Abstract
It is widely assumed that acute benefit of statin therapy is mediated especially by non-lipid effects. The immediate influence of statins on lipid levels in patients with acute coronary syndrome (ACS) is, however, not clear. A total of 64 consecutive patients with ACS were randomized at admission to fluvastatin 80 mg (Group 1, N = 32) or standard therapy without statin (Group 2, N = 32). The levels of total cholesterol (TC), low-density-lipoprotein cholesterol (LDL-C), high-density-lipoprotein cholesterol (HDL-C), and triglycerides (TG) were examined at admission and after 24 h. Baseline characteristics were comparable in both groups. In Group 1, fluvastatin significantly decreased the levels of TC by 14.5%, LDL-C by 17.2%, and HDL-C by 10.0% (P < 0.001); TG were not influenced. In Group 2 only marginal reductions in TC (by 4.1%, P = 0.03) and HDL-C (by 7.5%, P < 0.01) were detected; the levels of LDL-C and TG were not changed. As compared with Group 2, in Group 1 the final levels of TC (P = 0.02) and LDL-C (P = 0.01) were significantly lower. Fluvastatin therapy, when started at admission in patients with ACS, significantly reduces TC and LDL-C already after 24 h. We suggest that the lipid-lowering effect of statins in the therapy of ACS is probably as prompt as non-lipid effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) lower serum cholesterol by suppression of its endogenous synthesis. Statins are, therefore, successfully used in patients with high blood lipids and clinically manifested atherosclerosis worldwide. There is a strong evidence demonstrating their efficacy and thus supporting their use in both primary and secondary prevention of coronary artery disease for reduction of mortality and nonfatal cardiovascular events [1–3]. A number of authors concur, however, that this effect can be only partially explained by the lipid-lowering mechanisms. During the past decades substantial effort was exerted in the investigation of the non-lipid-mediated actions of statins, referred to also as “pleiotropic effects”. It has been found that besides the lipid-lowering activity, statins exert also anti-inflammatory, antithrombotic, and antioxidant effects, increase nitric oxide (NO) production or improve endothelial dysfunction [4].
Recently, marked progress has been achieved also in the understanding of pathogenesis of the acute coronary syndrome (ACS). Several pathways participating in the development of unstable coronary plaque may be simultaneously influenced by statins. Extension of our knowledge of the “pleiotropic effects” of statins together with the increasing understanding of pathogenesis of ACS shifted the initiation of the statin therapy closer to the onset of symptoms [4, 5]. However, recent experimental studies [6–8], similarly as the first clinical trials [9–11], have brought promising results, supporting the idea of the cardioprotective effect of statin administration not only in the early-initiated secondary prevention but also in the first-line therapy of ACS. Pleiotropic effects of statins, mainly rapid suppression of the inflammatory reaction, have been suggested by a number of authors as the major mechanism of the beneficial role of statins in the therapy of ACS [5, 12–15]. Pushing the statin research under the condition of ACS toward the non-lipid effects led, however, to marginalization of the lipid-lowering issue in ACS. Despite the fact that cholesterol lowering is a generally accepted mechanism of plaque stabilization and the cholesterol level is still the major target for statin therapy, the acute effect of statins on the lipid levels in ACS has not yet been investigated. The aim of the present study was, therefore, to analyze the acute effect of statin on lipid levels in patients with ACS.
Methods
Subjects
The study protocol was approved by the institutional ethics review committee and written informed consent was obtained from all participating patients. A total of 64 consecutive patients admitted to the Coronary Care Unit for ACS were enrolled to this study. Eligible patients with ST elevation ACS had rest chest pain less than 12 h before admission and ≥1 mm ST-segment elevation in two or more continuous leads or new left bundle branch block on ECG. Eligible patients with non-ST elevation ACS had rest chest pain during the past 24 h and ≥1 mm ST segment depression or negative T waves in two or more continuous leads. Exclusion criteria were: concomitant active liver disease or known persistent elevation of transaminases more than three times above the upper limit, history of lipid lowering therapy less than 30 days before the index event, known allergy for fluvastatin or any present additives in the drug, disability of oral drug administration, pregnancy or nursing, women of fertile age without effective contraception, suspicions of muscle disease, such as myositis, and subjects younger than 18 years.
Following signed informed consent, blood samples were taken for examination of total cholesterol (TC), triglycerides (TG), low-density-lipoprotein cholesterol (LDL-C), and high-density-lipoprotein cholesterol (HDL-C), as well as for routine laboratory investigation including troponin I, and patients were randomized in blocks to 80 mg fluvastatin (Lescol XL, Novartis, Switzerland) (Group 1, n = 32) or standard therapy (Group 2, n = 32). Exactly 24 h after admission, blood samples were taken for estimation of the final levels of TC, TG, LDL-C, and HDL-C. All patients underwent urgent coronary angiography and percutaneous coronary intervention, if necessary. Standard therapy included aspirin, heparin or low-molecular-weight heparin, and clopidogrel in all patients, nitrates, beta-blockers, and angiotensin-converting-enzyme inhibitors according to the clinical conditions. With the exception of fluvastatin in Group 1, no other lipid-lowering drug was administered during the first 24 h.
Laboratory assays
Non-fasting blood lipid levels were analyzed before randomization and after 24 h. TC, LDL-C, HDL-C, and TG levels were directly measured with a calibrated Advia 1650 automatic analyzer system (Bayer, Germany). Troponin I was assayed by an automated monoclonal-antibody solid-phase sandwich-type enzyme immunoassay on the Centaur instrument (Bayer, Germany).
Statistical analysis
The values are expressed as means ± standard error (SE). Statistical comparison of the groups was performed by the t-test, Fisher’s exact test, and Mann Whitney test for baseline characteristics, paired two-tailed t-test for intra-group analysis of lipid level differences, and unpaired two-tailed t-test for inter-group analysis of lipid levels. P < 0.05 was considered to be statistically significant.
Results
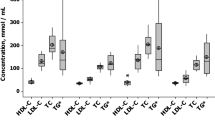
A total of 64 patients were enrolled and all completed the study. No significant side effect of fluvastatin was registered. Baseline characteristics are shown in Table 1: study groups were comparable with respect to the age, gender, history of CAD, diabetes, hypertension, hyperlipoproteinemia, smoking status, type of ACS, maximum troponin I level, and the number of stenosed coronary arteries (Table 1). Also baseline lipid levels were not significantly different between the groups. In Group 1, single-dose, 80 mg fluvastatin therapy significantly decreased the levels of TC (by 14.5%), HDL-C (by 10.0%), and LDL-C (by 17.2%), whereas the levels of TG remained unchanged (Table 2). In Group 2, marginal but statistically significant reduction of TC and HDL-C (by 4.1% and 7.5%) was detected and, additionally, a trend to decrease LDL-C and to increase TG was observed (Table 2). As compared with Group 2, the final level of TC was significantly lower in Group 1 (difference between means: 23.6 mg/dl (11.4%), 95% confidence interval: 3.5–43.3, P = 0.02) and LDL-C (difference between means: 21.7 mg/dl (15.3%), 95% confidence interval: 5.0–37.9, P = 0.01); the final levels of HDL-C and TG were comparable (Fig. 1).
Discussion
Our study shows that a single dose of 80 mg fluvastatin, when administered at admission, reduced TC and LDL-C in patients with ACS within 24 h. To our knowledge, this is the first clinical study showing an immediate effect of statins on the blood lipids in patients with ACS.
We realized that the analysis of non-fasting lipids represents a non-standard measurement. The assessment of non-fasting lipids was, however, used also in other studies focusing on the rapid effect of statins [16]; we have not observed TG levels high enough to interfere with the analysis of other lipid parameters. Possible further limitations include also the open-label design and the relatively low number of participants; moreover, in ACS patients, there are marked “spontaneous” changes in blood lipids during the first 24 h, probably as a consequence of stress conditions, resulting in a wide range of measured levels.
Our results are in accordance with several recently published studies demonstrating the lipid-lowering effect of statins in only several days: Michelena et al. [16] reported reduction of TC and LDL-C after 3 days of high-dose simvastatin therapy in stable patients with a high-risk for coronary events; similarly, decreased TC and LDL-C after one-week therapy with atorvastatin was observed by Zhou et al. [15] in patients with ACS and Marchesi et al. [17] in hypocholesterolemic women; furthermore, Li et al. [18] found decreased TC and LDL-C after a two-week therapy with simvastatin in patients with hypercholesterolemia. On the other hand, Tsunekawa et al. [19] did not observe any difference in blood lipids after a three-day therapy with cerivastatin in elderly diabetic patients; this discrepancy can be at least partly explained by the low dose of cerivastatin (0.15 mg/day) as well as by different patient characteristics.
During the past 5 years, large, randomized clinical trials were published, showing safety and in some points also efficacy of statin therapy, started several days after ACS [20–28]. A possible beneficial effect of statins, when administered soon after the beginning of symptoms of ACS, i.e., in the first-line therapy, has been shown recently by our group [9] and others [6–8, 10, 11]. This effect has been at least partly explained by rapid anti-inflammatory activity of statins [9, 11], as well as an increased production of NO [6, 7]. Our present results suggest, however, that besides the acute “pleiotropic effect” statins exert also an immediate classical, lipid-lowering effect. Obviously, this study cannot answer the question whether rapid reduction of TC and LDL-C during the first day of hospitalization for ACS may result in plaque stabilization and improved prognosis; it points out, however, that cholesterol lowering has to be taken into consideration.
It may be concluded that statins, when administered in the first-line therapy of ACS can have a beneficial effect caused not only by decreased inflammation and increased production of NO, but also by reduction in TC and LDL-C; large, controlled clinical trials are, however, required to confirm this hypothesis; some are already under way [29].
References
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) (1994) Lancet 344(8934):1383–1389
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels (1998) The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339(19):1349–1357
Sacks FM, Pfeffer MA, Moye LA et al (1996) The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 335(14):1001–1009
Ostadal P, Alan D, Vejvoda J (2005) Statins in the first-line therapy of acute coronary syndrome - similar to aspirin? Exp Clin Cardiol 10(1):9–16
Fang CH, Li JJ, Hui RT (2005) Statin, like aspirin, should be given as early as possible in patients with acute coronary syndrome. Med Hypotheses 64(1):192–196
Wolfrum S, Dendorfer A, Schutt M et al (2004) Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol 44(3):348–355
Bell RM, Yellon DM (2003) Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol 41(3):508–515
Hayashidani S, Tsutsui H, Shiomi T et al (2002) Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 105(7):868–873
Ostadal P, Alan D, Hajek P et al (2003) The effect of early treatment by cerivastatin on the serum level of C-reactive protein, interleukin-6, and interleukin-8 in the patients with unstable angina and non-Q-wave myocardial infarction. Mol Cell Biochem 246(1-2):45–50
Tousoulis D, Antoniades C, Katsi V et al (2005) The impact of early administration of low-dose atorvastatin treatment on inflammatory process, in patients with unstable angina and low cholesterol level. Int J Cardiol
Macin SM, Perna ER, Farias EF et al (2005) Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J 149(3):451–457
Ray KK, Cannon CP (2005) Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol 96(5A):54F–60F
Nair P, Roguin A (2006) Statins and the acute coronary syndrome: ‘the early bird catches the worm’. Int J Clin Pract 60(6):716–727
Kanadasi M, Cayli M, Demirtas M et al (2006) The effect of early statin treatment on inflammation and cardiac events in acute coronary syndrome patients with low-density lipoprotein cholesterol. Heart Vessels 21(5):291–297
Zhou T, Zhou SH, Qi SS et al (2006) The effect of atorvastatin on serum myeloperoxidase and CRP levels in patients with acute coronary syndrome. Clin Chim Acta 368(1-2):168–172
Michelena HI, Osorio LA, Citkowitz E (2005) Cholesterol levels after 3 days of high-dose simvastatin in patients at moderate to high risk for coronary events. Int J Cardiol 101(1):111–114
Marchesi S, Lupattelli G, Siepi D et al (2000) Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol 36(5):617–621
Li JJ, Chen MZ, Chen X et al (2003) Rapid effects of simvastatin on lipid profile and C-reactive protein in patients with hypercholesterolemia. Clin Cardiol 26(10):472–476
Tsunekawa T, Hayashi T, Kano H et al (2001) Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation 104(4):376–379
Kayikcioglu M, Turkoglu C, Kultursay H et al (1999) The short term results of combined use of pravastatin with thrombolytic therapy in acute myocardial infarction [abstract]. Circulation 100(suppl 1):I-303, Abstract 1586
Schwartz GG, Olsson AG, Ezekowitz MD et al (2001) Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285(13):1711–1718
Liem AH, van Boven AJ, Veeger NJ et al (2002) Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 23(24):1931–1937
Arntz HR, Agrawal R, Wunderlich W et al (2000) Beneficial effects of pravastatin (+/−colestyramine/niacin) initiated immediately after a coronary event (the randomized Lipid-Coronary Artery Disease [L-CAD] Study). Am J Cardiol 86(12):1293–1298
Cannon CP, Braunwald E, McCabe CH et al (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350(15):1495–1504
Thompson PL, Meredith I, Amerena J et al (2004) Effect of pravastatin compared with placebo initiated within 24 h of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J 148(1):e2
de Lemos JA, Blazing MA, Wiviott SD et al (2004) Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 292(11):1307–1316
Briel M, Schwartz GG, Thompson PL et al (2006) Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA 295(17):2046–2056
Hulten E, Jackson JL, Douglas K et al (2006) The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med 166(17):1814–1821
Ostadal P, Alan D, Hajek P et al (2005) Fluvastatin in the therapy of acute coronary syndrome: rationale and design of a multicenter, randomized, double-blind, placebo-controlled trial (The FACS Trial)[ISRCTN81331696]. Curr Control Trials Cardiovasc Med 6(1):4
Acknowledgments
This study is supported by the grant of the Czech Ministry of Health, Nr. 00000064203 and by the Charles University in Prague Research Project MSM 0021620817, awarded by the Ministry of Education, Youth and Physical Education of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ostadal, P., Alan, D., Vejvoda, J. et al. Immediate effect of fluvastatin on lipid levels in acute coronary syndrome. Mol Cell Biochem 306, 19–23 (2007). https://doi.org/10.1007/s11010-007-9549-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9549-8