Abstract
Although statin therapy is beneficial in the setting of acute coronary syndrome (ACS), a substantial proportion of patients with ACS still do not receive the guideline-recommended lipid management in contemporary practice. We hypothesize that the low-density lipoprotein cholesterol (LDL-C) level at the time of admission might affect patient management and the subsequent outcome. Nine-hundred and forty-two consecutive patients with ACS who underwent percutaneous coronary intervention were analyzed retrospectively. The study patients were first divided into two groups based on the LDL-C level on admission: group A (n = 267), with LDL-C < 100 mg/dL; and group B (n = 675), with LDL-C ≥ 100 mg/dL. Each group was then further divided into those who were prescribed statins or not at the time of discharge from the hospital. The primary endpoint was all-cause death. In addition, we analyzed the serial changes of LDL-C within 1 year. Patients in group A were significantly older and more likely to have multiple comorbidities compared with group B. The proportion of patients who were prescribed statin at discharge was significantly smaller in group A compared with group B (57.7 vs. 77.3%, p < 0.001). During the median 4-year follow-up, there were 122 incidents of all-cause death. Multivariate Cox proportional hazard analysis revealed that LDL-C < 100 mg/dL on admission [hazard ratio (HR), 1.61; 95% confidence interval (CI), 1.09–2.39; p < 0.05] and prescription of statins at discharge (HR, 0.52; 95% CI, 0.36–0.76; p < 0.001) were associated significantly with all-cause death. Under these conditions, increasing LDL-C levels were documented during follow-up in those patients in group A when no statins were prescribed at discharge (79 ± 15–96 ± 29 mg/dL, p < 0.001), whereas these remained unchanged when statins were prescribed at discharge (79 ± 15–77 ± 22 mg/dL, p = 0.30). These results demonstrate that decreased LDL-C on admission in ACS led to less prescription for statins, which could result in increased death, probably due to underestimation of the baseline LDL-C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the previous studies have suggested that lipid-lowering therapy with statins is beneficial in the setting of acute coronary syndrome (ACS), a substantial proportion of patients with ACS still do not receive the guideline-recommended lipid management in contemporary practice [1,2,3]. On the other hand, recent studies have suggested that low levels of low-density lipoprotein cholesterol (LDL-C) were paradoxically associated with the incidence of adverse outcomes in ACS [4,5,6,7]. Moreover, few studies have evaluated the effect of statin therapy on serial changes of LDL-C, particularly in those with low baseline LDL-C [8, 9]. Since the previous studies have shown that LDL-C in patients with ACS decreased spontaneously through the acute phase response [10,11,12,13], we hypothesized that the LDL-C level on admission might affect the patient management and the subsequent outcome. Thus, our objectives were: (1) to investigate whether decreased LDL-C on admission was associated with increased adverse events; (2) to describe the serial changes of LDL-C levels with or without statin therapy at discharge in ACS patients undergoing percutaneous coronary intervention (PCI).
Materials and methods
Patient population
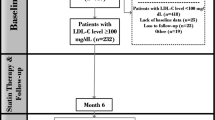
We reviewed the records from 1115 consecutive patients with ACS who underwent emergent coronary angiography at two institutions (Kanazawa University Hospital and Kanazawa Cardiovascular Hospital) from November 2006 and October 2014. Among these, five patients without coronary revascularization and forty-one patients who underwent emergent coronary artery bypass grafting were excluded from our analysis. Thus, 1069 consecutive ACS patients who underwent emergent PCI were analyzed retrospectively. All PCI procedures were performed using standard techniques. Of these, 24 patients whose LDL-C on admission was not available, 50 patients who died within 30 days, and 53 patients without follow-up information at 30 days were excluded from our study, resulting in a final sample size of 942 subjects. The study patients were first divided into two groups based on the LDL-C level on admission. Each group was then further divided into those who were prescribed statins or not at hospital discharge (Fig. 1). All patient data, including the clinical records, laboratory results, and angiograms, were independently reviewed by two cardiologists. This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Bioethical Committee on Medical Research, School of Medicine, Kanazawa University.
Biochemical analysis
In all patients, peripheral venous blood samples for laboratory analysis were drawn at the time of presentation before the patients were transferred to the catheter laboratory. Serum levels of total cholesterol, triglyceride, and high-density cholesterol were determined enzymatically (Qualigent, Sekisui Medical, Tokyo, Japan) using automated instrumentation based on assays, as described previously [14]. The LDL-C level was calculated as described by Friedewald et al., except when the triglyceride level exceeded 400 mg/dL [15]. In such instances, direct LDL-C measurement was used. Follow-up LDL-C was assessed at least 1 month after but within 1 year of the admission LDL-C.
Renal function was expressed as the estimated glomerular filtration rate (eGFR) on admission, and was calculated using the Modification of Diet in Renal Disease formula, as modified for Japanese patients [16]. Anemia was defined as admission hemoglobin < 13 g/dL in men or < 12 g/dL in women [17]. Hypoalbuminemia was defined as serum albumin < 3.5 g/dL [18].
Definition of risk factors and other variables
Hypertension was defined as a blood pressure ≥ 140/90 mmHg or requiring treatment with antihypertensive medications. Diabetes mellitus was defined as HbA1c ≥ 6.5%, plasma glucose ≥ 200 mg/dL, or requiring treatment with insulin or hypoglycemic agents. We defined ACS as ST-segment elevated myocardial infarction, non-ST-segment elevated myocardial infarction, or unstable angina pectoris. Patients with elevated creatine kinase, twofold greater than the normal reference, with elevated MB fraction were considered to have myocardial infarction [19]. Patients with ST-segment elevated myocardial infarction had ST-segment elevation > 1 mm or more contiguous leads. Non-ST-segment elevated myocardial infarction exhibited elevated cardiac enzymes, as noted above, without ST-segment elevation on the ECG. Unstable angina pectoris was defined by the following criteria: the presence of typical chest discomfort lasting at least 5 min and occurring within 96 h of (or during) hospital admission and having an unstable pattern of pain, consisting of either resting pain, new onset, severe or frequent angina, or accelerating angina [20]. Classification of the statin intensity was determined according to a previous study [21]. The strong statin regimen was comprised of rosuvastatin, atorvastatin, and pitavastatin. The standard statin regimen was comprised of pravastatin, fluvastatin, and simvastatin.
Outcome measurement
The endpoint of this study was all-cause death. Clinical information was obtained from a review of the hospital record or by telephone contact with the patients, the family members, or the primary care physicians. Cardiovascular death was defined as presumed cardiovascular death, unexpected death, death from myocardial infarction, arrhythmia, congestive heart failure or stroke, and other cardiovascular diseases, including abdominal aortic aneurysm rupture and pulmonary embolism [22]. Clinical follow-up was performed from January 1, 2017 to June 31, 2017. The endpoints were reviewed independently by two cardiologists.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation, and examined using unpaired t test or Mann–Whitney U test. Categorical data were presented as the frequency and percentage, and frequency analysis was performed using χ2 test. Paired variables obtained from the serial LDL-C levels were compared using paired t test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated with the use of a Cox proportional hazard model. The candidate variables that were entered into the multivariate model included age, gender, and variables with p < 0.05 on univariate analysis in consideration of potential confounding variables. Survival curves were constructed with Kaplan–Meier estimates and compared by log-rank tests. We performed a landmark survival analysis with the landmark set at 30 days. All analyses were 2-sided, and significance was established at p < 0.05. All analyses were performed using JMP pro Version 12 (SAS institute, Cary, NC, USA).
Results
Characteristics of the study patients
The baseline clinical characteristics of the patients in group A (n = 267; 28.3% of the 942 patients) and group B (n = 675) are shown in Table 1. The patients in group A were significantly older compared with group B (70 ± 12 vs. 67 ± 12 years, p < 0.001) and the eGFR was significantly lower in group A (63 ± 31 vs. 73 ± 27 mL/min/1.73 m2, p < 0.001). Of note, approximately half of the patients in group A were complicated with anemia (52.1 vs. 21.8%, p < 0.001). Furthermore, the patients in group A were more likely to have received prehospital drug treatment, including statins; nevertheless, the proportion of patients who were prescribed statins at discharge was significantly smaller in group A compared with group B (57.7 vs. 77.3%, p < 0.001).
The angiographic and procedural characteristics are presented in Table 2. While the culprit lesion and type of stent were well balanced, aspiration catheter (54.3 vs. 64.7%, p < 0.05), distal protection (26.6 vs. 33.0%, p = 0.052), intravascular ultrasound (86.5 vs. 91.4%, p < 0.05), and radial approach (50.9 vs 69.0%, p < 0.001) were less likely to be used in group A compared with group B.
Mortality
The median duration was 4.2 years (interquartile range 2.2–6.5 years; range 31–3465 days). Complete 1-, 2-, 4-year follow-up information was obtained for 89.6, 83.7, and 61.9% of all patients, respectively.
During follow-up, there were 122 incidents of all-cause death. Cardiovascular death occurred in 67 patients. Details of the death events in both groups A and B are shown in Table 3.
On multivariate Cox proportional hazard regression analysis, age > 70 years (HR 2.41; 95% CI 1.60–3.71; p < 0.001), diabetes mellitus (HR 1.83; 95% CI 1.26–2.68; p < 0.05), eGFR < 60 mL/min/1.73 m2 (HR 1.80; 95% CI 1.22–2.68; p < 0.05), anemia (HR 1.62; 95% CI 1.05–2.52; p < 0.05), left ventricular ejection fraction < 40% (HR 1.68; 95% CI 1.06–2.59; p < 0.05), and final post-PCI Thrombosis In Myocardial Infarction flow grade 3 (HR 0.39; 95% CI 0.21–0.79; p < 0.05) were associated significantly with all-cause death (Table 4). In addition, admission LDL-C < 100 mg/dL (HR 1.61; 95% CI 1.09–2.39; p < 0.05) and prescription of statins at discharge (HR 0.52; 95% CI 0.36–0.76; p < 0.001) were also independent predictors of all-cause death.
Moreover, predictors of cardiovascular mortality were also evaluated (Table 5). Prescription of statins was related significantly cardiovascular death (HR 0.59; 95% CI 0.35–0.98; p < 0.05), whereas admission LDL-C < 100 mg/dL was not (HR 1.34; 95% CI 0.78–2.28; p = 0.29). Prehospital statin therapy did not affect the long-term outcome (data not shown).
Kaplan–Meier estimates for death at 7 years were 47.5% in group A without statin prescription, 28.6% in group A with statin prescription, 21.3% in group B without statin prescription, and 12.5% in group B with statin prescription (Fig. 2). While statin prescription provided favorable outcomes in both groups A and B, there was a significant difference in the mortality between the groups among the patients prescribed statins at discharge.
Cumulative risk of death after percutaneous coronary intervention for patients stratified across the LDL-C level on admission and prescription of statins at discharge. *p < 0.001 vs. group B & statin (+) at discharge. †p < 0.05 vs. group B & statin (+) at discharge. ‡p < 0.05 vs. group A & statin (+) at discharge
Difference in the proportion of statin intensity
To investigate why group A, comprised of patients with LDL-C < 100 mg/dL, had worse outcomes than those with LDL-C ≥ 100 mg/dL, even under statin therapy, we assessed if there were any differences of the statin intensity between groups. We found that the prevalence of strong statins was significantly lower in patients in group A compared with group B among the patients prescribed statins at discharge (74.7 vs. 90.2%, p < 0.001; Fig. 3).
Difference in the proportion of statin intensity according to the LDL-C level on admission among those patients prescribed statins at discharge (n = 676). The strong statin group (586 patients) was comprised of those treated with rosuvastatin (279 patients; median daily dose 2.5 mg), atorvastatin (244 patients; median daily dose 10 mg), and pitavastatin (63 patients; median daily dose 1 mg). The standard statin group (90 patients) was comprised of those treated with pravastatin (45 patients; median daily dose 10 mg), fluvastatin (41 patients; median daily dose 20 mg), and simvastatin (4 patients; median daily dose 10 mg)
Serial changes in LDL-C
In our study population of 942 patients, serial LDL-C measurements were available from 819 patients (86.9%). The median time between admission and follow-up LDL-C assessment was 4.5 months (interquartile range: 3.3–6.5 months); the time to follow-up measurement of LDL-C was not statistically different between group A and group B, with or without statin prescription at discharge.
Interestingly, the LDL-C levels in patients in group A with no statin prescription at discharge increased significantly from 79 ± 15–96 ± 29 mg/dL (p < 0.001; Fig. 4a). In contrast, the LDL-C levels in group A with statins prescribed at discharge remained unchanged (79 ± 15–77 ± 22 mg/dL, p = 0.30; Fig. 4b). Moreover, the patients in group B with no statins prescribed at discharge did not experience a dramatic drop in LDL-C levels (127 ± 20–119 ± 27 mg/dL, p < 0.05; Fig. 4c) compared with those in group B who were prescribed statins at discharge (142 ± 32–89 ± 24 mg/dL, p < 0.001; Fig. 4d). The effects of statin intensity on serial changes of LDL-C level are shown in Fig. 5.
Serial changes of LDL-C levels between admission and short-term follow-up. Serial changes of LDL-C level in patients in group A [with no prescription of statins at discharge (a); with prescription of statins at discharge (b)] and group B [with no prescription of statins at discharge (c); with prescription of statins at discharge (d)] are shown
Serial changes of LDL-C levels between admission and short-term follow-up under statin therapy. Serial changes of LDL-C level in patients in group A [with prescription of strong statins (a); standard statins (b) at discharge] and group B [with prescription of strong statins (c); standard statins (d), at discharge] are shown
Discussion
This study provides new evidence in real-world patients with ACS, as follows; (1) Low admission LDL-C was an important predictor that was associated with death. (2) Low admission LDL-C tended to receive less statin therapy at discharge. (3) Increasing LDL-C levels were documented during follow-up in patients exhibiting low LDL-C level on admission and without statin therapy at discharge. The current data demonstrate that patients exhibiting decreased LDL-C on admission tended to receive less statin therapy, leading to a greater number of incidents of all-cause death than in whom the LDL-C was preserved, probably due to underestimation of the baseline LDL-C level.
The previous studies have shown that cholesterol levels begin to decrease within 24 h after ACS through the acute inflammatory response, reach a nadir at approximately 1 week, and recover gradually thereafter [10,11,12,13]. Thus, increasing the LDL-C level during short-term follow-up, which was observed in the group with low admission LDL-C and without statin prescription at discharge, was not particularly surprising. Since it is possible that the lipoprotein values obtained immediately upon admission were already lower as a baseline, attention must be paid to the assessment of LDL-C levels in ACS patients and to not forget to prescribe statins at discharge.
The Japanese guidelines state that the target LDL-C level in patients with coronary artery disease is < 100 mg/dL as secondary prevention [23]. In addition, the National Cholesterol Education Program recommends an optimal target LDL-C of < 70 mg/dL in patients in the high-risk group [24]. Under these conditions, recent studies have shown the benefit of aggressively reducing LDL-C to the target level in patients with ACS [25, 26]. Furthermore, in the present study, the proportion of patients prescribed strong statins was less in those with decreased LDL-C on admission compared with those with preserved LDL-C. The effects of strong statins, beyond LDL-C lowering, could be considered as a possible explanation for the reduction of cardiovascular disease in patients receiving strong statins [21]. Moreover, a recent intracoronary imaging study suggested that patients with decreased LDL-C on admission for ACS suffer from a greater number of residual risk factors [27]. Although a previous study already demonstrated that the aggressive use of strong statins could improve the outcome of ACS [28], further investigation is needed to elucidate the mechanism underling the beneficial effects of the strong statin and modifying the residual risk factors, especially in patients with decreased LDL-C on admission.
There are several limitations to the present study. First, this study was a retrospective analysis, and follow-up LDL-C levels were not available from all patients. Those patients without follow-up data or who died as a result of ACS before the serial lipid study were not included; therefore, some caution should be taken when extending our findings to other cohorts. Second, serum cholesterol is not only affected by ACS but also by other critical illnesses [29,30,31,32]. Although we performed risk-adjusted analyses to compensate for potential confounders factors, it is possible that we were not able to correct for some important unidentified variables, i.e., increased cholesterol absorption and tolerance of statin therapy, which might affect the outcome [33]. Third, we could not compare the efficacy of statins at different doses. We were also not able to assess the relationship between statin intensity and cardiovascular outcomes, due to the relatively small number of subjects in each subgroup. A long-term, large-scale trial evaluating the precise clinical events is needed, particularly in patients with ACS exhibiting low baseline LDL-C. Finally, this study did not include a detailed analysis regarding the onset of symptoms that could affect the LDL-C level on admission. If subjects with low baseline LDL-C experienced a longer delay before hospital admission, this might be a relevant factor. Despite these limitations, the present study was able to clearly show the importance of identifying patients with decreased LDL-C on admission as a high-risk subgroup of ACS.
In conclusion, our study showed that ACS patients exhibiting low LDL-C levels on admission represent a high-risk subgroup that tend to receive less statin therapy and subsequently experience adverse outcomes, likely due to underestimation of the baseline LDL-C level. It is not rare for LDL-C to remain low in patients with ACS, even when measured immediately at admission. Future investigations should determine whether more aggressive lipid-lowering therapy with strong statins can improve outcomes, in addition to modifying residual risk factors, especially in patients with decreased LDL-C level on admission.
References
Ko DT, Alter DA, Newman AM, Donovan LR, Tu JV (2005) Association between lipid testing and statin therapy in acute myocardial infarction patients. Am Heart J 150:419–425
Elbarouni B, Banihashemi SB, Yan RT, Welsh RC, Kornder JM, Wong GC, Anderson FA, Spencer FA, Grondin FR, Goodman SG, Yan AT, Canadian Global of Registry Acute Coronary Events (GRACE, GRACE(2)), Canadian Registry of Acute Coronary Events (CANRACE) Investigators (2012) Temporal patterns of lipid testing and statin therapy in acute coronary syndrome patients (from the Canadian GRACE experience). Am J Cardiol 109:1418–1424
Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T, PACIFIC investigators (2013) Management and 2-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J 77:934–943
Al-Mallah MH, Hatahet H, Cavalcante JL, Khanal S (2009) Low admission LDL-cholesterol is associated with increased 3-year all-cause mortality in patients with non ST segment elevation myocardial infarction. Cardiol J 16:227–233
Oduncu V, Erkol A, Kurt M, Tanboğa IH, Karabay CY, Şengül C, Bulut M, Özveren O, Fotbolcu H, Akgün T, Türkyilmaz E, Kırma C (2013) The prognostic value of very low admission LDL-cholesterol levels in ST-segment elevation myocardial infarction compared in statin-pretreated and statin-naive patients undergoing primary percutaneous coronary intervention. Int J Cardiol 167:458–463
Reddy VS, Bui QT, Jacobs JR, Begelman SM, Miller DP, French WJ, Investigators of National Registry of Myocardial Infarction (NRMI) 4b–5 (2015) Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol 115:557–562
Miura M, Yamasaki M, Uemura Y, Yoshikawa M, Miyauchi K, Tanaka H, Miyachi H, Yamashita J, Suzuki M, Yamamoto T, Nagao K, Komuro I, Takayama M (2016) Effect of statin treatment and low-density lipoprotein-cholesterol on short-term mortality in acute myocardial infarction patients undergoing primary percutaneous coronary intervention—multicenter registry from Tokyo CCU Network Database. Circ J 80:461–468
Giraldez RR, Giugliano RP, Mohanavelu S, Murphy SA, McCabe CH, Cannon CP, Braunwald E (2008) Baseline low-density lipoprotein cholesterol is an important predictor of the benefit of intensive lipid-lowering therapy: a PROVE IT-TIMI 22 (pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22) analysis. J Am Coll Cardiol 52:914–920
Kanadaşi M, Cayli M, Demirtaş M, Inal T, Demir M, Koç M, Avkaroğullari M, Dönmez Y, Usal A, Alhan CC, San M (2006) The effect of early statin treatment on inflammation and cardiac events in acute coronary syndrome patients with low-density lipoprotein cholesterol. Heart Vessels 21:291–297
Fyfe T, Baxter RH, Cochran KM, Booth EM (1971) Plasma-lipid changes after myocardial infarction. Lancet 2:997–1001
Pfohl M, Schreiber I, Liebich HM, Häring HU, Hoffmeister HM (1999) Upregulation of cholesterol synthesis after acute myocardial infarction—is cholesterol a positive acute phase reactant? Atherosclerosis 142:389–393
Rosenson RS (1993) Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol 22:933–940
Pitt B, Loscalzo J, Ycas J, Raichlen JS (2008) Lipid levels after acute coronary syndromes. J Am Coll Cardiol 51:1440–1445
Tada H, Kawashiri MA, Yoshida T, Teramoto R, Nohara A, Konno T, Inazu A, Mabuchi H, Yamagishi M, Hayashi K (2016) Lipoprotein(a) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ J 80:512–518
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Ennezat PV, Maréchaux S, Pinçon C, Finzi J, Barrailler S, Bouabdallaoui N, Van Belle E, Montalescot G, Collet JP (2013) Anaemia to predict outcome in patients with acute coronary syndromes. Arch Cardiovasc Dis 106:357–365
Oduncu V, Erkol A, Karabay CY, Kurt M, Akgün T, Bulut M, Pala S, Kirma C (2013) The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis 24:88–94
Nayak AK, Kawamura A, Nesto RW, Davis G, Jarbeau J, Pyne CT, Gossman DE, Piemonte TC, Riskalla N, Chauhan MS (2006) Myocardial infarction as a presentation of clinical in-stent restenosis. Circ J 70:1026–1029
Cannon CP, McCabe CH, Stone PH, Rogers WJ, Schactman M, Thompson BW, Pearce DJ, Diver DJ, Kells C, Feldman T, Williams M, Gibson RS, Kronenberg MW, Ganz LI, Anderson HV, Braunwald E (1997) The electrocardiogram predicts 1-year outcome of patients with unstable angina and non-Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in myocardial ischemia. J Am Coll Cardiol 30:133–140
Natsuaki M, Furukawa Y, Morimoto T, Nakagawa Y, Ono K, Kaburagi S, Inada T, Mitsuoka H, Taniguchi R, Nakano A, Kita T, Sakata R, Kimura T, CREDO-Kyoto PCI, CABG registry cohort-2 investigators (2012) Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization. Perspectives from the CREDO-Kyoto registry cohort-2. Circ J 76:1369–1379
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research Consortium (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115:2344–2351
Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T, Yokote K, Yokode M (2007) Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb 14:45–50
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, National Heart, Lung, and Blood Institute, American College of Cardiology Foundation, American Heart Association (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, Investigators IMPROVE-IT (2015) Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372:2387–2397
Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H, PRECISE–IVUS Investigators (2015) Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol 66:495–507
Takaoka N, Tsujita K, Kaikita K, Hokimoto S, Yamanaga K, Komura N, Chitose T, Ono T, Mizobe M, Horio E, Sato K, Nakayama N, Saito M, Iwashita S, Kojima S, Tayama S, Sugiyama S, Nakamura S, Ogawa H (2014) Intravascular ultrasound morphology of culprit lesions and clinical demographics in patients with acute coronary syndrome in relation to low-density lipoprotein cholesterol levels at onset. Heart Vessels 29:584–595
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350:1495–1504
Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ (2004) Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291:451–459
Nakayama A, Morita H, Miyata T, Hoshina K, Nagayama M, Takanashi S, Sumiyoshi T, Komuro I, Nagai R (2014) Predictors of mortality after emergency or elective repair of abdominal aortic aneurysm in a Japanese population. Heart Vessels 29:65–70
Karataş MB, Güngör B, İpek G, Çanga Y, Günaydın ZY, Onuk T, Durmuş G, Yelgeç NS, Yılmaz HY, Bolca O (2016) Association of serum cholesterol levels with short-term mortality in patients with acute pulmonary embolism. Heart Lung Circ 25:365–370
Phuah CL, Raffeld MR, Ayres AM, Viswanathan A, Greenberg SM, Biffi A, Rosand J, Anderson CD (2016) Subacute decline in serum lipids precedes the occurrence of primary intracerebral hemorrhage. Neurology 86:2034–2341
Hagiwara N, Kawada-Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H (2017) Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J 38:2264–2276
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nakahashi, T., Tada, H., Sakata, K. et al. Paradoxical impact of decreased low-density lipoprotein cholesterol level at baseline on the long-term prognosis in patients with acute coronary syndrome. Heart Vessels 33, 695–705 (2018). https://doi.org/10.1007/s00380-017-1111-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1111-3