Abstract
Enteroaggregative Escherichia coli (EAEC) is emerging as a cause of acute and persistent diarrhea in developing countries. An important feature of EAEC pathogenesis is the induction of profound inflammatory response in the intestinal epithelium. In this article, we have shown that EAEC-induced activation of mitogen-activated protein kinases (MAPK) (ERK-1/2, JNK and p38MAPK) in cultured human intestinal epithelial cells (INT-407) leads to the induction of DNA-binding activity of NF-κB and AP-1, resulting in IL-8 production. Plasmid-cured EAEC could also activate the MAPK and the transcription factors leading to IL-8 secretion, but to a lesser extent than that of wild-type EAEC. Further, pretreatment of these cells with the highly specific MEK inhibitor (PD 098059), the JNK inhibitor (SP 600125), and the p38MAPK inhibitor (SB 203580) resulted in inhibition of the IL-8 secretion by EAEC (wild type as well as plasmid cured)-infected INT-407 cells. These findings demonstrate that the inflammatory response induced by EAEC may be due to the specific stimulation of MAPK signaling pathways leading to nuclear responses. To our knowledge, this is the first article regarding the detailed mechanism of IL-8 secretion from the EAEC-infected human intestinal epithelial cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteroaggregative Escherichia coli (EAEC) is an important enteric pathogen responsible for acute and persistent diarrhea in both developing and industrialized countries [1, 2]. EAEC-induced pathogenicity is known to be associated with intestinal inflammation [3]. It has been reported that EAEC-infected children had significant elevation in faecal lactoferrin, IL-8, and IL-1β [4]. Further, flagellin as well as AAF/II of EAEC-042 strain were shown to induce IL-8 secretion from different human intestinal epithelial cell lines [5, 6]. Recently, we have reported that a galactose-specific adhesin of EAEC-T7 strain induced IL-8 secretion from INT-407 cells [7]. Thus, it is possible that multiple factors of EAEC may contribute to EAEC-induced inflammation.
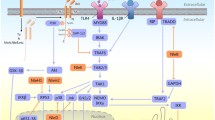
The IL-8 gene expression is regulated by several pathways. The promoter region of the gene contains the binding sequences for various transcription factors, including NF-κB and AP-1 [8]. In most cell types, NF-κB exists in an inactive form in the cytoplasm through its binding to inhibitory proteins IκB. Treatment of cells with various inducers activates a signalling cascade that culminates in phosphorylation of IκBs, resulting in degradation of IκB proteins. The bound NF-κB is released and translocates to the nucleus, where it activates appropriate set of target genes. The transcription factor AP-1 is composed of homodimer or heterodimer of proteins of the jun and fos families of DNA-binding proteins. Stimulation of AP-1 activity can result from an increase in the level of the components of this transcription factor or from an in increase in their activity due to specific phosphorylation.
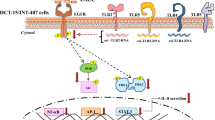
Activation of both NF-κB and AP-1 is dependent on mitogen-activated protein kinases (MAPK) (ERK-1/2, JNK and p38) that are central in various host responses including the regulation of cytokines. Studies have revealed that infection of cultured epithelial cells with different enteric organisms could lead to the activation of MAPK pathways resulting in the induction of DNA-binding activity of NF-κB and AP-1 with subsequent production of IL-8 [9–11]. Khan et al. [12] demonstrated that EAEC-flagellin-induced IL-8 secretion by colonic epithelial cells requires Toll-like receptor (TLR) 5-dependent activation of p38MAPK.
Not much has been studied on the signal transduction pathways involved in the EAEC-induced IL-8 secretion. Therefore, the aim of this study was to investigate the mechanisms implicated in the stimulation of IL-8 production in intestinal epithelial cells infected with EAEC. Since IL-8 gene transcription is regulated by the MAPK-mediated activation of transcription factors NF-κB and AP-1, we have assessed the level of expression of the activated MAPK (ERK-1/2, JNK, and p38) and the DNA-binding activity of NF-κB and AP-1 in EAEC-infected human intestinal epithelial cell line (INT-407). Further, the involvement of MAPK in EAEC-induced IL-8 secretion by these cells was also established in this study.
Materials and methods
Chemicals
All the chemicals used in the study were of analytical grade. The MEK1/2 inhibitor (PD 098059), the p38MAPK inhibitor (SB 203580), and the JNK inhibitor (SP 600125) were procured from Sigma-Aldrich Co. (St. Louis, MO, USA) and solubilized in DMSO. The anti-phospho-ERK-1/2 (sc-7976-R), anti-ERK-1/2 (sc-94), anti-phospho-p38MAPK (sc-7975-R), anti-phospho-JNK (sc-6254), and anti-JNK (sc-1648) antibodies, as well as the HRP-conjugated anti rabbit IgG (sc-2054) and HRP-conjugated anti mouse IgG (sc-2055) were obtained from Santa Cruz Biotechnology (CA, USA).
Bacterial strains and growth conditions
The EAEC-042 (prototype strain) and T8 (clinical isolate harbouring the AAF II plasmid) strains were procured from National Institute of Cholera and Enteric Diseases (Kolkata, India). The strains were grown at 37°C in Luria–Bertani medium. The T8 strain was cured of its plasmid by repeated passage in increasing concentration of acriflavine [13] and designated as −pT8. All the strains were characterized by the characteristic plasmid profile, PCR using EAEC-specific primers [14] as well as on the basis of HEp-2 adherence assay [15].
Cell culture
The INT-407 cells (an epithelial cell line derived from human embryo small intestine) were obtained from National Centre For Cell Science (Pune, India) and grown in Minimum Essential Medium (MEM) with 5 mM l-glutamine, Earle’s balanced salt solution (Gibco BRL, USA), 1.5 g/l sodium bicarbonate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (Biological Industries, Israel) at 37°C in a humidified atmosphere of 5% CO2–95% air. Cells were seeded at high density in tissue culture plates for further experiments.
Infection
Unless otherwise mentioned, bacteria were added to the INT-407 monolayers in 6-well/24-well tissue culture plates at 1:100 multiplicity of infection in MEM medium without serum and antibiotics. The cells were incubated in the CO2 incubator at 37°C for different time intervals as required for each experiment.
IL-8 assay
The INT-407 cells were infected with different EAEC strains for 3 h. Prior to infection, cells were washed and incubated in serum and antibiotic free MEM. The culture supernatant was collected by centrifugation and used for the estimation of IL-8 by sandwich ELISA, using monoclonal anti human IL-8 and biotinylated anti human IL-8 as the capture antibody and detection antibody (BD Pharmingen, USA), respectively. Where indicated, the inhibitors SB 203580 (10 μM, inhibitor for p38MAPK), SP 600125 (20 μM, inhibitor for JNK), and PD 098059 (50 μM, inhibitor for MEK-1/2) were added for 90 min prior to the infection and maintained throughout the infection period. In the assay performed with inhibitors, an EAEC control was pre-treated with DMSO, and DMSO was maintained during the infection.
Detection of IL-8 mRNA in INT-407 cells
The INT-407 cells grown in 75-cm2 flasks were infected with overnight grown bacterial culture and incubated for 3 h. The medium was removed, and the total RNA was isolated using RNeasy mini kit (Qiagen, USA). Reverse transcription was done on 2 μg of RNA from each sample at 42°C for 1 h using First strand cDNA synthesis kit (Roche, Germany). The reaction was terminated by 5-min incubation at 99°C and rapid chilling on ice.
The polymerase chain reaction (PCR) reaction was done by the addition of 2.5 μl of 10× PCR buffer, 2.0 μl of dNTP mix, 1.5 U of Taq DNA polymerase (Roche, Germany), 10 pmol of each primer (Sigma Genosys, USA), 5 μl of cDNA template, and the volume was made up to 25 μl with double autoclaved distilled water. For IL-8, the reaction was cycled 35 times at 95°C for 1 min, 60°C for 2.5 min, and 72°C for 1 min whereas for β-actin, the reaction was cycled 30 times at 95°C for 1 min, 58°C for 30 s, and 72°C for 45 s. A volume of 10 μl of each reaction product was electrophoresed on 1.5% agarose gel, stained with ethidium bromide, and visualized by UV light. The primers were obtained from Sigma Genosys (USA) and had the following sequences: 5′-tgacttccaagctggccgtggct-3′ and 5′-tctcagccctcttcaaaaacttctc-3′ for IL-8; 5′-tgacggggtcacccacactgtgcccatcta-3′ and 5′-ctagaagcattgcggtggacgatggaggg-3′ for β-actin.
The changes in band intensity in case of each parameter were quantified by scanning densitometry using Scion image 4.3 software (Bio-Rad, USA). Variations in the cDNA concentration were normalized by co-amplification with β-actin in each set. The increase/decrease (fold) in the expression of IL-8 in EAEC-infected INT-407 cells was calculated by taking the normalized value of the same transcript as 1 in cells cultured without bacteria.
Electrophoretic mobility shift assay
The INT-407 cells were seeded in six-well plates. At 70–80% confluence, the cells were washed and infected with different EAEC strains for 3 h in serum and antibiotic-free MEM. The DNA-binding activity of AP-1 as well as NF-κB was analyzed in total cleared cellular extracts prepared with NE-PER nuclear extraction reagent (Pierce, USA). The extracts (10 μg protein) were incubated separately for 25 min at 25°C with biotin-labeled double stranded oligonucleotide containing the AP-1 site (5′-TTCGTGACTCAGCGG-3′) and NF-κB site (5′-GATCCAAGGGGACTTTCCATG-3′). Complexes were separated by electrophoresis on a 6% non-denaturing polyacrylamide gel in 0.5× TBE [45 mM Tris/45 mM Borate/1 mM EDTA buffer (pH 8.0)]. The bands on the gel were electrophoretically transferred to nylon membrane in the presence of 0.5× TBE buffer, and the membrane was air dried. The transferred DNA in the membrane was cross linked by UV (120 × 103 J, 2 min), and the transblot was developed by using Chemiluminescent Electrophoretic mobility shift assay (EMSA) detection kit (Pierce, USA). The specificity of the complexes was analyzed by preincubation of the extracts with 50-fold excess of unlabelled AP-1 or NF-κB-binding oligonucleotides.
Western blotting
The INT-407 cells were seeded into six-well tissue culture plates and were grown up to 70–80% confluency. The cells were triggered with EAEC for different time intervals (1, 2, and 3 h). The cells were washed with PBS to remove the excess of unattached bacteria. Cells without bacteria served as control in all the assays. Subsequently, the cells were suspended in lysis buffer [10 mM HEPES (pH 7.5) containing 150 mM NaCl, 10% glycerol, 0.6% Triton X-100, 10 mM NaVO4, and a complete cocktail protease inhibitor (Roche, Germany)] followed by incubation for 30 min at 4°C and sonication. The debris was removed by centrifuging the homogenate at 10,000 rpm for 10 min. An aliquot of each lysate was removed for protein estimation by BCA assay.
Equal amount (30 μg) of each lysate was subjected to sodium dodecyl sulphate polyacrylamide gel (10%) electrophoresis [16]. The proteins were transferred to a 0.45-μm membrane (Hybond ECL, Amersham, UK) at 80 V for 2 h at 4°C [17]. The strips were placed in blocking buffer [5% skim milk in PBS (SM-PBS) containing 0.1% Tween-20] at 4°C for overnight and washed with PBST (PBS containing 0.1% Tween-20). Membranes were probed with antibodies to phospho-ERK1/2 (1:5,000), phospho-JNK (1:3,000), and phospho-p38MAPK (1:5,000) for 2 h at 37°C. After extensive washing, the blots were incubated with the respective HRP-conjugated secondary antibody (1:3,500) for 1 h at 37°C followed by detection with ECL Plus Western Blotting Detection System (Amersham, UK). In order to distinguish total cellular ERK1/2, JNK, and p38MAPK from their activated (phosphorylated) forms, respective blots were stripped and re-probed with antibodies to total ERK1/2 (1:6,000), JNK (1:4,000), and p38MAPK (1:5,000), respectively.
Statistical analysis
Data were presented as mean ± SD. Differences were analyzed by one-way analysis of variance (ANOVA) with subsequent Dunnett t-test. ** P < 0.01 was taken as significant.
Results
Characterization of bacterial strains
The agarose gel electrophoretic profile of EAEC-T8 [wild type and plasmid cured] and EAEC-O42 (prototype strain) revealed the presence of mega plasmid in both T8 and O42 strains, but not in the −pT8 strain. The T8 (wild type and plasmid cured) and 042 strains were also assessed for EAEC-specific PCR. The amplified PCR product of 630 bp confirmed the T8 strain to be enteroaggregative in nature, and the size of the amplified band was comparable to that of the prototype EAEC-042 [14]. The EAEC-specific gene from which the primers have been designed, are known to be present on the 60–65 MDa plasmid of EAEC [18]. Hence, in the case of the −pT8 strain, no band of 630 bp was detected in EAEC-specific PCR. Moreover, characteristic stacked-brick pattern of adherence of EAEC-T8 strain was observed with HEp-2 cells as well as with INT-407 cells (Fig. 1). However, no adherence of the −pT8 strain was observed with both the cell lines.
Aggregative adherence pattern of EAEC-T8 strain (wild type and plasmid cured) with a HEP-2 cells and b INT-407 cells after 3 h of infection. The cells were stained with Giemsa stain. Wild type T8 strain showed the characteristic stacked brick type of adherence pattern, whereas it was absent in case of −pT8 (plasmid-cured strain)
EAEC infection increases IL-8 release by INT-407 cells
It has been reported that EAEC-042 could induce IL-8 secretion from intestinal epithelial cells [4]. This observation prompted us to assess the extent of IL-8 secretion from EAEC-T8 [wild type and plasmid cured]-infected INT-407 cells. The cells infected with EAEC-042 were taken as positive control, and the uninfected cells were used as negative control. As shown in Fig. 2a, the wild-type T8 strain and the prototype 042 strain induced significant (P < 0.01) extent of IL-8 secretion (344.5 ± 9.22 pg/ml and 277.5 ± 8.6 pg/ml, respectively vs. 69.5 ± 0.9 pg/ml from uninfected cells) after 3 h. The −pT8 strain could also induce IL-8 (191.88 ± 4.3 pg/ml) release but to a much lesser extent than its wild-type counterpart.
a EAEC infection-induced IL-8 release by INT-407 cells. IL-8 content was estimated in the supernatant of INT-407 cells by ELISA after 3 h of infection with EAEC-T8 [wild-type and plasmid cured (−pT8)] and EAEC-042 separately. Errors bars indicate standard deviations. The asterisk indicates that the value is significantly different from the value of uninfected control cells (P < 0.01), as determined by one-way ANOVA with subsequent Dunnett t-test. b Agarose gel electrophoretic profile of IL-8 mRNA in EAEC-infected INT-407 cells. Total cellular RNA (2 μg) was reverse transcribed using cDNA synthesis kit. Reverse transcription products were amplified by PCR using Taq polymerase and primers for IL-8 and β-actin separately. PCR products were analyzed by electrophoresis in agarose gel. Bands of different intensity were observed in INT-407 cells infected with different strains of EAEC. c Level of expression of IL-8 transcripts after densitometric analysis followed by normalization with the values of β-actin
Detection of IL-8 mRNA in EAEC-infected INT-407 cells
Reverse transcriptase PCR analysis of RNA isolated from INT-407 cells showed 18.89, 3.49, and 9.2-fold increase in IL-8 mRNA expression after 3 h of infection with the wild-type T8 strain, −pT8 strain, and prototype EAEC-042 strain, respectively, as compared to that in the uninfected cells (Fig. 2b, c).
EAEC-induced activation of AP-1 and NF-κB in INT-407 cells
Since NF-κB and AP-1 are prime regulatory elements for IL-8 gene expression [8], EMSA was performed to investigate the ability of EAEC-T8 (wild type and plasmid cured) as well as EAEC-O42 to activate AP-1 and NF-κB in INT-407 cells. As shown in Fig. 3, the control lanes (0 h) corresponding to uninfected INT-407 cells revealed no AP-1 and NF-κB DNA-binding activity. However, both the transcription factors were found to be induced after 3 h in INT-407 cells infected with T8 and 042 strains of EAEC. The −pT8 strain also activated both the transcription factors but to a much lesser extent than that of wild-type EAEC-T8. In order to confirm the specificity of this signal, competition studies were performed in the presence of 50-fold excess of unlabeled AP-1 as well as NF-κB-binding oligonucleotides. Both AP-1 and NF-κB bindings to the respective biotinylated oligonucleotide probes were found to be inhibited.
EAEC infection-induced NF-κB and AP-1 DNA-binding activity in INT-407 cells. NF-κB and AP-1 DNA-binding activities were examined by EMSA using biotinylated probe corresponding to NF-κB and AP-1 sites, respectively. The specificity of each complex was analyzed by incubation with a 50-fold excess of unlabeled NF-κB probe and AP-1 probe, respectively. The control lanes (0 h) corresponding to uninfected INT-407 cells revealed no NF-κB and AP-1 DNA-binding activity
EAEC-induced activation of MAPK (ERK-1/2, p38, and JNK) in INT-407 cells
Regulation of NF-κB and AP-1 activities depends on the activation of the MAPK [9, 10]. Thus, in order to gain insight into the signaling mechanisms induced by EAEC, which led to AP-1 and NF-κB activation, we assessed the kinetics of activation of various MAPK modules in EAEC-infected INT-407 cells by Western blotting using antibodies that specifically recognize the phosphorylated forms of ERK-1/2, JNK, and p38MAPK. As depicted in Fig. 4, in the case of EAEC-T8 as well as EAEC-042-infected INT-407 cells, the activated ERK-1/2 (phosphorylated at Tyr 204 residue), activated p38MAPK (phosphorylated at Tyr182), and activated JNK (phosphorylated at Thr183 and Tyr185) were found to be expressed after 1 and 2 h of infection, respectively, and the extent of activation was found to be increased after 3 h. In the case of the −pT8 strain, the phosphorylated form of both ERK-1/2 and JNK was barely detectable after 1 h of infection of INT-407 cells. The activated p38MAPK was not detectable even after 2 h of infection. However, the activated form of all the three kinases was expressed appreciably after 3 h of infection of these cells. Moreover, the levels of expression of all the three MAPK in the −pT8 strain-infected cells were much less than that of the cells infected with EAEC-T8 under the same conditions. Our results also revealed that activation of the MAPK occurred at the same time as AP-1 and NF-κB activation in the EAEC-infected cells.
Activation of MAPK in EAEC-infected INT-407 cells. INT-407 cells were lysed at different time periods after infection with EAEC. Samples were resolved by SDS-PAGE and analyzed by immunoblotting using antibodies to phospho-ERK-1/2, ERK-1/2, phosphor-p38, phospho-JNK, and JNK. The control lanes (0 h) reveal the MAPK activity of the uninfected INT-407 cells
Prevention of EAEC-induced IL-8 secretion from INT-407 by inhibition of MAPK pathways
In order to determine the direct implication of MAPK pathways in EAEC-T8 (wild type and plasmid cured)-induced IL-8 secretion, we used a pharmacologic approach to block these pathways. For this purpose, INT-407 cells were pretreated with specific inhibitors of MEK, JNK, and p38MAPK separately before infection with EAEC. In order to rule out the effect of the vehicle (DMSO) of the inhibitors on IL-8 secretion, EAEC-induced IL-8 secretions were also assessed in presence of DMSO. As depicted in Figs. 2a and 5, the presence of DMSO could not significantly modify the extent of IL-8 secretion by EAEC-infected INT-407 cells. However, in presence of each inhibitor separately or in combination, IL-8 secretion was found to be reduced significantly (P < 0.01) by the EAEC-infected cells (Fig. 5). Our observations revealed that the activation of all the three MAPK was required for the production of IL-8.
EAEC infection-induced IL-8 secretion is related to MAPK signaling. INT-407 cells were pre-incubated with PD098059 (inhibitor of MEK)/SB203580 (inhibitor of p38MAPK)/SP 600125 (inhibitor of JNK) separately or in combination for 90 min prior to infection with EAEC for 3 h, and maintained throughout the infection period. IL-8 content was estimated by ELISA in the culture supernatant of INT-407 cells. In order to rule out the effect of the vehicle (DMSO) of the inhibitors on IL-8 secretion, EAEC-induced IL-8 secretions were also assessed in presence of DMSO. Error bars indicate standard deviation. The asterisk indicates that a value is significantly different from the value for EAEC-infected cells (P < 0.01), as determined by one way ANOVA followed by Students t test
Discussion
In this study, we have provided evidences that EAEC infection of cultured human intestinal epithelial cells induced activation of MAPK leading to nuclear responses and IL-8 production. Study on the EAEC-induced infection was mostly carried out on epithelial cells of human colonic origin [6, 19]. However, EAEC has been found to colonize human small intestinal as well as colonic epithelium [20]. Thus, in the present investigation, human embryonic small intestinal epithelial cell line (INT-407) was used to study the mechanism of EAEC-induced IL-8 release. In this study. we have used wild-type and plasmid-cured T8 strain of EAEC and the prototype EAEC-042 strain. The wild-type EAEC-T8 was cured of its plasmid using acriflavine [13]. Acriflavine intercalates between adjacent base-pairs in the double helix [21]. Such interaction is believed to account for the observed decrease in superhelix density of covalently closed circular DNA [22] and for the production of the frameshift mutations [23]. However, in this study, sub-lethal concentrations of acriflavine were used which is enough for curing of the plasmid but is not known to cause any chromosomal mutations [13]. The curing of plasmid was confirmed by the absence of the plasmid as well as amplified 630 bp EAEC-specific PCR product in agarose gel electrophoresis (data not shown).
Studies have demonstrated the association of EAEC infection and inflammatory responses in intestinal epithelial cells. Steiner et al. [5] have shown the release of IL-8 by Caco-2 cells infected with EAEC-042. Further, it was demonstrated that IL-8 releasing activity of this strain was due to flagellin, a chromosome-encoded protein [12]. Many of the EAEC virulence factors have been found to be localized within the 60–65 MDa plasmid, which include the aggregative adherence fimbrial adhesins, several other adhesins, enterotoxins, the transcriptional activator AggR, and dispersin [1]. A study by Jiang et al. [24] implicated an association between the possession of plasmid borne virulence factors of EAEC strains isolated from patients with diarrhea and the resultant fecal cytokine profile. Harrington et al. [6] suggested the involvement of Aaf B protein (the component of AAF fimbriae encoded by the genes present on AA plasmid) in the non-flagellar IL-8 response of polarized T84 cells infected with EAEC-042. It was also shown that EAEC strains harbouring aggR, aggA, and aap genes, were more likely to cause IL-8 secretion from non-polarized human colonic epithelial cell line, HCT-8 [25]. Recently, we have reported the galactose-specific adhesin (encoded by the plasmid-borne gene) of EAEC-T7 as another contributor of IL-8 secretion from human intestinal epithelial cells infected with the organisms [7]. All these evidences clearly indicated the involvement of both plasmid and chromosome-encoded factors of EAEC in the release of IL-8 from intestinal epithelial cells. This is well correlated with our observation in this study, which revealed a significant increase in IL-8 secretion by INT-407 cells after 3 h of infection with EAEC-T8 and an appreciable reduction in IL-8 release in case of plasmid-cured T8 strain. This finding was further substantiated by the level of expression of IL-8 mRNA in INT-407 cells infected with the wild-type and plasmid-cured strain of EAEC-T8. The variation in the extent of IL-8 release induced by EAEC-T8 and prototype EAEC-042 (used as positive control for IL-8 secretion) might be due to the difference in the virulence potential of these strains.
The DNA-binding sites of AP-1 and NF-κB are known to be present in the promoter region of IL-8 gene, and, thus, both these transcription factors participate in its expression. Studies have reported that infection of intestinal epithelial cells by different enteric organisms could lead to the activation of NF-κB and AP-1, which were responsible for the secretion of IL-8 from these cells [9, 10]. Our findings regarding EAEC-induced activation of NF-κB and AP-1 in INT-407 cells as well as IL-8 secretion from these cells, are in good agreement with the previous articles. It is likely that the inflammatory response induced by EAEC infection might be due to the induction of signal transduction pathways leading to the activation of both these transcription factors with subsequent stimulation of IL-8 gene expression. Further our observation regarding the decrease in the level of activation of NF-κB and AP-1 in the cells infected with the plasmid-cured T-8 strain are well correlated with the reduced level of IL-8 secretion induced by this strain. Our findings have clearly implicated the role of plasmid in the enhanced IL-8 secretion by the INT-407 cells infected with wild-type T-8 strain of EAEC.
The involvement of MAPK pathways in the activation of AP-1 and NF-κB has been well documented [9, 26, 27]. Previous studies have suggested that the release of IL-8 following infection of cultured epithelial cells with different enteric organisms [Salmonella enterica serovar Salmonella typhimurium, Helicobacter pylori, Enterohemorrhagic Escherichia coli (EHEC), and Enteropathogenic Escherichia coli (EPEC)] was dependent on the activation of MAPK pathways [9, 28–30]. In our study, an increased expression of activated ERK1/2, p38MAPK, and JNK was found in the EAEC-stimulated INT-407 cells. Further, the reduced level of the EAEC-induced IL-8 secretion in the presence of PD098059 (the specific inhibitor of MEK), SB203580 (the specific inhibitor of p38MAPK), and SP600125 (the specific inhibitor of JNK) separately and in combination, clearly indicated the involvement of all the three MAPK in the regulation of IL-8 release by these cells. It has been demonstrated that TLR-5-mediated activation of p38MAPK was required for IL-8 production in intestinal epithelial cells triggered by flagellin, the chromosomal gene-encoded protein of EAEC [12]. In our study, a reduction in MAPK activation in INT-407 cells infected by the plasmid-cured T8 strain as compared to the wild-type strain clearly indicated the involvement of the plasmid-borne factors of EAEC in the induction of IL-8 secretion involving the MAPK-mediated signal transduction pathways.
In this study, we have established the importance of the plasmid of EAEC-T8 in the activation of MAPK-mediated signal transduction pathways in INT-407 cells leading to IL-8 secretion. Based on our results, we propose that activation of both AP-1 and NF-κB essential in the synthesis of IL-8 must occur in concert with the activation of all the three MAPK in human intestinal epithelial cells. Thus, our contribution regarding the EAEC-mediated signaling undoubtedly leads to an improved understanding of the pathogenesis caused by this important enteric pathogen.
References
Huang DB, DuPont HL (2004) Enteroaggregative Escherichia coli: an emerging pathogen in children. Semin Pediatr Infect Dis 15:266–271
Pawlowski SW, Warren CA, Guerrant R (2009) Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 136:1874–1886
Okeke IN, Nataro JP (2001) Enteroaggregative Escherichia coli. Lancet Infect Dis 1:304–313
Steiner TS, Lima AA, Nataro JP, Guerrant RL (1998) Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis 177:88–96
Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL (2000) Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest 105:1769–1777
Harrington SM, Strauman MC, Abe CM, Nataro JP (2005) Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol 7:15651–15678
Goyal A, Bhattacharyya S, Majumdar S, Narang A, Ghosh S (2009) Cellular response induced by a galactose-specific adhesin of enteroaggregative Escherichia coli in INT-407 cells. FEMS Immunol Med Microbiol 55:378–387
Mukaida N, Okamoto S, Ishikawa Y, Matsushima K (1994) Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol 56:554–558
Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D (2002) Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect Immun 70:2304–2310
Hobbie S, Chen LM, Davis RJ, Galan JE (1997) Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol 9:5550–5559
Savkovic SD, Ramaswamy A, Koutsouris A, Hecht G (2001) EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am J Physiol Gastrointest Liver Physiol 281:6890–6898
Khan MA, Kang J, Steiner TS (2004) Enteroaggregative Escherichia coli flagellin-induced interleukin-8 secretion requires Toll-like receptor 5-dependent p38 MAP kinase activation. Immunology 112:651–660
Mesas JM, Carmen RM, Alegre MT (2004) Plasmid curing of Oenococcus oeni. Plasmid 51:37–40
Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H (1995) Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol 33:701–705
Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C (1991) Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 337:262–264
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Cerna JF, Nataro JP, Estrada-Garcia T (2003) Multiplex PCR for detection of three plasmid-borne genes of enteroaggregative Escherichia coli strains. J Clin Microbiol 41:2138–2140
Nataro JP, Hicks S, Phillips AD, Vial PA, Sears CL (1996) T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun 64:4761–4768
Hicks S, Candy DC, Phillips AD (1996) Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun 64:4751–4760
Lerman LS (1963) The structure of the DNA-acridine complex. Proc Natl Acad Sci USA 49:94–102
Waring MJ (1968) Drugs which affects the structure and function of DNA. Nature 219:1320–1325
Crick FHC, Barnett L, Brenner S, Watts-Tobin R (1961) General nature of the genetic code for proteins. Nature 192:1227–1232
Jiang ZD, Greenberg D, Nataro JP, Steffen R, DuPont HL (2002) Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J Clin Microbiol 40:4185–4190
Huang DB, Mohanty A, DuPont HL, Okhuysen PC, Chiang T (2006) A review of an emerging enteric pathogen: enteroaggregative Escherichia coli. J Med Microbiol 55:1303–1311
Meyer CF, Wang X, Chang C, Templeton D, Tan TH (1996) Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating kappaB enhancer activation. J Biol Chem 271:8971–8976
Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486
Hobert ME, Sands KA, Mrsny RJ, Madara JL (2002) Cdc42 and Rac1 regulate late events in Salmonella typhimurium induced interleukin-8 secretion from polarized epithelial cells. J Biol Chem 277:51025–51032
Kim SY, Lee YC, Kim HK, Blaser MJ (2006) Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol 8:97–106
Betis F, Brest P, Hofman V, Guignot J, Bernet-Camard MF, Rossi B, Servin A, Hofman P (2003) The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect Immun 71:1068–1074
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, K., Konar, M., Goyal, A. et al. Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in INT-407 cells. Mol Cell Biochem 337, 17–24 (2010). https://doi.org/10.1007/s11010-009-0282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0282-3