson A (2001) Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physol Heart Cric Physiol 281:H1422–H1432
Den Hartog GJ, Haenen GR, Boven E, van der Vijgh WJ, Bast A (2004) Lecithinized copper, zinc-superoxide dismutase as a protector against dexorubicin-induced cardiotoxicity in mice. Toxicol Appl Pharmacol 194:180–188
Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH (2003) NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fisher 344 rats. Am J physiol Heart Circ Physiol 285:H1015–H1022
Fukaya Y, Yamaguchi M (2004) Regucalcin increases superoxide dismutase activity in rat liver cytosol. Biol Pharm Bull 27:1444–1446
Ichikawa E, Yamaguchi M (2004) Regucalcin increases superoxide dismutase activity in the heart cytosol of normal and regucalcin transgenic rats. Int J Mol Med 14:691–695
Connelly PA, Sisk RB, Schulman H, Garrison JC (1987) Evidence for the activation of the multifunction Ca2+/calmodulin-dependent protein kinase in response to hormones that increase intracellular Ca2+. J Biol Chem 262:10154–10163
Mori S, Yamaguchi M (1990) Hepatic calcium-binding protein regucalcin decreases Ca2+/calmodulin-dependent protein kinase activity in rat liver cytosol. Chem Pharm Bull 38:2216–2218
Kurota H, Yamaguchi M (1997) Inhibitory effect of regucalcin on Ca2+/calmodulin-dependent protein kinase activity in rat renal cortex cytosol. Mol Cell Biochem 177:239–243
Hamano T, Hanahisa Y, Yamaguchi M (1999) Inhibitory effect of regucalcin on Ca2+-dependent protein kinase activity in rat brain cytosol: involvement of endogenous regucalcin. Brain Res Brain 50:187–192
Hamano T, Yamaguchi M (2001) Inhibitory role of regucalcin in the regulation of Ca2+-dependent protein kinase activity in rat brain tissues. J Neurol Sci 183:33–38
Omura M, Yamaguchi M (1998) Inhibition of Ca2+/calmodulin-dependent phosphatase activity by regucalcin in rat liver cytosol: involvement of calmodulin binding. J Cell Biochem 71:140–148
Yamaguchi M, Mori S (1990) Inhibitory effect of calcium-binding protein regucalcin on protein kinase C activity in rat liver cytosol. Biochem Med Metab Biol 43:140–146
Kurota H, Yamaguchi M (1998) Inhibitory effect of calcium-binding protein regucalcin on protein kinase C activity in rat renal cortex cytosol. Biol Pharm Bull 21:315–318
Hunter T (1995) Protein kinases and phosphatases: the Yin and Yang of protein phosphorylation and signaling. Cell 80:225–236
Wang Y, Santini F, Qin K, Huang CY (1995) A Mg2+-dependent, Ca2+-inhibitable seruin/throsine protein phosphatase from bovine brain. J Biol Chem 270:25607–25612
Pallen CJ, Wang JH (1983) Calmodulin-stimulated dephosphorylation of p-nitrophenylphosphate and free phosphotyrosine by calcineurin. J Biol Chem 258:850–855
Omura M, Yamaguchi M (1999) Effect of anti-regucalcin antibody on neutral phosphatase activity in rat liver cytosol: involvement of endogenous regucalcin. Mol Cell Biochem 197:25–29
Omura M, Kurota H, Yamaguchi M (1998) Inhibitory effect of regucalcin on Ca2+/calmodulin-dependent phosphatase activity in rat renal cortex cytosol. Biol Pharm Bull 21:440–443
Omura M, Yamaguchi M (1999) Enhancement of neutral phosphatase activity in the cytosol and nuclei of regenerating rat liver: role of endogenous regucalcin. J Cell Biochem 73:332–341
Omura M, Yamaguchi M (1999) Regulation of protein phosphatase activity by regucalcin localization in rat liver nuclei. J Cell Biochem 75:437–445
Morooka Y, Yamaguchi M (2001) Suppressive role of endogenous regucalcin in the regulation of protein phosphatase activity in rat renal cortex cytosol. J Cell Biochem 81:639–646
Morooka Y, Yamaguchi M (2001) Inhibitory effect of regucalcin on protein phosphatase activity in the nuclei of rat kidney cortex. J Cell Biochem 83:111–120
Morooka Y, Yamaguchi M (2002) Endogenous regucalcin suppresses the enhancement of protein phosphatase activity in the cytosol and nucleus of kidney cortex in calcium-administered rats. J Cell Biochem 85:553–560
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 17:215–228
Ichikawa E, Tsurusaki Y, Yamaguchi M (2004) Suppressive effect of regucalcin on protein phosphatase activity in the heart cytosol of normal and regucalcin transgenic rats. Int J Mol Med 13:289–293
Hamano T, Yamaguchi M (1999) Inhibitory effect of regucalcin on Ca2+/calmodulin-dependent protein phosphatase activity in rat brain cytosol. Int J Mol Med 3:615–619
Tobisawa M, Tsurusaki Y, Yamaguchi M (2003) Decrease in regucalcin level and enhancement of protein tyrosine phosphatase activity in rat brain microsomes with increasing age. Int J Mol Med 12:577–580
Tobisawa M, Yamaguchi M (2003) Suppressive effect of endogenous regucalcin on protein tyrosine phosphatase activity in the nucleus of rat brain: attenuation with increasing age. Int J Mol Med 11:205–210
Brostrom CO, Bocckino SB, Brostrom MA, Galuska EM (1983) Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem 258:14390–14399
Brostrom CO, Bocckino SB, Brostrom MA, Galuska EM (1986) Regulation of protein synthesis in isolated hepatocytes by calcium-mobilizing hormones. Mol Pharmacol 29:104–111
Menaya J, Parvilla R, Ayuso MS (1988) Effect of vasopressin on the regulation of protein synthesis initiation in liver cells. Biochem J 254:773–779
Thomas AP, Alexander J, Williamson JR (1984) Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem 259:5574–5584
Berthon B, Binet A, Mauger J-P, Claret M (1984) Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin 2. Effects of noradrenalin and vasopressin. FEBS Lett 167:19–24
Yamaguchi M, Mori S (1990) Effect of calcium-binding protein regucalcin on hepatic protein synthesis: inhibition of aminoacyl-tRNA synthetase activity. Mol Cell Biochem 99:25–32
Tsurusaki Y, Yamaguchi M (2000) Suppressive effect of endogenous regucalcin on the enhancement of protein synthesis and aminoacyl-tRNA synthetase activity in regenerating rat liver. Int J Mol Med 6:295–299
Wang KK, Villalobo A, Ronfogalis BD (1989) Calmodulin-binding protein as calpain substrates. Biochem J 262:693–706
Pontremoli S, Melloni E, Salamino F, Sparator B, Michetti M, Horecker BL (1984) Cytosolic Ca2+-dependent neutral proteinases from rabbit liver: activation of the proenzymes by Ca2+ and substrate. Proc Natl Acad Sci USA 81:53–56
Yamaguchi M, Tai H (1992) Calcium-binding protein regucalcin increases calcium-independent proteolytic activity in rat liver cytosol. Mol Cell Biochem 12:89–95
Yamaguchi M, Nishina N (1995) Characterization of regucalcin effect on proteolytic activity in rat liver cytosol: relation to cysteinyl-proteases. Mol Cell Biochem 148:67–72
Mellgren RI (1987) Calcium-dependent proteases: an enzyme system active at cellular membranes? FASEB J 1:110–115
Bode W, Huber R (1992) Natural protein proteinase inhibitors and their interaction with proteinase. Eur J Biochem 204:433–451
Rosser BG, Powers SP, Gores GJ (1993) Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J Biol Chem 268:23593–23600
Baba T, Yamaguchi M (1999) Stimulatory effect of regucalcin on proteolytic activity in rat renal cortex cytosol: involvement of thiol proteases. Mol Cell Biochem 195:87–92
Baba T, Yamaguchi M (2000) Stimulatory effect of regucalcin on proteolytic activity is impaired in the kidney cortex cytosol of rats with saline ingestion. Mol Cell Biochem 206:1–6
Croall DE, Demartino GN (1991) Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev 71:813–847
Melloni E, Pontremoli S, Michetti M, Sacco O, Sparatore B, Horecker BL (1986) The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem 261:4101–4105
Boynton AL, Whitfield JF, MacManus JP (1980) Calmodulin stimulates DNA synthesis by rat liver cells. Biochem Biohys Res Commun 95:745–749
Cruise J, Houck KA, Michalopoulos GK (1985) Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science 227:749–751
Pujol MJ, Soriano M, Alique R, Carafoli E, Bachs O (1989) Effect of alpha-adrenergic blockers on calmodulin associate with the nuclear matrix of rat liver cells during proliferative activation. J Biol Chem 264:18863–18865
Nakagawa T, Yamaguchi M (2008) Nuclear localization of regucalcin is enhanced in culture with protein kinase C activation in cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med 21:605–610
Tsurusaki Y, Yamaguchi M (2004) Role of regucalcin in liver nuclear function: binding of regucalcin to nuclear protein or DNA and modulation of tumor-related gene expression. Int J Mol Med 14:277–281
Jones DP, McConkey DJ, Nicotera P, Orrenius S (1989) Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem 264:6398–6403
Farber JL (1981) The role of calcium in cell death. Life Sci 29:1289–1295
Yamaguchi M, Sakurai T (1991) Inhibitory effect of calcium-binding protein regucalcin on Ca2+-activated DNA fragmentation in rat liver nuclei. FEBS Lett 279:281–284
Moroianu J, Blobel G (1995) Protein export from the nucleus requires the GTPase Ran and GTP hydrolysis. Proc Natl Acad Sci USA 92:4318–4322
Tsurusaki Y, Yamaguchi M (2001) Suppressive effect of endogenous regucalcin guanosine triphosphatase activity in rat liver nucleus. Biol Pharm Bull 24:958–961
Katsumata T, Yamaguchi M (1998) Inhibitory effect of calcium-binding protein regucalcin on protein kinase activity in the nuclei of regenerating rat liver. J Cell Biochem 71:569–576
Yamaguchi M, Kanayama Y (1996) Calcium-binding protein regucalcin inhibits deoxyribonucleic acid synthesis in the nuclei of regenerating rat liver. Mol Cell Biochem 162:121–126
Tsurusaki Y, Yamaguchi M (2002) Suppressive role of endogenous regucalcin in the enhancement of deoxyribonucleic acid synthesis activity in the nucleus of regenerating rat liver. J Cell Biochem 85:516–552
Morooka Y, Yamaguchi M (2002) Suppressive effect of endogenous regucalcin on deoxyribonucleic acid synthesis in the nuclei of rat renal cortex. Mol Cell Biochem 229:157–162
Yamaguchi M, Ueoka S (1997) Inhibitory effect of calcium-binding protein regucalcin on ribonucleic acid synthesis in isolated rat liver nuclei. Mol Cell Biochem 173:169–175
Tsurusaki Y, Yamaguchi M (2002) Role of endogenous regucalcin in nuclear regulation of regenerating rat liver: suppression of the enhanced ribonucleic acid synthesis activity. J Cell Biochem 87:450–457
Pardo JP, Fernandez F (1982) Effect of calcium and calmodulin on RNA synthesis in isolated nuclei from rat liver cells. FEBS Lett 143:157–160
Sturges MR, Peck LJ (1994) Calcium-dependent inactivation of RNA polymerase III transcription. J Biol Chem 269:5712–5719
Liu S, Shia D, Liu G, Chen H, Liu S, Hu Y (2000) Roles of Se and NO in apoptosis of hepatoma cells. Life Sci 68:603–610
Abou-Elella AM, Siendones E, Padillo J, Montero JL, De la Meta M, Relat JM (2002) Tumour necrosis factor-alpha and nitric oxide mediate apoptosis by d-galactosamine in a primary culture of rat hepatocytes: exacerbation of cell death by cocultured Kupffer cells. Can J Gastroenterol 16:791–799
Belloma G, Perotti M, Taddei F, Mirabelli F, Finardi G, Nicotera P, Orrenius S (1992) Tumor necrosis factor α induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res 52:1342–1346
Hukkanen M, Hughes FJ, Buttery LD, Gross SS, Evans TJ, Seddon S, Riveros-Moreno V, MacIntyre I, Polak JM (1995) Cytokine stimulated expression of inducible nitric oxide synthase by mouse, rat, and human osteoblast-like cells and its functional role in osteoblast metabolic activity. Endocrinology 136:5445–5453
Wink DA, Hanbauer I, Laval F, Cook JA, Krishna MC, Mifchell JB (1994) Nitric oxide protects against the cytotoxic effects of reactive oxygen species. Ann NY Acad Sci 738:265–278
Izumi T, Yamaguchi M (2004) Overexpression of regucalcin suppresses cell death in cloned rat hepatoma H4-II-E cells induced by tumor necrosis factor-α or thapsigargin. J Cell Biochem 92:296–306
Alikhani M, Alikhani Z, He H, Liu R, Popek BI, Graves DT (2003) Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J Biol Chem 278:52901–52908
Nalan Y, Vereker E, Lynch AM, Lynch MA (2003) Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Exp Neurol 184:794–804
Izumi T, Yamaguchi M (2004) Overexpression of regucalcin suppresses cell death and apoptosis in cloned rat hepatoma H4-II-E cells induced by lipopolysaccharide, PD98059, dibucaine, or Bay K 8644. J Cell Biochem 93:598–608
Zelivianshi S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Daris TL, Hauke R, Lin MF (2003) ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer 107:478–485
Arita K, Utsumi T, Kato A, Kanno T, Kobuchi H, Inoue B, Akiyama J, Utsumi M (2000) Mechanism of dibucaine-induced apoptosis in promyelocytic leukemia cells (HL-60). Biochem Pharmacol 60:905–915
Giuliano M, Bellacvia G, Lauricella M, D’Anneo A, Vassallo B, Vento R, Tesoriere G (2004) Staurosporine-induced apoptosis in Chang liver cells is associated with down-regulation of Bcl-2 and Bcl-XL. Int J Mol Med 13:565–571
Christensen SB, Andersen A, Kromann H, Treiman M, Tombal B, Denmeads S, Isaacs JT (1999) Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg Med Chem 7:1273–1280
Tombal B, Weeraratna AT, Denmeade SR, Isaacs JT (2000) Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate 43:303–317
Cohen JJ, Duke RC (1984) Glucocorticoid activation of a calcium-dependent endonuclease in thymocytes nuclei leads to cell death. J Immunol 132:38–42
Pereira M, Millot J-M, Sebille S, Manfait M (2002) Inhibitory effect of extracellular Mg2+ on intracellular Ca2+ dynamic changes and thapsigargin-induced apoptosis in human cancer MCF7 cells. Mol Cell Biochem 229:163–171
Cano-Abad MF, Villarroya M, Garcia AG, Gabilan NH, Lopez MG (2001) Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J Biol Chem 276:39695–39704
Fukaya Y, Yamaguchi M (2005) Overexpression of regucalcin suppresses cell death and apoptosis in cloned rat hepatoma H4-II-E cells induced by insulin or insulin-like growth factor-I. J Cell Biochem 96:145–154
Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignami F, Riccardi C, Nicoletti I (1994) The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res 18:431–439
Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Duport NA, Chevolleau S, Gasc N, Tulliez J, Terce F (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60:1426–1433
Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P (2002) Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 23:581–586
Singh AV, Xiao D, Lew KL, Dhir R, Singh SV (2004) Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 25:83–90
Fukaya Y, Yamaguchi M (2005) Overexpression of regucalcin suppresses apoptotic cell death in the cloned rat hepatoma H4-II-E cells induced by a naturally occurring isothiocyanate sulforaphane. Int J Mol Med 15:853–857
Nakagawa T, Yamaguchi M (2005) Hormonal regulation on regucalcin mRNA expression in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem 95:589–597
Nakagawa T, Yamaguchi M (2005) Overexpression of regucalcin suppresses apoptotic cell death in cloned normal rat kidney proximal tubular epithelial NRK52E cells: change in apoptosis-related gene expression. J Cell Biochem 96:1274–1285
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Zou H, Hanzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-l, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Widmann C, Gibson S, Johnson GL (1988) Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem 273:7141–7147
Dieguez-Acuna FJ, Polk WW, Ellis ME, Simmonds PL, Kushleika JV, Woods JS (2004) Nuclear factor kappaB activity determines the sensitivity of kidney epithelial cells to apoptosis: implications for mercury-induced renal failure. Toxicol Sci 82:1114–1123
Inagaki S, Yamaguchi M (2001) Suppressive role of endogenous regucalcin in the enhancement of protein kinase activity with proliferation of cloned rat hepatoma cells (H4-II-E). J Cell Biochem (Supplement) 36:12–18
Inagaki S, Misawa H, Yamaguchi M (2000) Role of endogenous regucalcin in protein tyrosine phosphatase regulation in the cloned rat hepatoma cells (H4-II-E). Mol Cell Biochem 213:43–50
Inagaki S, Yamaguchi M (2000) Enhancement of protein tyrosine phosphatase activity in the proliferation of cloned rat hepatoma H4-II-E cells: suppressive role of endogenous regucalcin. Int J Mol Med 6:323–328
Mackintosh C, Malintosh RW (1997) Inhibitors of protein kinases and phosphatases. Trends Biochem Sci 19:444–448
Cohen P, Cohen PTW (1989) Protein phosphatase come of age. J Biol Chem 264:21435–21438
Inagaki S, Yamaguchi M (2001) Regulatory role of endogenous regucalcin in the enhancement of nuclear deoxyribonucleic acid synthesis with proliferation of cloned rat hepatoma cells (H4-II-E). J Cell Biochem 82:704–711
Misawa H, Inagaki S, Yamaguchi M (2002) Suppression of cell proliferation and deoxyribonucleic acid synthesis in cloned rat hepatoma H4-II-E cells overexpressing regucalcin. J Cell Biochem 84:143–149
Yamaguchi M, Daimon Y (2005) Overexpression of regucalcin suppresses cell proliferation in cloned rat hepatoma H4-II-E cells: involvement of intracellular signaling factors and cell cycle-related genes. J Cell Biochem 95:1169–1177
Yamaguchi M (2005) Role of regucalcin in maintaining cell homeostasis and function (Review). Int J Mol Med 15:371–389
Meijer L, Borgne A, Mulner O, Chhong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP (1997) Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 243:527–536
Singh SV, Herman-Antosiewice A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R (2004) Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem 279:25813–25822
Hulla JE, Schneider RP (1993) Structure of the rat p53 tumor suppressor gene. Nucleic Acids Res 21:713–717
Charollais RH, Buquet C, Mester J (1990) Butyrate blocks the accumulation of CDc2 mRNA in late G1 phase but inhibits both early and late G1 progression in chemically transformed mouse fibroblasts BP-A31. J Cell Physiol 145:46–52
Curran T (1991) Fos and Jun: intermediary transcription factors. In: Cohen P, Foulkes JG (eds) The hormonal control of gene transcription. Elsevier Science Publisher, New York, pp 295–308
Tsurusaki Y, Yamaguchi M (2003) Overexpression of regucalcin modulates tumor-related gene expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem 90:619–626
Nakagawa T, Sawada N, Yamaguchi M (2005) Overexpression of regucalcin suppresses cell proliferation of cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med 16:637–643
Dang ZC, Lowik CW (2004) Differential effects of PD98059 and UO126 on osteogenesis and adipogenesis. J Cell Biochem 92:525–533
Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita E (1986) Staurosporine, a potent inhibitor of phospholipids/Ca2+-dependent protein kinase. Biochem Biophys Res Commun 135:397–402
Vincenzi FF (1982) Pharmacology of calmodulin antagonism. In: Godfranid T, Albertini A, Paoletti R (eds) Calcium modulators. Elsevier Biomedical Press, Amsterdam, pp 67–80
Park YC, Lee CH, Kang HS, Chung HT, Kim HD (1997) Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem Biophys Res Commun 240:692–696
Nakagawa T, Yamaguchi M (2006) Overexpression of regucalcin enhances its nuclear localization and suppresses L-type Ca2+ channel and calcium-sensing receptor mRNA expressions in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem 99:1064–1077
Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, Fujita T (2004) Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 15:2320–2333
Ba J, Friedman PA (2004) Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium 35:229–237
Xue JH, Takahashi H, Yamaguchi M (2000) Stimulatory effect of regucalcin on mitochondrial ATP-dependant calcium uptake activity in rat kidney cortex. J Cell Biochem 80:285–292
Nakagawa T, Yamaguchi M (2007) Overexpression of regucalcin suppresses cell response for tumor necrosis factor-α or transforming growth factor-β1 in cloned normal rat kidney proximal tubular epithelial NRK52E cells. J Cell Biochem 100:1178–1190
Fan JM, Ng Y-Y, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY (1999) Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 56:1455–1467
Higuchi O, Nakamura T (1991) Identification and change in the receptor for hepatocyte growth factor in rat liver after partial hepatectomy or induced hepatitis. Biochem Biophys Res Commun 176:599–607
Baffy G, Yang L, Michalopoulos GK, Williamson JR (1992) Hepatocyte growth factor induces calcium mobilization and inositol phosphate production in rat hepatocytes. J Cell Physiol 153:332–339
Higgins GM, Anderson RM (1931) Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12:186–202
Kanayama Y, Yamaguchi M (1995) Enhancement of nuclear Ca2+-ATPase activity in regenerating rat liver: involvement of nuclear DNA increase. Mol Cell Biochem 146:179–186
Pinol MR, Berchtold MW, Backs O, Heizmann CW (1988) Increased calmodulin synthesis in the pre-replicative phase of rat liver regeneration. FEBS Lett 231:445–450
Suzuki S, Asamoto M, Tsujimura K, Shirai T (2004) Specific differences in gene expression profile revealed by cDNA microarray analysis of glutathione S-transferase placental form (GST-P) immunohistochemically positive rat liver foci and surrounding tissue. Carcinogenesis 25:439–443
Yamaguchi M, Morooka Y, Misawa H, Tsurusaki Y, Nakajima R (2002) Role of endogenous regucalcin in transgenic rats: suppression of kidney cortex cytosolic protein phosphatase activity and enhancement of heart muscle microsomal Ca2+-ATPase activity. J Cell Biochem 86:520–529
Carpentier A, Taghibiglou C, Leung N, Szeto L, van Iderstine SC, Vffelman KD, Buckingham R, Adeli K, Lewis GF (2002) Ameliorated hepatic insulin resistance is associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J Biol Chem 277:28795–28802
Tsurusaki Y, Yamaguchi M (2003) Role of endogenous regucalcin in transgenic rats: suppression of protein tyrosine phosphatase and ribonucleic acid synthesis in liver nucleus. Int J Mol Med 12:207–211
Fukaya Y, Yamaguchi M (2004) Characterization of protein tyrosine phosphatase activity in rat liver microsomes: suppressive effect of endogenous regucalcin in transgenic rats. Int J Mol Med 14:427–432
Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhand-Sun G, Fantus IG, Ng JB, Hall DA, Lum BS, Shaver A (1994) Peroxovanadium compounds: a new class of potent phosphotyrosine phosphatase inhibits which are insulin mimetics. J Biol Chem 269:4596–4604
Ahmad F, Goldstein BJ (1995) Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model ofinsulin-resistance obesity and diabetes mellitus. Metabolism 44:1175–1184
Dylla SJ, Williams JP, Williford J, Hardy RW (2000) Phosphatase activity in rat adipocytes: effects of insulin and insulin resistance. J Cell Biochem 77:445–454
Nakashima C, Yamaguchi M (2006) Overexpression of regucalcin enhances glucose utilization and lipid production in cloned rat hepatoma H4-II-E cells: involvement of insulin resistance. J Cell Biochem 99:1582–1592
Nakashima C, Yamaguchi M (2007) Overexpression of regucalcin suppresses gene expression of insulin signaling-related proteins in cloned rat hepatoma H4-II-E cells: involvement of insulin resistance. Int J Mol Med 20:709–716
Yamaguchi M (2010) Regucalcin and metabolic disorder: osteoporosis and hyperlipidemia are induced in regucalcin transgenic rats. Mol Cell Biochem 341:119–133
Yamaguchi M, Kobayashi M, Uchiyama S (2005) Suppressive effect of regucalcin on cell differentiation and mineralization in osteoblastic MC3T3–E1 cells. J Cell Biochem 96:543–554
Yamaguchi M, Uchiyama S (2005) Regucalcin stimulates osteoclast-like cell formation in mouse marrow cultures. J Cell Biochem 94:794–803
Yamaguchi M, Otomo Y, Uchiyama S, Nakagawa T (2008) Hormonal regulation of regucalcin mRNA expression in osteoblastic MC3T3–E1 cells. Int J Mol Med 21:771–775
Otomo Y, Yamaguchi M (2006) Regulatory effect of exogenous regucalcin on cell function in osteoblastic MC3T3–E1 cells: involvement of intracellular signaling factor. Int J Mol Med 18:321–327
Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N (2002) Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha-and Fas-mediated apoptosis. Am J Pathol 161:1273–1281
Ishigami A, Kondo Y, Nanba R, Ohsawa T, Handa S, Kubo S, Akita M, Maruyama N (2004) SMP30 deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span. Biochem Biophys Res Commun 315:575–580
Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, Goto S, Nishikimi M, Maruyama N, Ishigami A (2006) Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci USA 103:5723–5728
Nejak-Bowen KN, Zeng G, Tan X, Cieply B, Monga SP (2009) β-Catenin regulates vitamin C biosynthesis and cell survival in murine liver. J Biol Chem 284:28115–28127
Acknowledgments
Regucalcin studies of the author were supported by a Grant-in-Aid for Scientific Research (C) No.63571053, No.02671006, No.04671362, No.06672193, No. 08672522, No.10672048, No.13672292 and No.17590063 from the Ministry of Education, Science, Sports, and Culture, Japan. Also, the author was awarded the Bounty of Encouragement Foundation in Pharmaceutical Research, 1994, Japan and the Bounty of the Yamanouchi Foundation for Research on Metabolic Disorders, 2004, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, M. Regucalcin and cell regulation: role as a suppressor protein in signal transduction. Mol Cell Biochem 353, 101–137 (2011). https://doi.org/10.1007/s11010-011-0779-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0779-4