Abstract
Perilipin A is the most abundant protein associated with the lipid droplets of adipocytes and functions to control both basal and stimulated lipolysis. Under basal or fed conditions, perilipin A shields stored triacylglycerols from cytosolic lipases, thus promoting triacylglycerol storage. When catecholamines bind to cell surface receptors to initiate signals that activate cAMP-dependent protein kinase (PKA), phosphorylated perilipin A facilitates maximal lipolysis. Mutagenesis studies have revealed that central sequences of moderately hydrophobic amino acids are required to target nascent perilipin A to lipid droplets and provide an anchor into the hydrophobic environment of lipid droplets. Sequences of amino acids in the unique carboxyl terminus of perilipin A and those in amino terminal sequences flanking the first hydrophobic stretch are required for the barrier function of perilipin A in promoting triacylglycerol storage. Site-directed mutagenesis studies of serine residues within six PKA consensus sites of perilipin A reveal functions for phosphorylation of at least three of the sites. Phosphorylation of one or more of the serines within three amino terminal PKA sites is required to facilitate hormone-sensitive lipase access to lipid substrates. Phosphorylation of serines within two carboxyl terminal sites is also required for maximal lipolysis. Phosphorylation of serine 492 (site 5) triggers a massive remodeling of lipid droplets, whereby large peri-nuclear lipid droplets fragment into myriad lipid micro-droplets that scatter throughout the cytoplasm. We hypothesize that perilipin A binds accessory proteins to provide assistance in carrying out these functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipocytes in white adipose tissue of mammals serve as a primary energy storage depot. Excess calories are metabolized to synthesize triacylglycerols, which are packaged into large cytosolic lipid droplets. These lipid droplets are coated with perilipins, the most abundant lipid droplet-associated proteins in adipocytes [1]. Perilipins localize exclusively to lipid droplets and are found in no other subcellular compartment [2]. The primary isoform of perilipin in adipocytes is perilipin A, which is the longest isoform with a unique carboxyl terminus of more than 100 amino acids that is absent in perilipins B and C [3, 4].
Studies conducted in cell culture models and perilipin null mice reveal that perilipins play a dual role in the regulation of lipolysis in adipocytes. Under fed conditions, when circulating insulin promotes triacylglycerol storage in adipocytes, perilipin A forms a barrier at the surfaces of lipid droplets to restrict the access of cytosolic lipases to the lipid droplet [5–7]; thus, triacylglycerol storage prevails over a very low rate of basal lipolysis. In contrast, during fasting or extended exercise, phosphorylated perilipin facilitates hormonally stimulated lipolysis through multiple mechanisms [8–13]. Consistent with these observations, perilipin null mice are lean and have ~30% of the fat mass of wild-type mice [6, 7]; adipocyte triacylglycerol stores turn over more rapidly under basal (or fed) conditions in the absence of the barrier function provided by perilipins. Interestingly, studies with perilipin null mice also revealed that perilipin plays an essential role in attaining maximally stimulated lipolysis; the release of glycerol and fatty acids is extremely attenuated from hormonally stimulated adipocytes isolated from perilipin null mice [6, 7].
Perilipin is a member of a gene family that includes five members in vertebrates and two members in insects; all of the proteins encoded by these genes associate with lipid droplets. The majority of cells in vertebrates have the capacity to store neutral lipids, triacylglycerols, or cholesterol esters, in cytoplasmic lipid droplets. In most cells, the ubiquitously expressed proteins adipophilin (also called ADFP, adipose differentiation-related protein or ADRP) and TIP47 coat the surfaces of lipid droplets [14–17]. Adipophilin and TIP47 have the greatest sequence similarity to each other (Fig. 1) and are likely the oldest members of the family [16]. The most recently described member of the family, OXPAT (also called MLDP and LSDP5), shows significant sequence similarity to adipophilin and TIP47; however, OXPAT expression is restricted to highly oxidative tissues that rely on β-oxidation of fatty acids for energy production, including the heart, muscle, brown adipose tissue, and, to a lesser extent, liver [18–20].
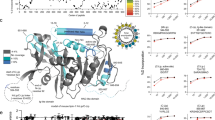
Structural features of the perilipin family of proteins. Conserved sequences of amino acids are depicted in color (in the online version of the figure); higher intensity of color represents greater conservation. Sequences of mouse proteins are depicted. An amino terminal sequence of 100 amino acids (green) is the most highly conserved peptide amongst members of the protein family, excluding S3-12. Proximal to this conserved peptide, the sequences of 11-mer repeats (maize) are predicted to form amphipathic helices; this sequence is expanded in S3-12 to include 29 tandem repeats of a 33-amino acid sequence. The carboxyl terminus of TIP47 folds into a 4-helix bundle of amphipathic helices shown in blue; adipophilin and OXPAT/MLDP have conserved sequences in their carboxyl termini that are not shared by perilipin A. Within the central region, three hydrophobic sequences (lilac) assist targeting and anchoring of nascent perilipin A into lipid droplets; a highly acidic sequence (cyan) of unknown function is found between the hydrophobic stretches of amino acids. Perilipin A has 6 consensus sites for phosphorylation of serine residues by PKA (charcoal); these sites are not conserved in other members of the protein family. (Illustration reproduced from Journal of Lipid Research [63] with permission of American Society for Biochemistry and Molecular Biology via Copyright Clearance Center.)
Perilipin is structurally divergent from adipophilin and TIP47. Perilipin is also the only vertebrate family member that has consensus sequences for phosphorylation of the protein by cAMP-dependent protein kinase (also called protein kinase A, PKA) [3, 4]. Significant expression of perilipin is limited to adipocytes of white and brown adipose tissue, and steroidogenic cells of the adrenal cortex, testes, and ovaries [1, 3, 6, 7, 21]. The least conserved member of the gene family is S3-12; the only conserved sequence in S3-12 is a long stretch of tandem repeats of 11-amino acid (11-mer) sequences [22] that are proposed to form amphipathic α-helices [23]. All of the other members of the family contain shorter sequences of 11-mer repeats. These sequences may assist in embedding the proteins into the surface phospholipid monolayer of lipid droplets. Significant expression of S3-12 is limited to white adipose tissue [24]. Insects express two related proteins, LSD1 and LSD2, in fat body [25–28], a tissue which resembles both adipose tissue and liver. Phosphorylation controls the functions of LSD1 and LSD2 in facilitating lipolysis and lipid droplet motility [27, 29]; hence, both LSD1 and LSD2 share functional similarities with perilipin A.
Structure/function studies of perilipin A
We have used mutagenesis strategies to study how the structure of perilipin A impacts its own function. We have shown that the central portion of the primary amino acid sequence comprises sequences required to target nascent perilipin A to lipid droplets [30, 31]. Within that region, no single short stretch of amino acids serves as a molecular zip code to send perilipin A to a lipid droplet address. Instead, three sequences of ~20 moderately hydrophobic amino acids cooperate in targeting and anchoring of perilipin A onto lipid droplets; deletion of any one of the three sequences fails to eliminate targeting of the mutated perilipin [31]. Removal of the entire central sequence of perilipin A is required to disrupt targeting to lipid droplets; conversely, when this central sequence is ligated to green fluorescent protein, the usually soluble green fluorescent protein relocates to the surfaces of lipid droplets [30]. Removal of the hydrophobic sequences also reduces the tight anchoring of perilipin A to lipid droplets.
The crystal structure of perilipin A has never been solved, and consequently, we understand little of how perilipin A assembles onto lipid droplets. The conformation of the hydrophobic stretches is unknown. Hence, it is unclear whether these sequences embed deeply or shallowly into the lipid droplet. The only structural information that is currently available for members of the gene family is the crystal structure of the carboxyl terminal region of TIP47 [32]. The carboxyl terminus of TIP47 folds into a 4-helix bundle of amphipathic helices that closely resembles the 4-helix bundle of the amino terminus of apolipoprotein E, an exchangeable apolipoprotein. Apolipoprotein E circulates in the blood both as a soluble protein and in association with lipoproteins. Although the 4-helix bundle assumes a closed conformation when apolipoprotein E is soluble, it opens up to embed the hydrophobic faces of the 4 helices into the acyl chains of phospholipid monolayers covering lipoproteins [33, 34]. Similarly, open and closed conformations of the corresponding 4-helix bundle may contribute to the exchangeable nature of TIP47. TIP47 not only remains stable as a soluble protein in the cytoplasm but also readily associates with lipid droplets, particularly following the incubation of cells with fatty acids to drive triacylglycerol synthesis and packaging [17, 35]. The amino acid sequence of adipophilin is similar to that of TIP47 in the region of the 4-helix bundle [32]; however, adipophilin is unstable in the cytoplasm, rapidly degraded in the absence of lipid droplets and stabilized upon binding lipids [36–38]. Thus, differences between the amino acid sequences of TIP47 and adipophilin are likely to contribute to the inability of adipophilin to collapse the bundle and dissociate from lipid droplets into the cytoplasm. Finally, the amino acid sequence of the carboxyl terminus of perilipin A diverges from that of TIP47; a highly acidic sequence in perilipin A is surrounded by hydrophobic sequences (Fig. 1). Like adipophilin, perilipin A is unstable as a soluble protein [39, 40], and is stabilized by its association with lipid droplets [39].
Using deletion mutagenesis strategies, we have identified portions of perilipin A that contribute to the barrier function in reducing lipase access to stored triacylglycerols. Expression of perilipin A in cultured fibroblasts increases triacylglycerol storage by reducing the rate of triacylglycerol hydrolysis [5]; ectopic perilipin A replaces endogenous adipophilin on lipid droplet surfaces to produce a more effective barrier to lipolysis [10, 12]. Thus, adipophilin is relatively permissive to lipolysis. Moreover, deletion of carboxyl terminal sequences unique to perilipin A eliminates the barrier function of perilipin and renders stored triacylglycerols susceptible to lipolysis [41]. This observation suggests that the perilipin B and C isoforms, which lack this peptide, are unlikely to facilitate triacylglycerol storage. Perilipin B is expressed at very low levels in adipocytes, while perilipin C is expressed only in steroidogenic cells [21]. The amino acid sequence of the unique carboxyl terminus of perilipin A contains polar and charged residues throughout; thus, this peptide is not likely to embed into the hydrophobic environment of the lipid droplet. Deletion of amino terminal sequences adjacent to the central hydrophobic sequences also eliminates the barrier function of perilipin A [41]. These sequences include 11-mer repeats (Fig. 1) that are predicted to form amphipathic helices, and, thus, may position perilipin A at the surfaces of lipid droplets. Through these mutagenesis studies, we have learned that the structure of perilipin is remarkably plastic, since the removal of relatively large portions of the sequence has little effect on the targeting of mutated perilipin to lipid droplets, or the function of perilipin A in reducing basal lipolysis. Surprisingly, the amino terminal sequence of ~100 amino acids that is highly conserved in four out of five mammalian (and both invertebrate) proteins within the gene family is not required for targeting to lipid droplets or facilitating triacylglycerol storage. Therefore, additional experimentation is required to reveal the function of this conserved sequence.
Phosphorylation of perilipin A by PKA
Adipocytes not only store excess calories as triacylglycerols but also mobilize fatty acids following lipolysis of stored triacylglycerols at times of caloric deficit and during extended exercise. One of the signaling pathways that initiate lipolysis begins when catecholamines bind to β-adrenergic receptors on the plasma membranes of adipocytes. Stimulatory G-proteins activate adenylyl cyclase, leading to an increased concentration of cAMP in the cytoplasm. PKA is then activated and phosphorylates multiple proteins. The phosphorylation of hormone-sensitive lipase (HSL) by PKA triggers rapid translocation of the lipase from the cytoplasm to the surfaces of lipid droplets, where it gains access to its lipid substrates [42–44]. HSL is an abundant lipase in adipocytes that displays significant hydrolytic activity against diacylglycerols, cholesterol esters, and triacylglycerols [45–48].
Perilipin A is the most highly phosphorylated protein in stimulated adipocytes [1]; the phosphorylation of perilipin A by PKA is necessary for maximal lipolysis [6–13]. The sequence of mouse perilipin A contains six consensus sites for the phosphorylation of serine residues by PKA (Fig. 1), although no one has yet demonstrated that all six sites are actually phosphorylated. Evidence from mutagenesis studies suggests that at least three of these serine residues are phosphorylated by PKA, including one serine in the amino terminus and two serines in the carboxyl terminus.
Mutation of the first three PKA site serines to alanines in combination reduces lipolysis in cells expressing the mutated perilipin [8, 10–13], particularly when HSL is present. Sztalryd et al. [11] have shown that perilipin is required for HSL docking on lipid droplets, and phosphorylation of one or more of the three amino terminal PKA sites of perilipin A facilitates HSL docking and maximal lipolysis. Workers in the Greenberg laboratory have also demonstrated reduction of HSL-catalyzed lipolysis when all three amino terminal PKA site serines of perilipin A were mutated [10, 13]; however, HSL translocated from the cytoplasm and docked on lipid droplets in stimulated adipocytes expressing a mutated form of perilipin lacking all six phosphorylation sites [8]. Furthermore, fluorescence resonance energy transfer experiments have suggested that HSL docks on lipid droplets via a protein–protein interaction with perilipin that does not require phosphorylation of any of these six sites [49]. Thus, there are currently two hypotheses for the mechanism by which phosphorylation of one or more of three amino terminal PKA sites of perilipin A facilitates HSL-catalyzed lipolysis: (1) phosphorylation is required to promote a binding interaction between HSL and perilipin A that brings HSL to its lipid substrates, or (2) HSL binds to perilipin A in a phosphorylation-independent mechanism, and phosphorylated perilipin A then assists HSL in gaining access to lipid substrates. Additional experimentation is required to solve this puzzle.
Phosphorylation of serine residues in the carboxyl terminal PKA sites of perilipin A also contributes to mechanisms regulating lipolysis. Mutation of all three of the carboxyl terminal PKA site serines to alanines reduces maximal lipolysis in both cultured fibroblasts that express mutated perilipin A, but lack HSL [13], and adipocytes differentiated from perilipin null mouse embryonic fibroblasts that express mutated perilipin A and endogenous lipases, including HSL [9]. Experiments with perilipin A lacking individual PKA sites show reduction of maximal lipolysis when either site 5 or 6 is mutated, providing evidence that these sites are physiological substrates for PKA [9]. These studies implicate perilipin A in the control of lipolysis catalyzed by lipases other than HSL. The cytosolic lipases of cultured fibroblasts have not been well characterized, but likely include members of the recently described patatin-like phospholipase A domain containing (PNPLA) family of proteins; this family includes adipose triglyceride lipase (ATGL, also called desnutrin), adiponutrin, GS2, and GS2-like [50–53]. ATGL, the best characterized member of the family, is highly expressed in adipocytes, hydrolyzes triacylglycerol, and plays an important role in both basal and stimulated lipolysis [50–59]. The phosphorylation of perilipin A facilitates lipolysis catalyzed by a variety of cytosolic lipases by several mechanisms that are not yet fully understood.
Chronic stimulation of lipolysis by addition of β-adrenergic agonists to cultured adipocytes induces release of glycerol and fatty acids, but also triggers massive remodeling of lipid droplets. Large centrally located lipid droplets fragment into myriad tiny lipid micro-droplets that scatter throughout the cytoplasm [60–62]. Our laboratory has been interested in gaining understanding of this remodeling process. To simplify the cell model, we expressed perilipin A in cultured fibroblasts and observed that perilipin A coated lipid droplets aggregate into clusters of uniformly sized lipid droplets in one or two areas of the cytoplasm [5]. When forskolin and isobutylmethylxanthine (IBMX) are added to the cells to increase cAMP and activate PKA, perilipin A coated lipid droplets disperse throughout the cytoplasm [62]. In contrast, adipophilin coated lipid droplets of control cells are dispersed throughout the cytoplasm in both the presence and absence of forskolin and IBMX. Thus, perilipin A organizes lipid droplets into aggregates that break apart when PKA is activated; other adipocyte factors are not required. Lipolysis is not required for the observed dispersion of lipid droplets, since addition of the lipase inhibitor diethylumbelliferyl phosphate fails to inhibit remodeling. Finally, by studying cells expressing perilipin A with PKA site mutations, we discovered that phosphorylation of serine 492 (site 5) is required for lipid droplet remodeling. Interestingly, substitution of a glutamic acid residue (for serine) to add negative charge to the site fails to trigger lipid droplet scattering under basal conditions and prevents remodeling, when forskolin and IBMX are added to the cells. Thus, the addition of a phosphate moiety to serine 492 is uniquely required for the remodeling of perilipin A coated lipid droplets.
We hypothesize that lipid droplet remodeling facilitates lipolysis by massively increasing the surface area of lipid droplets available for lipase binding. The observed reduction in stimulated lipolysis in adipocytes expressing perilipin A with a serine 492 to alanine mutation [9] supports the importance of this site in control of lipolysis. Additionally, the dispersion of lipid droplets implies increased motility of lipid micro-droplets as they move away from the large peri-nuclear lipid droplets to locations throughout the cytoplasm.
The role of perilipin A in the control of lipolysis
Data from our laboratory and others have contributed to a new model for how lipolysis in adipocytes is controlled by perilipin A at the surfaces of lipid droplets in response to nutritional status. This model replaces the long-standing view that PKA-mediated phosphorylation of HSL controls adipocyte lipolysis through activation of lipase activity; the current model is considerably more complex. When animals are in the fed state, perilipin A is minimally phosphorylated and forms a barrier at the surfaces of lipid droplets that restricts the access of cytosolic lipases to stored triacylglycerols. Lipolysis occurs at a very low rate and is likely catalyzed by ATGL, a lipase that constitutively associates with lipid droplets. When catecholamines activate the β-adrenergic signaling cascade, PKA-phosphorylated HSL translocates from the cytoplasm to the surfaces of lipid droplets where it docks by binding to PKA-phosphorylated perilipin A. This potent lipase gains access to triacylglycerol and diacylglycerol substrates to catalyze extensive lipolysis, working in collaboration with ATGL. PKA-mediated phosphorylation of one or more of three serines in the amino terminus of perilipin A contributes to the mechanism by which HSL gains access to lipid substrates. The phosphorylation of additional serine residues of perilipin A by PKA is required for maximal lipolysis. Phosphorylation of serine 492 (site 5) triggers a massive remodeling of lipid droplets that increases surface area available to lipases; the mechanisms controlling fragmentation and dispersion of lipid droplets are not yet understood. Finally, the mechanisms by which phosphorylation of serine 517 (site 6) promotes lipolysis are also currently unknown.
We hypothesize that perilipin A fulfills its various functions by forming a dynamic scaffold at the surfaces of adipocyte lipid droplets [60, 63]. This scaffold serves as an organizing center to recruit enzymes required for metabolism of lipids stored in lipid droplets, and proteins involved in the maintenance, motility, and remodeling of lipid droplets. Under fed conditions, the perilipin scaffold may bind proteins that support triacylglycerol packaging and stabilization of lipid droplets, while permitting a low level of lipolysis. When hormones stimulate lipolysis, PKA-phosphorylated perilipin A then disperses these proteins to recruit HSL and other proteins that facilitate elevated rates of lipolysis, lipid droplet remodeling, and increased motility of lipid micro-droplets.
HSL is an example of a protein that binds to the perilipin A scaffold under lipolytically stimulated, but not basal conditions; PKA-mediated phosphorylation of both HSL and perilipin A is required to facilitate optimal access of HSL to substrate. In contrast, CGI-58 (also called ABHD5 for α/β hydrolase domain 5) binds to perilipin A under basal conditions, and disperses into the cytoplasm following activation of PKA [49, 64, 65]. CGI-58 plays an important but poorly understood role in triacylglycerol catabolism. Mutations in CGI-58 cause a neutral lipid storage disorder called Chanarin-Dorfman Syndrome, characterized by ichthyosis and excessive storage of triacylglycerols in many cells and tissues [66]. Although CGI-58 is required to maintain triacylglycerol homeostasis in many cells, it lacks lipase activity [67]. Zechner and colleagues have proposed that CGI-58 functions as an activating co-factor for ATGL [67, 68]. Additional experimentation is required to elucidate why CGI-58 localizes to lipid droplets under basal conditions, but disperses into the cytoplasm, away from lipid droplet-bound ATGL, when lipolysis is stimulated. One hypothesis is that CGI-58 may promote triacylglycerol hydrolysis under basal, but not stimulated conditions [63]. Many other questions regarding the mechanisms by which phosphorylation of perilipin A facilitates lipolysis remain to be answered; phosphorylation of each of the six serines within PKA sites may contribute to separate mechanisms. Finally, identification of additional proteins that bind to perilipin A will undoubtedly reveal new aspects of the complex mechanisms by which adipocytes maintain lipid homeostasis.
Abbreviations
- ATGL:
-
Adipose triglyceride lipase
- HSL:
-
Hormone-sensitive lipase
- IBMX:
-
Isobutylmethylxanthine
- PKA:
-
Protein kinase A (cAMP-dependent protein kinase)
References
Greenberg AS, Egan JJ, Wek SA et al (1991) Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266:11341–11346
Blanchette-Mackie EJ, Dwyer NK, Barber T et al (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36:1211–1226
Greenberg AS, Egan JJ, Wek SA et al (1993) Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci USA 90:12035–12039. doi:10.1073/pnas.90.24.12035
Lu E, Gruia-Gray J, Copeland NG et al (2001) The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome 12:741–749
Brasaemle DL, Rubin B, Harten IA et al (2000) Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem 275:38486–38493. doi:10.1074/jbc.M007322200
Martinez-Botas J, Anderson JB, Tessier D et al (2000) Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 26:474–479. doi:10.1038/82630
Tansey JT, Sztalryd C, Gruia-Gray J et al (2001) Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98:6494–6499. doi:10.1073/pnas.101042998
Miyoshi HS, Souza SC, Zang HH et al (2006) Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 281:15837–15844. doi:10.1074/jbc.M601097200
Miyoshi H, Perfield JW, Souza SC et al (2007) Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 282:996–1002. doi:10.1074/jbc.M605770200
Souza SC, Muliro KV, Liscum L et al (2002) Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem 277:8267–8272. doi:10.1074/jbc.M108329200
Sztalryd C, Xu G, Dorward H et al (2003) Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103. doi:10.1083/jcb.200210169
Tansey JT, Huml AM, Vogt R et al (2003) Functional studies on native and mutated forms of perilipins: a role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem 278:8401–8406. doi:10.1074/jbc.M211005200
Zhang HH, Souza SC, Muliro KV et al (2003) Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem 278:51535–51542. doi:10.1074/jbc.M309591200
Brasaemle DL, Barber T, Wolins NE et al (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263
Heid HW, Moll R, Schwetlick I et al (1998) Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 294:309–321. doi:10.1007/s004410051181
Miura S, Gan JW, Brzostowski J et al (2002) Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 277:32253–32257. doi:10.1074/jbc.M204410200
Wolins NE, Rubin B, Brasaemle DL (2001) TIP47 associates with lipid droplets. J Biol Chem 276:5101–5108. doi:10.1074/jbc.M006775200
Dalen KT, Dahl T, Holter E et al (2007) LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta 1771:210–227
Wolins NE, Quaynor BK, Skinner JR et al (2006) OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55:3418–3428. doi:10.2337/db06-0399
Yamaguchi TS, Matsushita S, Motojima K (2006) MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem 281:14232–14240. doi:10.1074/jbc.M601682200
Servetnick DA, Brasaemle DL, Gruia-Gray J et al (1995) Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J Biol Chem 270:16970–16973. doi:10.1074/jbc.270.28.16970
Scherer PE, Bickel PE, Kotler M et al (1998) Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat Biotechnol 16:581–586. doi:10.1038/nbt0698-581
Bussell R Jr, Eliezer D (2003) A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol 329:763–778. doi:10.1016/S0022-2836(03)00520-5
Wolins NE, Skinner JR, Schoenfish MJ et al (2003) Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem 278:37713–37721. doi:10.1074/jbc.M304025200
Gronke S, Beller M, Fellert S et al (2003) Control of fat storage by a Drosophila PAT domain protein. Curr Biol 13:603–606. doi:10.1016/S0960-9822(03)00175-1
Gronke S, Muller G, Hirsch J et al (2007) Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol 5:1248–1256. doi:10.1371/journal.pbio.0050137
Patel RT, Soulages JL, Hariharasundaram B et al (2005) Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem 280:22624–22631. doi:10.1074/jbc.M413128200
Teixeira L, Rabouille C, Rorth P et al (2003) Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech Dev 120:1071–1081. doi:10.1016/S0925-4773(03)00158-8
Welte MA, Cermelli S, Griner J et al (2005) Regulation of lipid-droplet transport by the perilipin homolog LSD2. Curr Biol 15:1266–1275. doi:10.1016/j.cub.2005.06.062
Garcia A, Sekowski A, Subramanian V et al (2003) The central domain is required to target and anchor perilipin A to lipid droplets. J Biol Chem 278:625–635. doi:10.1074/jbc.M206602200
Subramanian V, Garcia A, Sekowski A et al (2004) Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res 45:1983–1991. doi:10.1194/jlr.M400291-JLR200
Hickenbottom SJ, Kimmel AR, Londos C et al (2004) Structure of a lipid droplet protein; the PAT family member TIP47. Structure 12:1199–1207. doi:10.1016/j.str.2004.04.021
Hatters DM, Peters-Libeu CA, Weisgraber KH (2006) Apolipoprotein E structure: insights into function. Trends Biochem Sci 31:445–454. doi:10.1016/j.tibs.2006.06.008
Saito HS, Lund-Katz S, Phillips MC (2004) Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog Lipid Res 43:350–380. doi:10.1016/j.plipres.2004.05.002
Wolins NE, Quaynor BK, Skinner JR et al (2005) S3-12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem 280:19146–19155. doi:10.1074/jbc.M500978200
Gross DN, Miyoshi H, Hosaka T et al (2006) Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol 20:459–466. doi:10.1210/me.2005-0323
Masuda Y, Itabe H, Odaki M et al (2006) ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res 47:87–98. doi:10.1194/jlr.M500170-JLR200
Xu G, Sztalryd C, Lu X et al (2005) Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem 280:42841–42847. doi:10.1074/jbc.M506569200
Brasaemle DL, Barber T, Kimmel AR et al (1997) Post-translational regulation of perilipin expression Stabilization by stored intracellular neutral lipids. J Biol Chem 272:9378–9387. doi:10.1074/jbc.272.14.9378
Xu G, Sztalryd C, Londos C (2006) Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta 1761:83–90
Garcia A, Subramanian V, Sekowski A et al (2004) The amino and carboxyl termini of perilipin A facilitate the storage of triacylglycerols. J Biol Chem 279:8409–8416. doi:10.1074/jbc.M311198200
Brasaemle DL, Levin DM, Ader-Wailes DC et al (2000) The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta 1483:251–262
Egan JJ, Greenberg AS, Chang MK et al (1992) Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA 89:8537–8541. doi:10.1073/pnas.89.18.8537
Su CL, Sztalryd C, Contreras JA et al (2003) Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem 278:43615–43619. doi:10.1074/jbc.M301809200
Holm C, Kirchgessner TG, Svenson KL et al (1998) Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science 241:1503–1506. doi:10.1126/science.3420405
Cook KG, Lee FT, Yeaman SJ (1981) Hormone-sensitive cholesterol ester hydrolase of bovine adrenal cortex: identification of the enzyme protein. FEBS Lett 132:10–14. doi:10.1016/0014-5793(81)80416-4
Fredrikson GP, Stralfors P, Nilsson NO et al (1981) Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem 256:6311–6320
Pittman RC, Khoo JC, Steinberg D (1975) Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 250:4505–4511
Granneman JG, Moore HP, Granneman RL et al (2007) Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282:5726–5735. doi:10.1074/jbc.M610580200
Jenkins CM, Mancuso DJ, Yan W et al (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279:48968–48975. doi:10.1074/jbc.M407841200
Lake AC, Sun Y, Li JL et al (2005) Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res 46:2477–2487. doi:10.1194/jlr.M500290-JLR200
Villena JA, Roy S, Sarkadi-Nagy E et al (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 279:47066–47075. doi:10.1074/jbc.M403855200
Zimmermann R, Strauss JG, Haemmerle G et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386. doi:10.1126/science.1100747
Haemmerle G, Lass A, Zimmermann R et al (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734–737. doi:10.1126/science.1123965
Kershaw EE, Hamm JK, Verhagen LA et al (2006) Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 55:148–157. doi:10.2337/diabetes.55.01.06.db05-0982
Langin D, Dicker A, Tavernier G et al (2005) Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 54:3190–3197. doi:10.2337/diabetes.54.11.3190
Mairal A, Langin D, Arner P et al (2006) Human adipose triglyceride lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia 49:1629–1636. doi:10.1007/s00125-006-0272-x
Ryden M, Jocken J, van Harmelen V et al (2007) Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab 292:E1847–E1855. doi:10.1152/ajpendo.00040.2007
Smirnova E, Goldberg EB, Makarova KS et al (2006) ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 7:106–113. doi:10.1038/sj.embor.7400559
Brasaemle DL, Dolios G, Shapiro L et al (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J Biol Chem 279:46835–46842. doi:10.1074/jbc.M409340200
Londos C, Brasaemle DL, Schultz CJ et al (1999) Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol 10:51–58. doi:10.1006/scdb.1998.0275
Marcinkiewicz A, Gauthier D, Garcia A et al (2006) The phosphorylation of serine 492 of perilipin A directs lipid droplet fragmentation and dispersion. J Biol Chem 281:11901–11909. doi:10.1074/jbc.M600171200
Brasaemle DL (2007) Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48:2547–2559. doi:10.1194/jlr.R700014-JLR200
Subramanian V, Rothenberg A, Gomez C et al (2004) Perilipin A mediates the reversible binding of CGI–58 to lipid droplets in 3T3–L1 adipocytes. J Biol Chem 279:42062–42071. doi:10.1074/jbc.M407462200
Yamaguchi T, Omatsu N, Morimoto E et al (2007) CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res 48:1078–1089. doi:10.1194/jlr.M600493-JLR200
Lefevre C, Jobard F, Caux F et al (2001) Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet 69:1002–1012. doi:10.1086/324121
Lass A, Zimmermann R, Haemmerle G et al (2006) Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3:309–319. doi:10.1016/j.cmet.2006.03.005
Schweiger M, Scheriber R, Haemmerle G et al (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241. doi:10.1074/jbc.M608048200
Acknowledgments
Research has been supported by NIH R01 DK54797, an Established Investigator Award from the American Heart Association, a Research Award from the American Diabetes Association, and Johnson & Johnson Discovery Awards administered through Rutgers University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brasaemle, D.L., Subramanian, V., Garcia, A. et al. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem 326, 15–21 (2009). https://doi.org/10.1007/s11010-008-9998-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9998-8